Synthesis and Evaluation of Bile Acid–Ribavirin Conjugates as Prodrugs to Target the Liver
Abstract
Ribavirin is used to treat hepatitis C but causes serious hemolytic anemia. The objective of the study was to develop a ribavirin prodrug to achieve liver-specific drug delivery and to reduce its off-target effect in red blood cells (RBCs). The approach aimed to target the human sodium taurocholate cotransporting polypeptide (NTCP), which is a bile acid transporter predominately expressed in the liver. Six prodrugs with ribavirin conjugation at C-3 or C-24 of the bile acids were synthesized. In vitro uptake studies indicated that all six prodrugs were NTCP substrates. Metabolic studies in vitro indicated that ribavirin–l-Val–glycochenodeoxycholic acid (GCDCA) was able to release ribavirin in the mouse liver S9 fraction. Additionally, in vitro studies showed that ribavirin in RBC was reduced by 16.7-fold from prodrug compared with parent drug incubation. Moreover, almost no prodrug was present in RBC. In vivo study in mice also showed that ribavirin–l-Val–GCDCA could provide almost the same ribavirin exposure in the liver as ribavirin administration, but with about 1.8-fold less exposure of ribavirin in RBC, plasma, and kidney. Overall, the study suggested that ribavirin–l-Val–GCDCA has the potential to achieve ribavirin-specific liver delivery. © 2015 Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 104:2864–2876, 2015
Abbreviations used
-
- ASBT
-
- sodium-dependent bile acid transporter
-
- CA
-
- cholic acid
-
- CDCA
-
- chenodeoxycholic acid
-
- DCM
-
- dichloromethane
-
- DEPC
-
- diethyl cyanophosphonate
-
- DIPEA
-
- N,N-diisopropylethylamine
-
- DMF
-
- dimethyl formamide
-
- DMP
-
- 2,2-dimethoxypropane
-
- EtOAc
-
- ethyl acetate
-
- GCDCA
-
- glycochenodeoxycholic acid
-
- HBSS
-
- Hank's balanced salts solution
-
- LTI
-
- liver targeting index
-
- NTCP
-
- sodium taurocholate cotransporting polypeptide
-
- Pd
-
- palladium
-
- PdOH
-
- palladium hydroxide
-
- p-TsOH
-
- p-toluenesulfonic acid
-
- Pybop
-
- benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate
-
- RBC
-
- red blood cell
-
- SFB
-
- sodium-free buffer
-
- TEA
-
- triethylamine
-
- TFA
-
- trifluoroacetic acid
-
- UDCA
-
- ursodeoxycholic acid
INTRODUCTION
Ribavirin is a guanosine analog for treating hepatitis C, which is the disease that primarily affects the liver and may eventually cause liver cirrhosis or liver cancer.1, 2 It is usually administered along with interferon alpha or a protease inhibitor (e.g., boceprevir) to enhance the sustained virological response, where a higher dose of ribavirin is more effective.3 Recent studies suggest that ribavirin may also be used to treat rhinovirus and acute myeloid leukemia.4, 5 However, its usefulness is limited by the dose-dependent hemolytic anemia, which results in dose reduction or treatment termination in approximate 20% and 5% treated patients, respectively.6 This serious side effect is caused by ribavirin accumulation in the anucleate red blood cells (RBCs), where ribavirin undergoes irreversible phosphorylation. Ribavirin phosphorylation in RBCs causes depletion of ATP in a competitive manner and exerts oxidative damage on the cell membrane.7 Targeted delivery of ribavirin to the liver may lower its accumulation in the RBCs and reduce its dose-limiting side effects.
Human sodium taurocholate cotransporting polypeptide (NTCP) is a sodium-dependent bile acid transporter that is involved in the enterohepatic circulation of bile acids. NTCP is predominantly expressed at the basolateral membrane of hepatocytes and probably responsible for transporting more than 80% of conjugated bile acids from portal blood into the liver.8 Because of its liver specificity and high capacity for transporting conjugated bile acids, bile acid conjugates have potential to serve as prodrugs to achieve liver-specific drug delivery.9 Previously, bile acid conjugates were shown to deliver the drug of interest to the liver.10, 11 In the current study, we aim to develop novel bile acid ribavirin prodrugs in order to reduce ribavirin accumulation in RBCs and hence alleviate ribavirin's most concerned side effect of hemolytic anemia.
Briefly, six bile acid–ribavirin prodrugs were synthesized by conjugating ribavirin to bile acids via an amino acid linker. These prodrugs were then evaluated for their in vitro NTCP uptake, metabolic stability, and potential of ribavirin accumulation in RBCs. After in vitro evaluation, a prodrug of ribavirin conjugated to glycochenodeoxycholic acid (GCDCA) via an l-valine linker was selected for in vivo assessment. The in vivo distribution of ribavirin–l-Val–GCDCA was examined in mice and compared with that of ribavirin itself. Results indicated that this bile acid–ribavirin conjugate has the potential to achieve liver-specific delivery of ribavirin and reduce its accumulation in nontarget organs.
EXPERIMENTAL
Figure 1 illustrates the overall approach and scope of compounds tested. Six bile acid–ribavirin prodrugs were collectively evaluated for liver-specific delivery. It should be noted that ribavirin itself is a prodrug, with the phosphorylated form being the active moiety. Nevertheless, for simplicity, ribavirin is referred to as a drug and ribavirin conjugates as prodrugs.
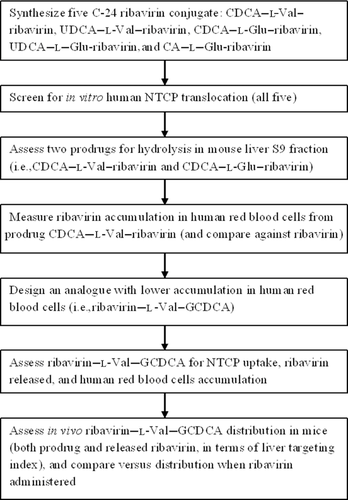
Initially, five ribavirin conjugates were synthesized by conjugating the drug to CDCA, ursodeoxycholic acid (UDCA), or cholic acid (CA) at the C-24 position, via a linker. Prodrug was then screened in vitro for human NTCP-mediated uptake across the cell membrane. All five were NTCP substrates. CDCA–l-Glu-ribavirin and CDCA–l-Val–ribavirin showed the higher normalized Jmax and were thus subjected to ribavirin release assays in mouse S9 fraction. CDCA–l-Val–ribavirin released more than 60% of ribavirin in mouse S9 fraction, and was assessed for accumulation into human RBCs as compared with ribavirin itself. Because of the unsatisfying CDCA–l-Val–ribavirin accumulation into human RBCs, ribavirin–l-Val–GCDCA was synthesized to reduce passive prodrug permeability. This sixth prodrug was also assessed for NTCP-mediated uptake, ribavirin release, and human RBCs accumulation. The prodrug had the lowest RBC accumulation and highest stability in whole blood, and was thus intravenously administrated to mice to evaluate the distribution of both released ribavirin and intact prodrug. Its pharmacokinetics was compared with that of intravenously administrated ribavirin.
Materials
Ribavirin was purchased from Carbosynth (Berkshire, England). CDCA was purchased from AK Scientific, Inc. (Union City, California). UDCA was purchased from Spectrum Chemical (New Brunswick, New Jersey). Benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (Pybop) was purchased from EMD Millipore (Billerica, Massachusetts). All other chemicals were purchased from Sigma–Aldrich (St. Louis, Missouri). Geneticin, fetal bovine serum (FBS), trypsin, Dulbecco's modified Eagle medium (DMEM), and pooled Balb-c mice S9 fraction were obtained from Invitrogen (Rockville, Maryland). Human whole blood was obtained from Innovative Research (Novi, Michigan).
Synthetic Method Overview
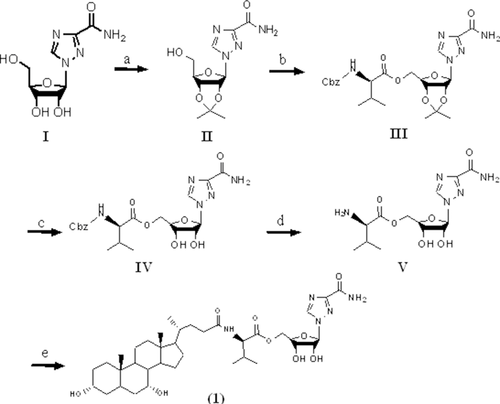
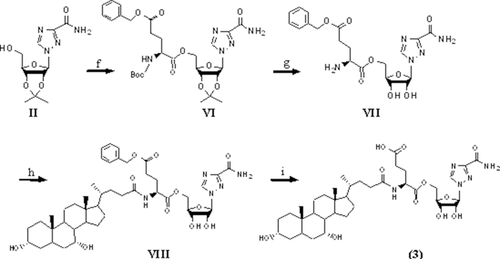
Schemes 1 and 2 show the synthesis of ribavirin prodrug conjugated to the C-24 position of bile acids. Briefly, the hydroxyl groups of ribavirin were initially protected with an acetonide group. Following protection, the intermediate was conjugated to an N-protected amino acid via an ester bond. After removal of the acetonide group and the protecting group at the N-terminal, the compound was conjugated to the amino acid's carboxylic acid at the C-24 position of CDCA, UDCA, or CA via amide bond. Hydrogenation was carried out when necessary to yield final prodrugs. Ribavirin was conjugated to the bile acids using either l-valine or l-glutamic acid, as shown in Schemes 1 and 2.
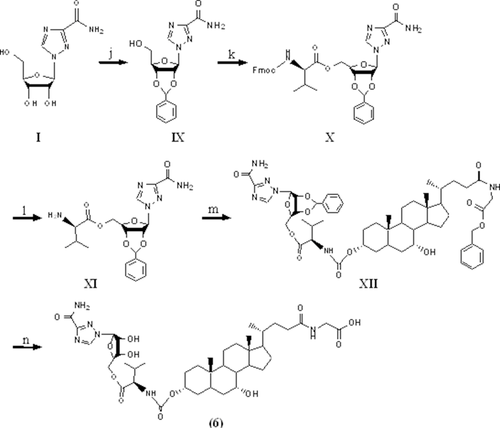
Scheme 3 shows the synthesis of ribavirin prodrugs conjugated at the C-3 position of bile acids. Briefly, the hydroxyl groups of ribavirin were initially protected by benzylidene acetals. The resulting intermediate was then conjugated to l-valine via an ester bond. The compound was then coupled to the hydroxyl group at the C-3 position of GCDCA benzyl ester via a carbamate bond. Hydrogenation was carried out to remove the benzylidene acetals and the benzyl ester protection group, yielding the final prodrug.
The identity and purity of all prodrugs were confirmed by thin layer chromatography, mass spectrometry (MS), and nuclear magnetic resonance (Table S1).
Synthesis of Ribavirin Acetonide (Compound II)
To the suspension of ribavirin (compound I in Scheme 1; 2.0 g, 8.2 mmol) in acetone (40 mL), p-toluenesulfonic acid monohydrate (p-TsOH; 0.30 g, 1.58 mmol), and 2, 2-dimethoxypropane (DMP; 2 mL, 16 mmol) were added. The reaction was heated to 60°C and stirred for 6 h, then cooled to room temperature. The acetone was evaporated under vacuum and purified by silica gel column chromatography using a mobile phase of dichloromethane (DCM) and methanol (v/v, 14:1) to afford the intermediate II as a white solid with 73% yield. MS showed an appropriate peak of [M+Na] 307.2.
Synthesis of l-Valine–Ribavirin Conjugates of Bile Acids (Compounds 1 and 2)
Intermediate II (1.2 g, 4.1 mmol) was coupled to N-Cbz–l-valine (1.0 g, 4.1 mmol) using Pybop (2.1 g, 4.1 mmol) as the coupling reagent in 10 mL dimethyl formamide (DMF) along with N,N-diisopropylethylamine (DIPEA; 1.4 mL, 8.1 mmol). The reaction was stirred at room temperature overnight. The reaction was then quenched by adding 50 mL water followed by extraction with ethyl acetate (EtOAc) (3×). The combined organic layer was washed with saturated sodium bicarbonate solution (1×), water (3×), brine (1×), and dried over anhydrous sodium sulfate. The solvent was evaporated to yield a white solid intermediate (compound III; Scheme 1) with 99% yield. MS showed an appropriate peak of [M+H] 518.9.
The intermediate III was dissolved in 5 mL DCM and trifluoroacetic acid (TFA; v/v, 1:9) and stirred at room temperature for 48 h. After excess solvent evaporation under vacuum, saturated sodium bicarbonate solution was added, followed by extraction with EtOAc (3×). The combined organic layer was washed with water (3×), brine (1×), and dried over anhydrous sodium sulfate. The solvent was evaporated and the residue was purified with silica gel column chromatography using a mobile phase of DCM and methanol (v/v, 14:1) to yield a white solid intermediate (compound IV; Scheme 1) with 20% yield.
The intermediate IV was subjected to catalytic hydrogenation to remove the Cbz-protecting group using 10% palladium (Pd)/charcoal in methanol for 4 h. The solution was filtered through Celite and the solvent was evaporated yielding a white solid intermediate (compound V; Scheme 1) with 97% yield. MS showed an appropriate peak of [M+H] 344.1.
To the solution of the intermediate V (0.40 g, 1.2 mmol) in 10 mL DMF, CDCA (0.46 g, 1.2 mmol) was added along with diethyl cyanophosphonate (DEPC; 0.2 mL, 1.3 mmol) and triethylamine (TEA; 1.7 mL, 12 mmol). The reaction was stirred at room temperature overnight. The reaction was quenched by adding 50 mL of water followed by extraction with EtOAc (3×). The combined organic layer was washed with saturated sodium bicarbonate solution (1×), water (3×), brine (1×), and dried over anhydrous sodium sulfate. The solvent was evaporated to yield a white solid. The crude product was further purified by silica gel column chromatography using a mobile phase of DCM–methanol–acetic acid (v/v, 10:1:0.001) that yielded a final white solid product (compound 1; Scheme 1). MS of compound 1 showed appropriate peaks of [M+Na] 740.2 with 78% yield. Compound 2 was synthesized in the same manner, but with UDCA substituted for CDCA. MS of compound 2 showed appropriate peaks of [M+Na] 740.2 with 81% yield.
Synthesis of l-Glutamate–Ribavirin Amide Conjugates of Bile Acids (Compounds 3–5)
Intermediate II in Scheme 1 (1 g, 3.52 mmol) was coupled to Boc–l-glutamic acid 5-benzyl ester (1.19 g, 3.52 mmol) using Pybop (1.83 g, 3.52 mmol) as the coupling reagent in 10 mL DMF along with DIPEA (1.22 mL, 7.04 mmol). The reaction was stirred at room temperature overnight. The reaction was quenched by adding 50 mL water followed by extraction with EtOAc (3×). The combined organic layer was washed with saturated sodium bicarbonate solution (1×), water (3×), brine (1×), and dried over anhydrous sodium sulfate. The solvent was evaporated to yield a white solid intermediate (compound VI; Scheme 2) with 47% yield. MS showed an appropriate peak of [M+Na] 626.1.
The intermediate VI was dissolved in 5 mL DCM and TFA (v/v, 1:9) and stirred at room temperature for 48 h to remove both the acetonide- and Boc-protecting groups. Excess DCM and TFA were evaporated under vacuum to yield an orange oily intermediate (compound VII; Scheme 2), which was used without further purification with 44% yield. MS showed an appropriate peak of [M+H] 464.1.
To the solution of intermediate VII (0.13 g, 0.28 mmol) in 4 mL DMF, CDCA (0.11 g, 0.28 mmol) was added along with DEPC (47 μL, 0.31 mmol) and TEA (390 μL, 2.8 mmol). The reaction was stirred at room temperature overnight. The reaction was quenched by adding 50 mL water followed by extraction with EtOAc (3×). The combined organic layer was washed with saturated sodium bicarbonate solution (1×), water (3×), brine (1×), and dried over the anhydrous sodium sulfate. The solvent was evaporated to yield a white solid intermediate (compound VIII; Scheme 2) with 76% yield. MS showed an appropriate peak of [M+H] 838.3.
Intermediate VIII was subjected to catalytic hydrogenation to remove the 5-benzyl ester group using 10% Pd/charcoal in methanol for 4 h. The solution was filtered through Celite and the solvent was evaporated to give a white solid product. The crude product was further purified by preparative high-performance liquid chromatography using a ZORBAX 300SB C18 PrepHT column (7 μm, 21.2 × 250 mm2; Agilent, Santa Clara, California). The mobile phase was composed of 68% methanol and 32% water with a flow rate of 5 mL/min. UV absorbance was monitored at 210 nm. The fractions containing the product were collected and freeze dried to yield a final white fluffy solid product (compound 3; Scheme 2). Compounds 4 and 5 were synthesized in the same manner, but with UDCA and CA substituted for CDCA, respectively. MS showed appropriate peaks of [M−H] 746.3 with 88% yield, [M−H] 746.3 with 87% yield, and [M−H] 762.3 with 75% yield.
Synthesis of Ribavirin Benzylidene Acetal (Compound IX)
To the suspension of ribavirin (compound I in Scheme 3; 2 g, 8.2 mmol) in benzaldehyde (20 mL), ZnCl2 (2 g, 14.8 mmol) was added. The reaction was stirred at room temperature for 6 h and poured into 250 mL diethyl ether, yielding a white precipitate. After suction filtration, the precipitate was purified by silica gel column chromatography using a mobile phase of DCM and methanol (14:1) to give a white solid intermediate (compound IX; Scheme 3) with 55% yield. MS showed an appropriate peak of [M+Na] 355.1.
Synthesis of l-Valine–Ribavirin Carbamate Conjugate of CDCA (Compound 6)
Intermediate IX (1.49 g, 4.50 mmol) was then coupled to Fmoc–l-valine (1.53 g, 4.50 mmol) using Pybop (2.34 g, 4.50 mmol) in 15 mL DMF along with DIPEA (1.57 mL, 9.00 mmol). The reaction was stirred at room temperature overnight. The reaction was quenched by adding 50 mL water followed by extraction with EtOAc (3×). The combined organic layer was washed with water (3×), brine (1×), and dried over anhydrous sodium sulfate. The solvent was evaporated to yield a white solid intermediate (compound X; Scheme 3) with 84% yield.
The intermediate X was dissolved in 10 mL of DMF with 5% piperidine and reacted for 2 h to remove the Fmoc-protecting group. The resulting product was acidified with 50 mL 2 M HCl and extracted with 50 mL EtOAc. The aqueous layer was immediately alkalized with saturated sodium bicarbonate solution followed by extraction with EtOAc (3×). The combined organic layer was washed with water (3×), brine (1×), and dried over anhydrous sodium sulfate. The solvent was evaporated to yield a white solid intermediate (compound XI; Scheme 3) with 55% yield.
Glycine benzyl ester was conjugated to CDCA with DEPC as the coupling reagent, as described above with 72% yield. To the solution of glycine benzyl ester, protected CDCA (1 g, 1.86 mmol) in 80 mL anhydrous DCM in the presence of the 0.5 mL pyridine (6.21 mmol), triphosgene (0.275 g, 0.93 mmol) in anhydrous DCM was added dropwise. The reaction was stirred at room temperature for 4 h and intermediate XI (0.80 g, 1.86 mmol) was subsequently added. The reaction was stirred for 48 h. After the solvent was evaporated, extraction was conducted with water and EtOAc (3×). The combined organic layer was washed with brine (1×) and dried over anhydrous sodium sulfate. The solvent was evaporated and the residue was subjected to further purification by silica gel column chromatography using a mobile phase of DCM and methanol (v/v, 33:1) to afford the intermediate XII (Scheme 3) as a white solid. MS showed an appropriate peak of [M+Na] 1019.3 with 32% yield.
The intermediate XII was subjected to catalytic hydrogenation to remove the benzylidene acetal- and benzyl ester-protecting groups using 20% palladium hydroxide (PdOH)/charcoal in methanol overnight. The solution was filtered through Celite and solvent was evaporated. The crude product was purified by C18 column chromatography using a mobile phase of water and methanol (v/v, 1.85:1), yielding a final white solid product (compound 6; Scheme 3) with 58% yield. MS showed an appropriate peak of [M−H] 817.4.
Cell Culture
Stably transfected human NTCP-HEK293 cells were grown in a 37°C, 5% CO2 atmosphere with 90% relative humidity and fed every 2 days as previously described.12 The medium was composed of DMEM supplemented with 10% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin, and geneticin (1 mg/mL). Cells were passaged when they reach 80% confluence.
Uptake of Prodrugs by NTCP into Cells
Uptake studies were conducted on NTCP-HEK293 cells. Cells were seeded at the density of 300,000 cells/well in 24-well Biocoat plates. On day 2 after seeding, cells were exposed to various concentrations of prodrugs (0–500 μM), which were dissolved in either Hank's balanced salts solution (HBSS) with 137 mM sodium chloride (pH 6.8) or sodium-free buffer (SFB) where sodium chloride was replaced with tetraethylammonium chloride (pH 6.8). Cells were exposed to the incubation buffer solutions for 5 min. The uptake was terminated by adding ice-cold SFB buffer after incubation buffer was removed. Cells were lysed using acetonitrile. After acetonitrile was evaporated, the lysate were then dissolved in the acetonitrile–H2O (1:1) with 500 nM internal standard (i.e., taurocholate or carbamazepine). The samples were stored at −80°C until further analysis.
 (1)
(1)In Vitro Stability Study
Metabolic stability of prodrug was assessed in mouse liver S9 fraction. Briefly, 5 μM of prodrug were incubated with 1 mg/mL mouse liver S9 fraction supplemented with 1 mM nicotinamide adenine dinucleotide phosphate in Dulbecco's phosphate-buffered saline at 37°C. At designated time points (i.e., 0, 0.5, 1, 3, and 5 h), 100 μL samples were collected and quenched immediately with 400 μL acetonitrile containing 1 μM internal standard. Samples were votexed for 30 sand centrifuged at 20,000 g for 10 min. Supernatant was dried overnight and reconstituted in 200 μL water and stores at −80°C until further analysis.
In Vitro Accumulation of Ribavirin and Prodrug in RBCs
Accumulation of the ribavirin, CDCA–l-Val–ribavirin and ribavirin–l-Val–GCDCA in RBC was determined as previous described with minor changes.13 Briefly, human RBCs were separated from plasma by centrifugation at 400 g at room temperature for 10 min. Plasma was spiked with ribavirin or prodrug and gently mixed with RBC and incubated at 37°C. The final concentration of initial ribavirin or initial prodrug in the whole blood was 100 μM. At designated time points (i.e., 0 and 5 h), 50 μL aliquots of whole blood were collected and centrifuged at 3500 g at 4°C for 2 min to separate RBCs from plasma. RBCs were lysed by adding 100 μL 0.1% triton X-100. Protein was precipitated with the addition of 300 μL and 400 μL of acetonitrile containing 1 μM internal standard to the lysed RBC and plasma, respectively. After votexing for 30 s, the samples were centrifuged at 20,000 g for 10 minutes. After centrifugation, 300-μL supernatant were transferred to 24-well plates and evaporated overnight at room temperature. Dry extracts were reconstituted in 200 μL 5 mM ammonium acetate buffer (pH 4.8). RBC samples were treated with 1 unit of phosphatase and incubated at 37°C overnight to convert phosphorylated ribavirin to ribavirin, which was measured. Both plasma and RBC samples were stored at −80°C until further analysis.
In Vivo Distribution of Ribavirin and Prodrug in Mice
The study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health (Bethesda, Maryland) and was approved by the University of Maryland Institutional Animal Care and Use Committee.
Normal nonfasted Balb/c mice (male, 12 weeks) were tail injected (i.v. bolus) with a dose of 20 mg/kg (0.08 mmol/kg) ribavirin and 67 mg/kg (0.08 mmol/kg) ribavirin–l-Val–GCDCA, respectively, to yield an equimolar amount of drug or prodrug. The compounds were dissolved in 60% saline and 40% polyethylene glycol (PEG 400). At designated time points (10 and 30 min, and 1, 2, 4, and 8 h), mice (n = 3 per time point) were euthanized by CO2 (carbon dioxide) asphyxiation, followed by cervical dislocation. Blood was drawn immediately by cardiac puncture and collected in heparinized vials. Samples were then centrifuged at 3500 g and 4°C for 2 min to separate plasma and RBC. Liver, small intestine, and kidney were isolated and weighed before homogenization in 2 mL water using Digital SLP cell disruptor (Branson Ultrasonics, Danbury, Connecticut).
Four-hundred microliter acetonitrile containing 1 μM internal standard was added to 50 μL tissue homogenate or plasma to precipitate protein. Samples were votexed for 30 s and centrifuged at 20,000 g for 10 minutes. After centrifugation, 300 μL supernatant was transferred to 24-well plates and evaporated overnight at room temperature. Dry extracts were reconstituted in 300 μL water and stored at −80°C until further analysis.
For RBC, 25-μL RBCs were first lysed by 100 μL 0.1% triton X-100. Three-hundred microliter acetonitrile containing 1 μM internal standard was then added to the lysate and votexed for 30 s. Samples were centrifuged at 20,000 g for 10 min. After centrifugation, 300 μL supernatant from each sample was transferred to 24-well plates and evaporated overnight at room temperature. Dry extracts were reconstituted in 300 μL 5 mM ammonium acetate buffer (pH 4.8). Samples were treated with 1 unit of phosphatase at 37°C overnight and stored at −80°C until further analysis.
 (2)
(2)The LTI of ribavirin employed the AUC of ribavirin in the liver and plasma over 8 h (t = 8 h).
Analytical Method
Both ribavirin and prodrugs were quantified by liquid chromatography–mass spectrometry (LC–MS), which was performed on Waters ACQUITY UPLC/TQD system (Waters, Milford, Massachusetts). All the samples were centrifuged at 20,000 g for 5 minutes and 5 μL supernatant were injected into LC–MS. Taurocholate was used as the internal standard for CA–l-Glu–ribavirin, whereas carbamazepine was used as the internal standard for all other compounds.
To determine the prodrug concentration in the cell, a Luna C8 (2) column (3 μm, 2.0 × 50 mm2; Torrance, California) was used. A gradient mobile phase employed methanol and water (v/v) with 0.1% formic acid, respectively. The flow rate was 0.7 mL/min and the compounds were detected under positive electrospray ionization mode [M+H]+, except for CA–l-Glu–ribavirin, which was detected under negative mode [M−H]−. Multiple reaction monitoring was used for all methods. The lower limit of quantification for all compounds was between 1 and 10 nM. Mass transitions and parameter of mass spectrometer for all six prodrugs and internal standards are detailed in Table 1.
| Compound | Multiple Reaction Monitoring Transition | Collision Energy (V) |
|---|---|---|
| Ribavirin | 245.13→112.87 | 16 |
| Carbamazepine (internal standard) | 237.13→193.99 | 26 |
| Taurocholate (internal standard) | 514.26→79.96 | 100 |
| CDCA–l-Val–ribavirina | 718.67→97.00 | 35 |
| UDCA–l-Val–ribavirina | 718.67→97.00 | 35 |
| CDCA–l-Glu–ribavirina | 748.54→97.06 | 72 |
| UDCA–l-Glu–ribavirina | 748.54→97.06 | 72 |
| CA–l-Glu–ribavirina | 762.33→128.01 | 68 |
| Ribavirin–l-Val–GCDCAa | 819.48→96.93 | 74 |
- All compounds were detected under positive electrospray ionization mode [M+H]+ except CA–l-Glu–ribavirin that was detected under negative mode [M−H]−.
- a Accuracy for CDCA–l-Val–ribavirin, UDCA–l-Val–ribavirin, CDCA–l-Glu–ribavirin, UDCA–l-Glu–ribavirin, CA–l-Glu–ribavirin, and ribavirin–l-Val–GCDCA were 1.83%, 0.318%, −3.58%, 1.99%, 3.87%, and 0.508%, respectively.
Quantification of ribavirin and prodrug concentrations in liver S9 fraction, RBC, and mice tissues was conducted using the same mass transition as in Table 1. A Xterra RP18 column (5 μm, 4.6 × 150 mm2; Waters) was used in order to separate endogenous compound (most likely uridine) from the compounds of interest.14 The mobile phase was composed of water and methanol with 0.1% acetic acid. The flow rate was 1 mL/min. The limit of quantification for all compounds was between 100 and 500 nM.
As ribavirin–l-Val–GCDCA was studied in vivo, the extraction efficiency of the ribavirin–l-Val–GCDCA from plasma, RBC, liver, kidney, and small intestine was 98%, 99%, 100%, 98%, and 93% respectively. The accuracy of quantification of ribavirin–l-Val–GCDCA in plasma, RBC, liver, kidney, and small intestine was 1.96%, 7.77%, −6.92%, 8.37%, and 0.121%, respectively. Ribavirin–l-Val–GCDCA stability in plasma, RBC, liver, kidney, and small intestine for 30 min on ice showed greater than 80% stability. Similarly, a freeze–thaw cycle (−80°C and subsequent room temperature for 48 h) of ribavirin–l-Val–GCDCA in plasma, RBC, liver, kidney, and small intestine showed greater than 80% stability.
Statistical Analysis
Student's t-test was used to evaluate difference of prodrug uptake in the presence and absence of sodium. It was also used to assess different ribavirin accumulation in RBCs and plasma when ribavirin or prodrug (e.g., CDCA–l-Val–ribavirin or ribavirin–l-Val–GCDCA) were incubated with human whole blood. A p value of less than 0.05 denoted statistical significance.
RESULTS
NTCP Uptake of Prodrugs with C-24 Conjugation
Ribavirin was first conjugated to carboxylic acid at the C-24 position of CDCA, UDCA, or CA using either l-valine or l-glutamic acid as the linker to yield five neutral or monoanionic compounds: CDCA–l-Val–ribavirin, UDCA–l-Val–ribavirin, CDCA–Glu–ribavirin, UDCA–Glu–ribavirin, and CA–l-Glu–ribavirin. Concentration-dependent uptake of all five compounds using NTCP-HEK293-stably transfected cells are shown in Figure 2. Studies were conducted in the presence and absence of sodium, where uptake in the absence of sodium served as the negative control as NTCP is a sodium-dependent transporter.
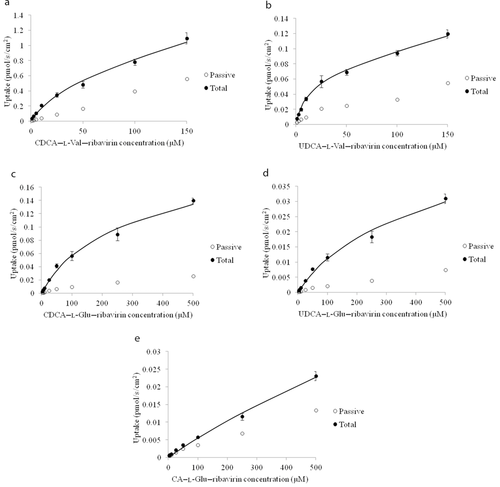
Chenodeoxycholic acid–l-Val–ribavirin and UDCA–l-Val–ribavirin are neutral compounds, whereas CDCA–l-Glu–ribavirin, UDCA–l-Glu–ribavirin, and CA–l-Glu–ribavirin each possess a single negative charge at the C-24 side chain. Despite these charge differences, all five compounds were NTCP substrates, where flux differed (Student's t-test, p < 0.05) between passive and total uptake at each concentration, except for the lowest concentration (2.5 μM) of CA–l-Val–ribavirin.
Prodrug uptake kinetic parameters are listed in Table 2. The transport capacities (i.e., normalized Jmax) of all prodrugs were significantly lower than that of the native substrate taurocholate, with the normalized Jmax values ranging from 0.011 to 0.302. CDCA–l-Val–ribavirin provided the highest normalized Jmax (i.e., 0.302, or 30% that of taurocholate), followed by CDCA–l-Glu–ribavirin (normalized Jmax = 0.075). The two prodrugs using l-valine as the linker yielded higher NTCP affinity (i.e., lower Kt) than their corresponding l-glutamic acid-linked prodrugs. UDCA–l-Val–ribavirin had the highest affinity toward NTCP, with a Kt value of 11.6 μM. The l-glutamic acid conjugates had much lower passive permeability than the neutral l-valine conjugates, possibly because of the negative charge on the linker.
| Prodrug | Kt (μM) | Jmax (pmol s−1 cm−2) | Normalized Jmaxa | Pp × 106 (cm/s) |
|---|---|---|---|---|
| CDCA–l-Glu–ribavirin | 182 ± 26 | 0.145 ± 0.009 | 0.0752 ± 0.047 | 0.0567 ± 0.0026 |
| UDCA–l-Glu–ribavirin | 244 ± 41 | 0.0330 ± 0.0026 | 0.0171 ± 0.0013 | 0.0154 ± 0.0004 |
| CA–l-Glu–ribavirin | 659 ± 261 | 0.0208 ± 0.0053 | 0.0108 ± 0.0028 | 0.0274 ± 0.0007 |
| CDCA–l-Val–ribavirin | 35.0 ± 7.4 | 0.592 ± 0.044 | 0.302 ± 0.031 | 3.76 ± 0.05 |
| UDCA–l-Val–ribavirin | 11.6 ± 1.4 | 0.0654 ± 0.0020 | 0.033 ± 0.002 | 0.376 ± 0.019 |
- Data are summarized as mean (±SEM) of three measurements.
- a Jmax of each prodrug was normalized to Jmax of taurocholate that was measured in parallel to each study in order to accommodate the variation in NTCP expression level across the studies.
Chenodeoxycholic acid–l-Val–ribavirin and CDCA–l-Glu–ribavirin were the two compounds with the highest normalized Jmax values and hence were subjected to further evaluation for ribavirin release in mouse liver S9 fraction.
Release of Ribavirin from Prodrugs with C-24 Conjugation
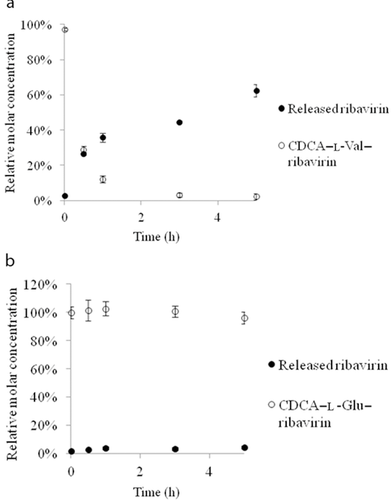
In order to possess efficacy in the liver to treat hepatitis C, ribavirin needs to be released from the prodrugs after hepatic uptake by NTCP. Thus, mouse liver S9 fraction, which contains a wide variety of liver enzymes, was used to assess ribavirin release from the prodrugs. CDCA–l-Val–ribavirin and CDCA–l-Glu–ribavirin were incubated with mouse liver S9 fraction over 5 h. As shown in Figure 3, by 5 h, 63% of ribavirin were released from the neutral CDCA–l-Val–ribavirin, whereas the prodrug itself was largely degraded by 1 h. By contrast, very little ribavirin (4%) was released from the anion CDCA–l-Glu–ribavirin and 96% of the prodrug remained intact after 5 h. The results suggest that CDCA–l-Val–ribavirin has the potential to release the drug in the liver, whereas CDCA–l-Glu–ribavirin showed little promise.
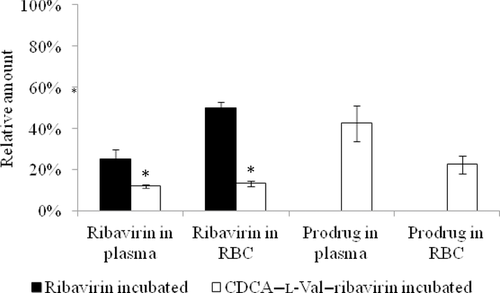
Reduced Ribavirin Accumulation in RBCs with CDCA–l-Val–Ribavirin
Given its susceptibility to release drug, CDCA–l-Val–ribavirin was incubated with human whole blood to assess ribavirin and prodrug distribution in plasma and RBC. The RBC is an off-target of ribavirin therapy, with hemolytic anemia limiting ribavirin dosing. By 5 h, 42% of initial prodrug remained intact in plasma (Fig. 4). Twenty-two percent of initial prodrug was distributed into RBC, and the prodrug remained intact in the RBC. Relative to initial prodrug, 13% of prodrug was converted to ribavirin and present in plasma and RBC, respectively. By contrast, the incubation of same molar amount of ribavirin itself with human whole blood resulted in 25% of ribavirin in plasma and 50% ribavirin accumulation in RBC. The comparison can be translated that CDCA–l-Val–ribavirin was able to reduce ribavirin accumulation in RBC by 3.8-fold (i.e., 13% vs. 50%). However, 22% of the prodrug permeated into RBC, reflecting the high-passive permeability of CDCA–l-Val–ribavirin. This high-passive permeability may result in the passive diffusion of the prodrug into organs or tissues other than the liver, as observed here in RBC. This potential wide distribution may promote prodrug hydrolysis before it enters the liver, thus reducing the amount of ribavirin delivered into the liver.
In Vitro Characterization of Ribavirin–l-Val–GCDCA
In order to reduce a prodrug's passive permeability as observed for CDCA–l-Val–ribavirin and preserve its ability to release ribavirin in the liver, ribavirin was conjugated to the C-3 position of GCDCA using l-valine as the linker. GCDCA was selected over CDCA as GCDCA has higher capacity to be taken up by NTCP than CDCA.15 This strategy retains the ester bond between l-valine and ribavirin and introduces one negative charge at the C-24 region to reduce the passive permeability, as observed from the above for l-glutamic-linked prodrugs.
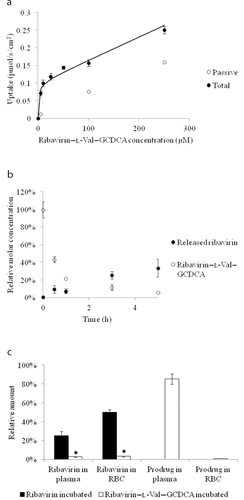
Ribavirin–l-Val–GCDCA was assessed for NTCP uptake, ribavirin release in mouse liver S9 fraction, and drug distribution in human whole blood. As shown in Figure 5a, the passive permeability across HEK293 cells was reduced by 5.7-fold as compared with CDCA–l-Val–ribavirin. Interestingly, the transport capacity also decreased significantly (normalized Jmax = 0.0538). However, the prodrug had much higher affinity toward NTCP than CDCA–l-Val–ribavirin, with a Kt value of 1.63 vs. 35.0 μM for CDCA–l-Val–ribavirin. Metabolic stability in mouse liver S9 fraction (Fig. 5b) suggests that ribavirin–l-Val–GCDCA has the potential to release ribavirin in the liver. After 5 h, 34% of ribavirin were released from the prodrug. The prodrug itself largely degraded within 1 h. Prodrug distribution in human whole blood demonstrated that 85% of the prodrug remained intact in the plasma, whereas almost no prodrug accumulated in the RBC (1%). Meanwhile, 3% of the released ribavirin were present in both the plasma and RBC (Fig. 5c), which was 16.7-fold lower in RBC than when ribavirin alone was tested. On the basis of these in vitro studies, ribavirin–l-Val–GCDCA possessed overall greater potential to achieve in vivo liver targeting with reduced ribavirin accumulation in RBC.
Pharmacokinetics of Ribavirin–l-Val–GCDCA and Ribavirin in Mice
A pharmacokinetic study of ribavirin–l-Val–GCDCA and ribavirin was conducted in mice in order to assess the liver-targeting potential of the prodrug in vivo. Administration entailed 0.08 mmol/kg intravenous tail vein injection in mice. After prodrug or ribavirin administration, prodrug, and/or ribavirin concentrations were measured in the plasma, RBC, liver, kidney, and small intestine over 8 h.
Previous studies suggested that ribavirin was present in RBCs in the form phosphorylated ribavirin.6 Thus, RBC samples were treated with phosphatase, which converted phosphorylated ribavirin to ribavirin; total ribavirin concentration measurement reflects the sum of ribavirin and phosphorylated ribavirin. Meanwhile, pilot studies here indicated that nonphosphorylated ribavirin was the predominant form in the liver sample (Fig. S1). Hence, for liver samples, no treatment of phosphatase was needed, yet ribavirin concentration is interpreted to reflect the sum of ribavirin and phosphorylated ribavirin.
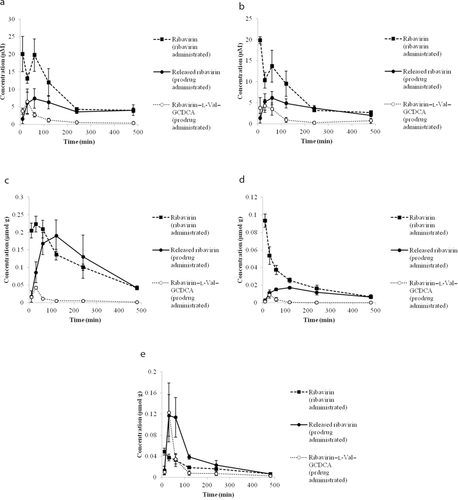
As shown in Figure 6, in comparison to ribavirin, administration of ribavirin–l-Val–GCDCA resulted in less ribavirin exposure in the plasma, RBC, and kidney, increased exposure in the intestine, and similar exposure in the liver. The Tmax of ribavirin in the liver was shifted from 30 to 120 min when prodrug was administered, likely because of the time required for ribavirin to be released from the prodrug. The AUC of both ribavirin and ribavirin–l-Val–GCDCA was calculated for the plasma, RBC, liver, kidney, and intestine, as shown in Table 3. The LTI for ribavirin was 0.0125 from ribavirin–l-Val–GCDCA administration and 0.00695 from ribavirin administration, indicating that the prodrug has achieved better ribavirin liver targeting by 1.80-fold. This enhancement was largely because of prodrug administration yielding about a 1.73-fold reduction in ribavirin exposure in plasma, compared with parent drug administration. Prodrug and drug administration afforded about the same ribavirin exposure in the liver.
| Compartment or Tissue | AUC0–8 h of Ribavirin After Ribavirin Administration (μM min or μmol min/g Tissue)a | AUC0–8 h of Ribavirin After Ribavirin–l-Val–GCDCA Administration (μM min or μmol min/g Tissue)a | AUC0–8 h of Ribavirin–l-Val–GCDCA After Its Administration (μM min or μmol min/g Tissue)a |
|---|---|---|---|
| Plasma | 3857 | 2221 | 600 |
| RBC | 2940 | 1788 | 547 |
| Liver | 53.6 | 55.6 | 3.28 |
| Kidney | 10.4 | 5.43 | 0.476 |
| Intestine | 8.47 | 16.6 | 7.03 |
- Six time points (n = 3 mice per time point) were employed in measuring AUC.
- a Units are μM min for plasma and RBC and μmol min/g tissue for liver, intestine, and kidney.
DISCUSSION
In the current study, we have developed and characterized six ribavirin prodrugs with the goal of achieving liver-specific delivery of ribavirin via targeting NTCP, thereby reducing toxic ribavirin accumulation in the RBCs. Bile acid–ribavirin prodrugs were synthesized with either l-valine or l-glutamic acid as the linker. Bile acids are native substrates of NTCP, and both l-valine and l-glutamic acid are endogenous compounds to impart either neutral or anionic character. Both in vitro and in vivo tests were conducted to characterize the six prodrugs among which ribavirin–l-Val–GCDCA was shown to achieve ribavirin liver-specific delivery in mice.
NTCP Uptake
Human NTCP uptake studies were conducted to screen the synthesized prodrugs for potential liver targeting. A previous study suggested that a negative charge at the C-24 side chain of bile acids is essential for NTCP transport.16 Bile acid conjugates with monoanionic side chains (i.e., CDCA–l-Glu–ribavirin, UDCA–l-Glu–ribavirin, CA–l-Glu–ribavirin, and ribavirin–l-Val–GCDCA) were substrates of NTCP. Interestingly, conjugates with no charge on the C-24 side chain (i.e., CDCA–l-Val–ribavirin and UDCA–l-Val–ribavirin) also showed favorable uptake, with Kt values comparable to that of taurocholate (i.e., 22.7 μM),12 suggesting that a monoanion at the C-24 side chain may not be necessary for the transport by human NTCP. Potentially, there may be unknown physicochemical or structural variables other than charge that affect NTCP transport, such as hydrophobicity that has been shown to impact sodium-dependent bile acid transporter (ASBT) transport activity.17
A previous study from our laboratory showed that bile acid conjugates coupled at the C-3 position afford translocation by NTCP.18 This C-3 coupling was also observed in the current study where the C-3-conjugated prodrug (i.e., ribavirin–l-Val–GCDCA) was a potent substrate with Kt of 1.63 μM. In terms of transport capacity, all six prodrugs possess a significantly reduced NTCP capacity compared with taurocholate. Of the six prodrugs investigated, ribavirin–l-Val–GCDCA had the lowest Kt value and a Jmax of and about 75% transport efficiency (Jmax/Kt) of taurocholate, suggesting moderately efficient uptake by NTCP.12 In addition, CDCA conjugates were shown to have higher transport capacity (i.e., normalized Jmax) than their corresponding UDCA or CA conjugates.
Prodrug Metabolism in Mouse Liver S9 Fraction
Metabolic stability studies were conducted to assess ribavirin release when exposed to mouse liver S9 fraction in vitro. All of the synthesized prodrugs possessed an ester bond between the amino acid linker and ribavirin. However, only CDCA–l-Val–ribavirin and ribavirin–l-Val–GCDCA showed favorable drug release when exposed to mouse liver S9 fraction, whereas CDCA–l-Glu–ribavirin was stable, suggesting that l-valine is more susceptible to enzymatic hydrolysis than l-glutamic acid. Although about 94% of CDCA–l-Val–ribavirin and ribavirin–l-Val–GCDCA were degraded after 5 h, only 63% and 34% of ribavirin was recovered, respectively. It has been reported that there are two metabolic pathways for ribavirin.19 One is the reversible phosphorylation pathway where ribavirin is phosphorylated to mono-, di-, and tri-phosphorylated ribavirin. The other pathway is the degradation involving deribosylation and amide hydrolysis to yield a triazole carboxylic acid metabolite. Our results suggest that ribavirin might undergo an irreversible metabolism by 5 h, resulting in incomplete recovery of ribavirin (Fig. S1).
Prodrug Distribution in Whole Blood
Both prodrug and ribavirin were incubated in human whole blood to assess ribavirin accumulation in RBC and stability of the prodrug in whole blood. It has been shown that ribavirin is phosphorylated in RBC where triphosphorylated ribavirin is the predominant metabolite.20 Phosphatase was used to convert phosphorylated ribavirin to ribavirin so that ribavirin concentration measured in RBC by LC–MS represents all of the ribavirin accumulated in cells.20 Ribavirin is actively transported into RBCs by equilibrative nucleoside transporter 1.21 Likely, the coupling of a bulky bile acid would hamper ribavirin's uptake into RBC that represents an off-target in clinical use of ribavirin. This reduced uptake was observed in our study where both CDCA–l-Val–ribavirin and ribavirin–l-Val–GCDCA caused significantly reduced ribavirin accumulation in RBC. However, because of the high-passive permeability, 22% of CDCA–l-Val–ribavirin was also present in RBC. The subsequent introduction of a single negative charge (i.e., ribavirin–l-Val–GCDCA) decreased the prodrug's passive permeability and reduced the accumulation of prodrug to only 1%. Meanwhile, the presence of prodrug in the plasma increased from 42% to 85% (CDCA–l-Val–ribavirin vs. ribavirin–l-Val–GCDCA).
In Vivo Study in Mice
The potential advantage of targeting NTCP to achieve liver-specific drug delivery motivated the in vivo evaluation of ribavirin–l-Val–GCDCA. A prodrug of ribavirin that targets NTCP may allow for preferential uptake into the liver. Hence, with subsequent release of ribavirin from prodrug, ribavirin may preferentially accumulate in the liver, which is the site of drug action in treating hepatitis C. Correspondingly, liver targeting has promise to minimize ribavirin accumulation in the RBC. Of course, all released ribavirin in the liver may not be retained in the liver. A pharmacokinetic study was conducted to evaluate the liver-targeting potential of ribavirin–l-Val–GCDCA in vivo. The study here showed that ribavirin exposure was reduced in the plasma, RBC, and kidney by about 40%, whereas an approximately equal amount of ribavirin was seen in the liver as compared with the administration of ribavirin itself. It should be noted that the specific location of ribavirin exposure within the liver (e.g., hepatocyte, bile canaliculi) was not evaluated. Ribavirin exposure was increased in the intestine, perhaps because of the enterohepatic circulation of bile acids where the apical ASBT may be responsible for reabsorbing unmetabolized prodrug from the lumen of the small intestine. Prodrug enterohepatic circulation could potentially increase the amount of prodrug delivered to the liver, if the prodrug is not quickly released in hepatocytes and instead is circulated. However, prodrug hydrolysis in the intestine may result in increased intestinal ribavirin exposure. Thus, a prodrug with faster hydrolysis in the liver may reduce ribavirin exposure in the intestine.
CONCLUSIONS
Six bile acid–ribavirin prodrugs were synthesized. All six demonstrated NTCP uptake, but had low-transport capacity compared with that of taurocholate. Of these six prodrugs, ribavirin–l-Val–GCDCA had the highest affinity toward NTCP, whereas CDCA–l-Val–ribavirin had the highest transport capacity (i.e., normalized Jmax). Metabolic stability in mouse liver S9 fraction indicated that CDCA–l-Val–ribavirin and ribavirin–l-Val–GCDCA efficiently released ribavirin in the liver. Of the two, ribavirin–l-Val–GCDCA had more favorable drug distribution in human whole blood, with a high amount of prodrug present in the plasma but a favorably low ribavirin accumulation in RBC. The in vivo pharmacokinetic study in mice showed that i.v. administration of ribavirin–l-Val–GCDCA allowed for greater liver targeting of ribavirin while reducing ribavirin accumulation in RBC. Results indicate that a study of oral administration of this prodrug for liver targeting is merited. Overall, the current study suggests that ribavirin–l-Val–GCDCA has the potential to achieve liver-specific delivery of ribavirin.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grant DK093406 and US FDA grant U01FD004320-01. The authors kindly acknowledge Dr. Keiser (University of Greifswald) for providing the human NTCP-HEK293 cell line used in this study.




