Primary sclerosing cholangitis in children with inflammatory bowel disease: An ESPGHAN position paper from the Hepatology Committee and the IBD Porto group
CME module may be found at https://learnonline.naspghan.org/jpgn2
Patrick F. van Rheenen, Kaija-Leena Kolho, and Richard K. Russell contributed equally and share the first authorship.
Marianne Samyn and Giuseppe Indolfi contributed equally and share the last authorship.
Disclaimers: Although this paper is produced by the ESPGHAN Hepatology Committee and the IBD Porto Group it does not necessarily represent ESPGHAN policy and is not endorsed by ESPGHAN.
ESPGHAN is not responsible for the practices of physicians and provides guidelines and position papers as indicators of best practice only. Diagnosis and treatment is at the discretion of the healthcare provider.
Abstract
Objective
We aimed to provide an evidence-supported approach to diagnose, monitor, and treat children with inflammatory bowel disease (IBD) and primary sclerosing cholangitis (PSC).
Methods
The core group formulated seven PICO-structured clinical questions. A systematic literature search from inception to December 2022 was conducted by a medical librarian using MEDLINE and EMBASE. Core messages from the literature were phrased as position statements and then circulated to a sounding board composed of international experts in pediatric gastroenterology and hepatology, histopathology, adult gastroenterology and hepatology, radiology, and surgery. Statements reaching at least 80% agreement were considered as final. The other statements were refined and then subjected to a second online vote or rejection.
Results
Regular screening for gamma-glutamyltransferase (GGT) is essential for detecting possible biliary disease in children with IBD. MR cholangiopancreatography is the radiological modality of choice for establishing the diagnosis of PSC. Liver biopsy is relevant in the evaluation of small duct PSC or autoimmune hepatitis. Children who do not have known IBD at the time of PSC diagnosis should undergo initial screening with fecal calprotectin for asymptomatic colitis, and then at least once yearly thereafter. Children with a cholestatic liver enzyme profile can be considered for treatment with ursodeoxycholic acid and can continue if there is a meaningful reduction or normalization in GGT. Oral vancomycin may have a beneficial effect on GGT and intestinal inflammation, but judicious use is recommended due to the lack of long-term studies. Children with PSC–IBD combined with convincing features of autoimmune hepatitis may benefit from corticosteroids and antimetabolites.
Conclusions
We present state-of-the-art guidance on the diagnostic criteria, follow-up strategies, and therapeutic strategies and point out research gaps in children and adolescents with PSC–IBD.
Graphical Abstract
Highlights
What is Known
- •
Primary sclerosing cholangitis (PSC) is an uncommon condition in childhood-onset inflammatory bowel disease (IBD) with severe outcomes, and there is no consensus on diagnostic and treatment approaches.
What is New
- •
The European Society for Paediatric Gastroenterology, Hepatology and Nutrition Hepatology Committee and the Pediatric IBD Porto group formulated clinical questions considered to be relevant for the care of children with PSC-IBD.
- •
An evidence-supported approach to diagnose, monitor, and treat children with PSC–IBD is presented here, as well as suggestions for future studies based on current knowledge gaps.
1 INTRODUCTION
Primary sclerosing cholangitis (PSC) is a progressive liver disease with a chronic course, characterized by the destruction of the intra- and extrahepatic bile ducts. Approximately 5%–10% of patients with childhood-onset inflammatory bowel disease (IBD) have or will develop PSC.1-3 Conversely, children who do not have known IBD at the time of PSC diagnosis are likely to develop IBD in the years thereafter. In a cohort of children with both PSC and IBD (PSC–IBD), 60% were diagnosed with both conditions simultaneously. In 26%, PSC was diagnosed after IBD, and in 14%, IBD was diagnosed after PSC.4 Thirty to sixty percent of children with PSC will also display features of autoimmune hepatitis (AIH).4, 5
With long-term complications such as cirrhosis, hepatopancreatobiliary and colorectal malignancies, which can become evident in young adulthood, children and adolescents with childhood-onset PSC–IBD carry a high disease burden with uncertain outcomes including disability and impact on their work and/or education.
Several practice guidelines have been published on the management of adult PSC,6-8 but currently, there is no standardized guidance for the management of patients with childhood-onset IBD and PSC. To address this, the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) governing board commissioned a core group of experts from the Hepatology Committee and the Paediatric IBD Porto group to provide a position article. The aim of this position article is to provide pediatric gastroenterologists with an evidence-supported overview of the diagnostic and therapeutic approach of PSC associated with IBD (Box 1).
Box 1.. Nomenclature
The International primary sclerosing cholangitis (PSC) Study Group recently provided the following working definitions12:
Large duct PSC: High-quality magnetic resonance cholangiopancreatography (MRCP) or endoscopic retrograde cholangiopancreatography (ERCP) with features compatible with sclerosing cholangitis in the absence of apparent causes of secondary sclerosing cholangitis.
Small duct PSC: histologic features typical of PSC with normal and recent (<1 year) high-quality MRCP or ERC in the absence of other cholestatic disorders.
PSC with features of autoimmune hepatitis: PSC with biochemical, serological, or histological features of autoimmune hepatitis.
In most pediatric studies the commonly used term for the overlap syndrome between sclerosing cholangitis and autoimmune hepatitis is “autoimmune sclerosing cholangitis” or “juvenile sclerosing cholangitis”, where the latter term acknowledges the significantly higher prevalence of this condition in children as compared to adults.
2 METHODOLOGY
The core group formulated seven clinical questions (see Box 2), structured by the Population-Intervention-Comparator-Outcome or Population-Exposure-Outcome format, to assimilate evidence from the literature and to prepare core messages as position statements. The position statements and supporting text were circulated to a sounding board composed of international experts in pediatric gastroenterology and hepatology, histopathology, adult gastroenterology and hepatology, radiology, and surgery. Following evaluation through an online platform, position statements reaching at least 80% agreement were considered as final. The other statements were refined and then subjected to a second online vote or rejection.
Box 2.. Clinical questions
- 1.
In patients with suspected inflammatory bowel disease (IBD), is the risk of coexistence of primary sclerosing cholangitis (PSC) or autoimmune hepatitis (AIH) higher in the case of elevated liver enzymes? If so, what is the predictive value of the liver enzyme tests?
- 2.
In patients with IBD and elevated gamma-glutamyltransferase (GGT), which of the two diagnostic modalities—liver biopsy or magnetic resonance cholangiography—best confirms sclerosing cholangitis? Which of the two has the best predictive value?
- 3.
In children with PSC, with or without overlapping AIH, can fecal calprotectin be used to screen for IBD?
- 4.
What is the time-to-cancer diagnosis in children and adolescents with PSC?
- 5.
Should children and adolescents with PSC be treated with ursodeoxycholic acid (UDCA) to prevent or reduce liver-related complications?
- 6.
Should children and adolescents with PSC be treated with vancomycin to prevent or reduce liver-related complications?
- 7.
In patients with IBD–PSC with features of AIH, is long-term treatment with corticosteroids and antimetabolites justified? See Supporting Information: Table S1 for an elaborated version.
2.1 Data sources and searches
We searched MEDLINE (through PubMed) and EMBASE from inception to December 2, 2022. The search strategies were developed in collaboration with a medical information specialist. The search strategies for each of the electronic databases are shown in Supporting Information: Tables S2– S8. No language restrictions were applied.
2.2 Classification of the quality of evidence
The quality of evidence was classified into four categories following the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Table 1). We categorized recommendations as weak or strong, in favor of or against an intervention. Weak recommendations imply that there was a significant variation in the decision of the voters and that physicians need to engage in a shared decision-making process with patients. Strong recommendations suggest that almost all voters would choose that intervention. In such a case, physicians usually do not need to present an alternative intervention.
| Certainty | What it means |
|---|---|
| High | We have a lot of confidence that the true effect is similar to the estimated effect |
| Moderate | We believe that the true effect is probably close to the estimated effect |
| Low | The true effect might be markedly different from the estimated effect |
| Very low | The true effect is probably markedly different from the estimated effect |
2.3 Position statements
For readability, this paper is divided into four main sections: diagnostic criteria, follow-up strategies, therapeutic strategies, and research agenda. Each position statement (with the quality of evidence and, if applicable, the strength of recommendation) is framed and followed by a discussion of the evidence. Practical guidance sections complement the evidence by providing additional information not covered by the position statements.
2.4 Practice points
Practice points are included based on the consensus of the core group and reflect common practice where evidence is lacking. The practice points are framed, but they are not accompanied by statements about the quality of the evidence and the strength of the recommendation.
3 RESULTS
3.1 Screening process
Supporting Information: Figures S1–S7 show the literature screening process. A total of 2121 records were screened in duplicate, after which 309 reports were sought for retrieval. In total, 39 studies with sufficient methodological quality to answer our clinical questions were used to formulate position statements (Supporting Information: Tables S9–S15).
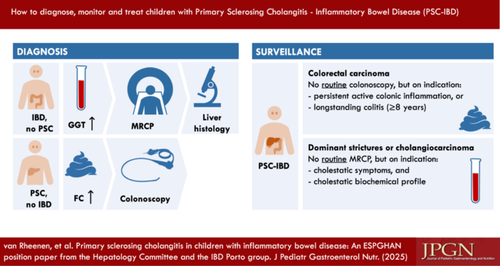
4 DIAGNOSTIC CRITERIA
4.1 Basic liver test panel
Position statement 1
In children with suspected or confirmed IBD the risk of coexistent liver disease is significantly higher in patients with elevated liver enzymes. We recommend testing liver enzymes (ALT and GGT) regularly to screen for associated liver disease. Significant or persistent elevation of liver enzymes prompts further diagnostic work-up into an underlying liver disease.
Moderate certainty | Agreement: 95% | Strong recommendation
Practice point
Screening is usually performed at 3 to 6 months intervals and a work-up for underlying liver disease is most commonly initiated when liver enzymes exceed 2x the upper limit of normal.
4.1.1 Evidence
Liver enzyme elevations are common in children with IBD, but little is known about the action threshold that justifies a diagnostic work-up for underlying liver disease. We searched for studies that assessed the diagnostic accuracy of elevated liver enzymes in patients who were not known to have liver disease. We found four studies that explored liver enzyme testing in IBD (Supporting Information: Table S9). There was considerable heterogeneity among the study cohorts regarding differences in the prevalence of PSC (ranging from 3% to 47%), group size, and the timing of blood sampling relative to the IBD diagnosis.3, 9-11 Nevertheless, the negative predictive value of GGT was high in all cohorts (99% to 100%), indicating that a value below 50 U/L can reliably exclude cholestatic liver disease.
4.1.2 Practical guidance
In adults, it is recommended to evaluate a diagnosis of PSC when serum markers of cholestasis, including alkaline phosphatase, GGT and/or bilirubin, are elevated.8 Due to its variation with age because of increased bone turnover and growth, alkaline phosphatase is not a reliable marker of biliary injury in children, hence should not be included in diagnostic testing.12
In children with IBD, abnormalities in alanine aminotransferase (ALT) and GGT frequently occur.1 This usually involves mild elevations (less than twice the upper limit of normal [ULN]) associated with a transient and undefined etiology. If mild liver enzyme elevations persist for more than 1 month, further evaluation is required. Moderate to marked liver enzyme elevations (≥2x ULN) warrant prompt investigation of etiology.13-15 Table 2 illustrates the common causes of liver enzyme abnormalities in IBD. As corticosteroids and antimetabolites could affect the presentation and course of the liver disease, liver enzymes should be checked at the time of IBD diagnosis and at regular intervals thereafter. All patients with IBD should have their liver enzymes tested half yearly as a minimum,14 although in practice the majority of the children with IBD will have them screened much more frequently than this.
| Causes | Biochemical profile | ||
|---|---|---|---|
| Hepatocellular | Cholestatic | Mixed type | |
ALT ↑↑ to ↑↑↑ GGT normal to ↑ |
ALT ↑ GGT ↑↑-↑↑↑ |
||
| Immune-related liver disease | |||
| Autoimmune hepatitis (AIH) | ☑ | □ | □ |
| Primary sclerosing cholangitis (PSC) | □ | ☑ | □ |
| PSC with features of AIH | □ | □ | ☑ |
| Viral hepatitis | |||
| Primary infection (Hepatitis A/B/C/E, EBV, CMV) | ☑ | □ | □ |
| Reactivation (Hepatitis B, EBV, CMV) associated with anti-TNF or steroid use | ☑ | □ | □ |
| Drug-induced liver injurya | |||
| Mesalamine, thiopurines, allopurinol, methotrexate and anti-TNF | ☑ | ☑ | □ |
| metabolic-dysfunction associated steatotic liver disease | |||
| Hepatic steatosis caused by obesity or starvation | ☑ | □ | □ |
| Biliary obstruction | |||
| Bile stones (unrelated to PSC) | □ | ☑ | □ |
| Other, e.g.b | |||
| Coeliac disease | ☑ | □ | □ |
| Wilson disease | ☑ | □ | □ |
| Alpha-1-antitrypsin deficiency | ☑ | □ | □ |
| Undefined | |||
| Rapid normalisation of liver enzymes | □ | □ | ☑ |
- Note: ↑: mild elevation (<2x ULN); ↑↑: moderate elevation (2 to 5x ULN); ↑↑↑: marked elevation (>5x ULN).
- Abbreviations: ALT, alanine aminotransferase; CMV, cytomegalovirus; EBV, Epstein-Barr virus; GGT, gamma-glutamyltransferase; TNF, tumor necrosis factor; ULN, upper limit of normal.
- a Here, the focus is on IBD medication. However, many more drugs are potentially hepatotoxic. For a complete overview see livertox.nih.gov.
- b These diseases may present with mild ALT elevation.
The most common cause of significantly elevated liver enzymes in children with IBD is drug-induced liver injury (DILI). Most drugs used for the treatment of IBD,16 as well as antibiotics, are known to to be hepatotoxic and can be found on a searchable database maintained by the National Institute of Diabetes and Digestive, and Kidney Diseases, called LiverTox.17 Attributing abnormalities in ALT and GGT to any of these drugs requires systematic evaluation, including time to liver test abnormality after the implicated drug has been started (latency), resolution after the drug is stopped (dechallenge), recurrence on re-exposure (rechallenge) and information on the drug's potential for hepatotoxicity (likelihood).13, 18
The ratio between ALT and GGT determines the biochemical profile and may be useful to narrow down the extensive etiology checklist (Table 2). A hepatocellular biochemical profile is characterized by a disproportionate elevation of ALT compared with GGT. Common causes include viral hepatitis and AIH. The latter has a weak bidirectional association with IBD. In adult patients with IBD, AIH coexists in less than 0.5% of cases, but the prevalence is 3–5 times higher in children and adolescents.14, 19 In patients with IBD and AIH, the presence of additional sclerosing cholangitis should be actively evaluated with magnetic resonance cholangiopancreatography (MRCP) (see Section 4.3), as PSC–IBD with AIH features is more common than IBD with isolated AIH.20
A cholestatic biochemical profile is a disproportionate elevation of GGT compared with ALT, which is typically seen in PSC. PSC is characterized by inflammation and fibrosis of the entire biliary tree, which ultimately leads to multifocal bile duct strictures and dilations. PSC with features of AIH exhibits a mixed-type biochemical profile.
4.2 Immunology
AIH is characterized by elevated immunoglobulin G levels (>1x ULN) and presence of circulating autoantibodies. Autoantibodies can be present in other liver disorders, including PSC, and are not diagnostic in isolation.21 Testing for autoantibodies by indirect immunofluorescence on a freshly prepared rodent substrate should include antinuclear antibody (ANA), antismooth muscle antibody (anti-SMA), antiliver kidney microsomal type 1 antibody (anti-LKM-1), antiliver cytosol type 1 antibody (anti-LC1), and antisoluble liver antigen (anti-SLA), if available.21 Antinuclear cytoplasmatic antibody (ANCA) positivity is common in colonic IBD and therefore not useful when screening for AIH.8 Remission of AIH is considered complete when transaminases and IgG levels are normal, and when autoantibodies are negative or low-titer (i.e., <1:20 for ANA and SMA and <1:10 for anti-LKM1 and anti-LC1).22
4.3 Imaging
Position statement 2
Use MRCP as the radiological modality of choice for diagnosing PSC.
Moderate certainty | Agreement: 100% | Strong recommendation
4.3.1 Evidence
Transabdominal ultrasound is recommended as a baseline imaging study when evaluating elevated liver enzymes. In PSC, bile duct wall thickening and focal bile duct dilatations may be demonstrated on ultrasound, however formal imaging of the biliary tree is essential for the diagnosis and classification of PSC (Box 1). Historically, direct cholangiography was performed via the endoscopic route (ERCP), which has now been largely replaced by MRCP. The latter is less invasive and easier to perform. The role of ERCP is currently mainly therapeutic, which will be further discussed in Section 6.5. Supporting Information: Table S10 shows the results of four pediatric studies in which the diagnostic yield of MRCP (index test) was compared with liver biopsy (reference test).23-26 It is unsurprising that children presenting solely with small duct PSC, either alone or with overlapping features of AIH, had a negative MRCP.
4.3.2 Practical guidance
The presence of a cholestatic liver enzyme profile and typical radiological findings of intra- or extrahepatic bile duct abnormalities on MRCP (Figure 1), in the absence of biochemical and serological evidence of AIH, supports a diagnosis of PSC. MRCP should be reviewed by experienced radiologists to exclude other possible diagnoses such as choledochal malformations. The Imaging working group of the International PSC Study Group recently provided a consensus document with definitions and suggested reporting standards for MRCP features of PSC, which allows for a standardized approach to diagnosis, assessment of disease severity, follow-up, and detection of complications.27
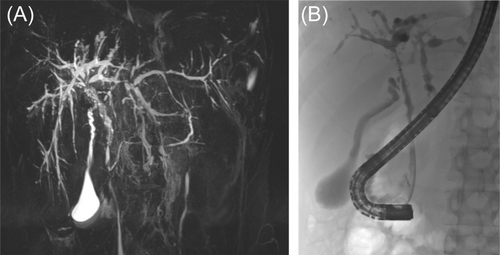
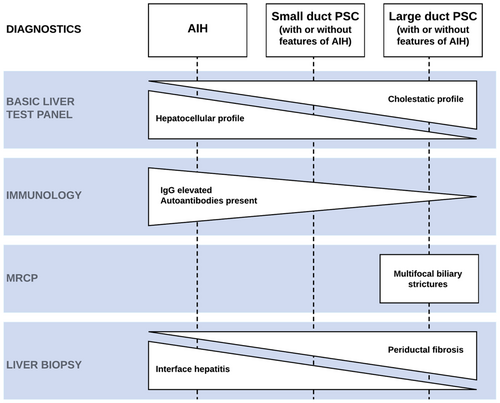
4.4 Liver biopsy
Position statement 3
Consider performing a liver biopsy in children with IBD and suspected PSC in the following circumstances:
- Normal biliary tree at MRCP, or
- Raised immunoglobulin G and the presence of liver-specific autoantibodies, or
- Clinical uncertainty before steroid induction therapy for IBD.
Low certainty | Agreement: 84% | Weak recommendation
4.4.1 Evidence
Typical histological features of PSC are periductal fibrosis, ductular reaction, periductal inflammation and less commonly fibro-obliterative cholangitis (Figure 2A). A liver biopsy specimen showing interface hepatitis, characterized by a dense infiltrate of plasma cells and lymphocytes which invade the surrounding parenchyma beyond the limiting plate, confirms AIH (Figure 2B) or PSC with features of AIH (Figure 2C). It is worth noting that portal inflammation and interface hepatitis may also be observed in patients with PSC.12 Histological criteria to decide whether inflammatory abnormalities in the presence of periductal fibrosis justify treatment with corticosteroids and antimetabolites do not exist. In addition to standard hematoxylin and eosin (H&E) staining as is shown in Figure 2, cytokeratin 7 immunohistochemical staining is employed to facilitate a more comprehensive examination of biliary structures.28

4.4.2 Practical guidance
Liver histology is required to evaluate the presence of small duct PSC in children with a predominant cholestatic biochemical profile and a normal biliary tree at MRCP, and it is the most accurate tool to demonstrate AIH (Figure 3). Children with a pure cholestatic liver enzyme profile and large duct PSC on MRCP may not require a liver biopsy, but the decision whether a liver biopsy is appropriate in this scenario is at the discretion of the professionals managing the patient.
4.5 Fecal calprotectin
Practice point
Children who do not have known IBD at the time of PSC diagnosis should undergo initial screening with fecal calprotectin for asymptomatic colitis.
Position statement 4
Perform fecal calprotectin screening at least once yearly in children with isolated PSC and/or AIH to select patients for diagnostic endoscopy for suspected inflammatory bowel disease.
Low certainty | Agreement: 90% | Strong recommendation
Practice point
There is no clear cutoff value of fecal calprotectin to predict colonic inflammation in patients with PSC. The guideline panel suggests that values ≥150 µg/g usually justify an ileocolonoscopy.
4.5.1 Evidence
Most children with PSC and IBD have colitis with a predilection for more severe inflammation in the right colon, as opposed to ulcerative colitis (UC) which is characterized by uniform inflammation of the colon and rectum, or increasing severity of inflammation towards the rectum. Children with PSC whose IBD is labeled Crohn's disease usually have colonic involvement. Isolated ileitis or extensive small bowel involvement is very rare in PSC–IBD.29 Figure 4 shows the typical distribution of inflammation with rectal sparing and/or backwash ileitis in the setting of pancolitis.29, 30
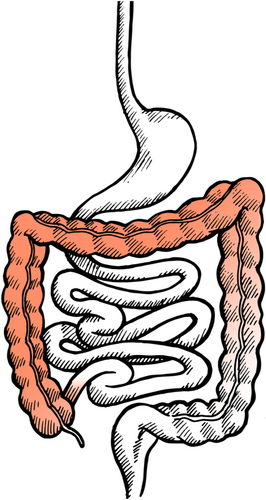
Persistent rectal bleeding or perianal disease should prompt endoscopic assessment of the gut.31 When the indication for endoscopy is less obvious, as in the case of children with chronic abdominal pain or nonbloody diarrhea, measuring fecal calprotectin may help to distinguish who is in need of a colonoscopy. A raised value signifies the presence of active inflammation warranting colonoscopy, whereas a normal value reduces the probability of finding inflammation to almost zero.32 It is unknown if this test strategy performs equally well in children with PSC and asymptomatic colitis, where there is typically mild intestinal disease, a reversed right to left gradient of inflammation, and rectal sparing.29, 33 Despite milder colonic inflammation in PSC–IBD, fecal calprotectin values may be very similar to that in patients with “non-PSC IBD.”34 This apparent paradox has been provisionally explained in a study among adult patients with active PSC, in whom endoscopically quiescent colitis (assessed with the UC endoscopic index of severity [UCEIS]) was correlated with fecal calprotectin, biliary calprotectin (samples collected during ERCP) and markers of cholestasis.35 It was hypothesized that fecal calprotectin may not necessarily reflect only the severity of colonic inflammation, but also the severity of the concurrent biliary inflammation.
4.5.2 Practical guidance
There is no consensus on the ideal fecal calprotectin cutoff point to justify a colonoscopy. In a prospective case–control study performed at the Hospital for Sick Children in Toronto fecal calprotectin values in children with PSC and colitis (cases, n = 37) and children with colitis without liver disease (controls, n = 50) were correlated with the UCEIS. The colonoscopy was executed as part of routine clinical care. The decision to perform a colonoscopy was irrespective of the fecal calprotectin result. The authors concluded that a fecal calprotectin level <93 µg/g best predicted endoscopic healing (UCEIS = 0) in children with PSC–IBD.36 This application of fecal calprotectin (to predict endoscopic remission) differs from the intended use as stated in Box 2, where calprotectin screening is used to predict active inflammation in the colon. The guideline panel suggests that values ≥150 µg/g usually justify endoscopy, which is in line with the European Crohn's and Colitis Organization (ECCO)–European Society for Gastrointestinal and Abdominal Radiology (ESGAR) guideline for diagnostic assessment in IBD.37
5 FOLLOW-UP STRATEGIES
5.1 Natural progression of PSC
A considerable proportion of children and adolescents with PSC–IBD have a slowly progressive liver disease characterized by hepatobiliary fibrosis, biliary strictures, intermittent bacterial cholangitis, and, eventually, the development of cirrhosis and end-stage liver disease. In a large multicenter cohort study in which 781 patients with childhood-onset PSC were followed longitudinally, approximately 50% developed these complications within the first 10 years after the diagnosis of PSC. Once these complications arose, the median time to liver transplantation was 3–4 years.5
Patients with PSC who have features of AIH and those with small-duct PSC seem to have a more extended period until the development of complications. There is a debate among experts whether these patients have an earlier stage of the same disease or rather a distinct PSC phenotype.38 The first concept assumes that the stage with immune-mediated hepatitis can be suppressed with steroids and thiopurines, while the biliary disease continues to deteriorate. In the end, the extent of biliary damage determines the long-term outcome.
5.2 Counseling
Various prognostic markers have been explored to predict patient outcomes. Achieving spontaneous or treatment-associated normalization of GGT (i.e., <50 IU/L) or a percentage decrease of >25% within the first year after a PSC diagnosis has been linked to a reduced occurrence of complications in the first 5-year period.39, 40
In the adult PSC literature, the presence of fibrosis or cirrhosis on liver biopsy, as well as increased liver stiffness (measured through vibration-controlled transient elastography), are cited as unfavorable predictors.7 The use of elastography for monitoring liver fibrosis in children is rapidly advancing but currently lacks clear guidance on interpreting test results.
The Pediatric PSC Consortium developed the Sclerosing Cholangitis Outcomes in Pediatrics (SCOPE) index based on the retrospective data of over 1000 children. The SCOPE index combines the results of four blood markers (total bilirubin, albumin, platelet count, and GGT) with cholangiography and may help to assess the child's risk for liver transplantation at diagnosis and over time.41 Children in the low-risk SCOPE category are unlikely to require a liver transplant in the next decade, while those in the medium- or high-risk SCOPE category probably require a referral to a pediatric hepatologist with expertise in PSC. The SCOPE index has not yet been prospectively validated. Until that time, the decision to refer a child to a liver transplant center should not be based on the SCOPE index. More information about referral criteria for liver transplantation can be found in Section 5.6.
5.3 Monitoring of disease progression
Monitoring of the progression of PSC is based on the regular reappraisal of clinical signs and symptoms, inflammatory activity in the gut and the liver, biliary involvement, and parenchymal fibrosis/cirrhosis (Table 3). The latter may be evaluated with elastography or radiological imaging. Liver biopsy to evaluate progression of fibrosis in isolation is not recommended.
| Category | Test | Test frequency |
|---|---|---|
| Clinical evaluation | Fatigue Pain right upper quadrant Bleeding tendency Jaundice Pruritis Fever of unknown origin Nutritional state Quality of life |
Every 3–6 months |
| Blood tests | ALT AST GGT TSB Albumin INR Platelets CRP |
Every 3–6 months |
IgG AFP Lipid soluble vitamins |
Every 12 months | |
| Autoantibodies (ANA, anti-SMA, anti-LKM-1, anti-LC1, and anti-SLA) | In patients with negative tests at diagnosis and increased IgG during follow-up | |
| Serum bile acids | Optional | |
| Imaging | Ultrasonography (liver parenchyma and bile ducts); in case of cirrhosis combined with doppler of portal circulation | Every 12 months |
| MRCP | In case of progression of biliary disease | |
| ERCP | For therapeutic interventions or tissue sampling for evaluation of dysplasia or cholangiocarcinoma | |
| Elastography | Liver stiffness | Optional |
- Abbreviations: AFP, alpha-fetoprotein; ALT, alanine aminotransferase; ANA, antinuclear antibody; AST, aspartate aminotransferase; anti-LC1, antiliver cytosol type 1 antibody; anti-LKM-1, antiliver kidney microsomal type 1 antibody; anti-SLA, antisoluble liver antigen; anti-SMA, antismooth muscle antibody; CRP, C-reactive protein; ERCP, endoscopic retrograde cholangiopancreatography; GGT, gamma-glutamyl transferase; IgG, immunoglobulin G; INR, international normalized ratio; MRCP, magnetic resonance cholangiopancreatography; PSC, primary sclerosing cholangitis; TSB, total serum bilirubin.
Performing an annual MRCP in children with PSC is not indicated due to the extremely low risk of cholangiocarcinoma (CCA) before the age of 18 (see Section 5.4.2). In the case of biochemical deterioration of cholestasis, an MRCP is recommended to assess the presence of a dominant stricture.
5.4 PSC and cancer risk
In adults with PSC–IBD, the inherent risk of colorectal carcinoma (CRC) and CCA plays a significant role in both morbidity and mortality. The likelihood of these malignancies manifesting before the 18th year of life is extremely low (see Sections 5.4.1 and 5.4.2). There is a significant difference between pediatric and adult practice regarding cancer screening. Due to the lower risk and practicalities for organizing endoscopy, yearly surveillance in children with PSC–IBD is less self-evident. Once the transfer to adult-oriented care has taken place, annual surveillance colonoscopy with biopsies is recommended in all adults with PSC–IBD.8 It is important to sensitively educate children and adolescents with PSC–IBD and their families about their elevated cancer risk at the appropriate time and prepare them for this more intense screening strategy.
5.4.1 CRC surveillance
Position statement 5
In children with PSC–IBD the risk of high-grade dysplasia or colorectal cancer is greater than in isolated IBD, however, the absolute risk of being diagnosed with colorectal cancer before the age of 18 is very low.
Moderate certainty | Agreement: 100%
Practice point
Surveillance colonoscopy should be considered in children with PSC–IBD and the following risk factors of colorectal cancer:
- persistent active colonic inflammation, or
- longstanding colitis (≥8 years), or
- a family history of colorectal cancer in a first-degree relative <50 years.
5.4.1.1 Evidence
The risk of high-grade dysplasia and CRC in adult patients with PSC is 4–5 times greater compared to patients with IBD without PSC.7 Leading adult guidelines recommend to perform yearly surveillance colonoscopy from the time of diagnosis of PSC. Dye-based or virtual chromoendoscopy with targeted biopsies is increasingly recommended, although it has not been proven that these techniques are superior to high-definition endoscopy without chromoendoscopy and random biopsies. Children with PSC–IBD seem to develop CRC at similar rates as adults, with approximately 5% affected 10 years after PSC diagnosis (see Figure 5). However, the absolute risk of being diagnosed with CRC before the age of 18 is low (0.2%).42
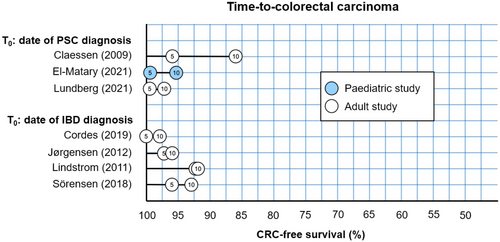
It is important to note that there is a lack of substantial evidence supporting the effectiveness of surveillance colonoscopy. Current practice guidelines offer little guidance regarding the age to start cancer surveillance, the colonoscopy technique, and the screening interval (as outlined in Supporting Information: Table S16). As a result, there is a large variation in clinical practice, as was shown in a recently published survey among Dutch pediatric gastroenterologists.43
5.4.1.2 Practical guidance
Recommendations for malignancy screening in adults with PSC–IBD are outlined in the ECCO Malignancy in IBD Guidelines.44 Strictly following these recommendations for children and adolescents with PSC–IBD would lead to unacceptably frequent procedures with no treatment consequences. We recommend including only those patients with a high-risk profile in a surveillance colonoscopy program. This high-risk profile includes individuals with ongoing colonic inflammation (fecal calprotectin >150 µg/g or histologic active disease), a history of long-standing colitis (8 years or more), or a family history of CRC in a first-degree relative under the age of 50. When one of these risk factors is present, surveillance colonoscopy is usually conducted every other year. Pediatricians involved in CRC screening are advised to familiarize themselves with the “best practice advice” to enhance the quality of screening.45, 46 Some centers involve endoscopists from the adult GI department in CRC screening for adolescents.
5.4.2 CCA surveillance
In adults CCA can occur any time during the disease course, but the most consistent risk factor for CCA is older age. CCA is rarely diagnosed in the pediatric population or in those with small-duct PSC.7 In a multicenter, international cohort of 781 children with PSC, eight were diagnosed with CCA at an age range of 15–18 years, a median of 6 years after initial PSC diagnosis.5 Three patients with metastatic cancer died under palliative care within 5 months of CCA diagnosis. Five patients with localized disease underwent surgery or liver transplantation, with all five alive at a median follow-up of 2.5 years after diagnosis. All patients who developed CCA had PSC without AIH overlap. Routine CCA surveillance for patients with PSC under 18 years of age is not recommended. New onset jaundice with a cholestatic biochemical profile should prompt MRCP and carbohydrate antigen 19.9 (CA 19.9) testing, and findings of distal common bile duct strictures will require ERCP with biliary brushings for cytology. CA 19.9 testing is not suitable for surveillance purposes due to its insufficient accuracy.7, 8
5.5 Referral criteria for liver transplantation
As mentioned in Section 5.1, approximately 50% of the patients with PSC eventually require a liver transplantation. A population-based study from the Netherlands among adults showed a transplant-free interval of 15–20 years after the diagnosis of PSC,47 whereas in specialized hepatology centers transplant-free survival is usually shorter (10–15 years), due to a different case mix.48 Although liver transplantation is more likely to occur after transfer to adult services, assessment for liver transplantation should be considered in patients with PSC and intractable severe pruritus, recurrent bacterial cholangitis, portal hypertension or relevant bile-duct strictures. This advice is consistent with a recently published consensus guideline of the European Society of Organ Transplantation.49 Prioritization for liver transplantation was traditionally based on the model for end-stage liver disease (MELD) score that included three blood parameters: international normalized ratio, creatinine, and bilirubin. The current revised MELD also includes serum sodium. The MELD-score, which is a proxy for the severity of hepatic dysfunction, underestimates the severity of PSC complicated by recurrent cholangitis.50, 51 Consequently, waiting times on transplant lists for patients with PSC have been typically longer and outcomes worse than those for other forms of liver disease.50, 51 We suggest referral of children with newly diagnosed PSC–IBD to a pediatric hepatologist with expertise in liver transplantation at an early stage. Thereafter, joint care with the IBD team and the local pediatric hepatologist should continue. This advice is in accordance with the latest ECCO guideline on extraintestinal manifestations in IBD.14
Recurrence of PSC (rPSC) after liver transplantation is not uncommon. The International Pediatric PSC Consortium described a cohort of 140 children with PSC who were transplanted before 18 years of age. rPSC occurred in 27% of the patients in the first 5 years after liver transplantation. IBD was more prevalent in the rPSC group as compared to those without recurrence.52 In an international retrospective adult cohort study of 531 liver transplant recipients, those with increased IBD activity after transplantation had a greater risk of rPSC compared to those with stable IBD (hazard ratio 1.7, 95% confidence interval 1.1–2.8; p = 0.021).53 A systematic review published in 2018 suggested a protective role of colectomy when carried out before or at the time of liver transplantation.54 The quality of the included papers was too low to routinely recommend colectomy for rPSC prevention, but colectomy may be considered on a case-by-case basis, especially if IBD has been difficult to manage.
6 THERAPEUTIC STRATEGIES
Currently, there is no medication available to slow down PSC disease progression, although some improvement in liver biochemistry may occur. We present here the most frequently used therapies.
6.1 UDCA
Position statement 6
UDCA may be prescribed at doses of 15–20 mg/kg/day. Despite evidence of improvement of liver enzymes, its long-term effect on disease progression has not been demonstrated.
Low certainty | Agreement: 90% | Weak recommendation
Practice point
Consider a 6-months therapeutic trial of UDCA, either immediately after PSC diagnosis or when spontaneous normalization of GGT does not occur in the first 6 months postdiagnosis. Continue UDCA treatment if there is a meaningful reduction or normalization of GGT or improvement of symptoms.
6.1.1 Evidence
UDCA is a hydrophilic bile acid with anticholestatic, anti-inflammatory, and antifibrotic actions. It is widely used in the treatment of PSC in children and adolescents. Similar to adult studies, pediatric research shows an improvement in liver biochemistry after introducing the drug (Supporting Information: Table S13). However, there is currently no evidence that UDCA slows down the progression of PSC.
There is no agreement on what constitutes a minimum effective dosage. In a retrospective multicenter study including 46 international centers the median UDCA dose administered to children with PSC was 15 mg/kg/day (interquartile range 15–19).55 Notably, an adult trial with high-dose UDCA (28–30 mg/kg/day) was prematurely terminated for its negative impact on esophageal varices, time-to-transplantation and survival, despite improvement in liver biochemistry.56, 57 Provided that UDCA is adequately dosed, its long-term safety profile is excellent. Up to 10% of users report loose stools and it has few interactions with other drugs.58
A prospective study among 22 children with PSC evaluated UDCA withdrawal. The participants had been on UDCA maintenance therapy with normal biochemistry. The UDCA dose was reduced by 50% for 4 weeks, after which it was discontinued for 8 weeks. A third of the participants showed a GGT increase above 100 IU/L during withdrawal with a normalization of GGT upon UDCA reinstitution. In another third GGT and ALT remained in the normal range during withdrawal. The remaining group had a mild increase in GGT up to a maximum of 100 IU/L. The significance of a biochemical flare is unclear and underscores the need for a more reliable biomarker of disease progression.59
6.1.2 Practical guidance
Despite lack of evidence of long-term effects, UDCA is prescribed long-term in over 80% of children with PSC across many countries.5 Many experts feel there is at least some role for a 6-month therapeutic trial of UDCA, with continuation of the drug in patients with a substantial biochemical response,60 while others recommend monitoring GGT for 6 months before initiating UDCA because levels can spontaneously normalize.7 Patients with persistently elevated GGT levels can then be considered for UDCA treatment at 15–20 mg/kg/day, and treatment can be continued if there is a meaningful reduction or normalization of GGT or improvement of symptoms with 6 months of treatment.
6.2 Oral vancomycin
Position statement 7
Oral vancomycin may be prescribed for a potential improvement in liver biochemistry as well as bowel inflammation. Its long-term effect on disease progression has not been demonstrated.
Low certainty | Agreement: 85% | Weak recommendation
6.2.1 Evidence
Dysregulation of the gut microbiome has been postulated as one facet of the intricate pathogenesis of PSC, and modulation of the gut microbiome may have a favorable effect on the course of PSC.61 Oral vancomycin is the most extensively investigated antibiotic in the context of childhood-onset PSC. It is poorly absorbed from the gut and may diminish the concentration of bacteria in the gut, or their potentially harmful metabolites in the portal circulation. Prospective and retrospective studies conducted in children (Supporting Information: Table S14) have demonstrated improvements in liver biochemistry following oral vancomycin treatment. In a small-scale retrospective study, liver function tests improved or normalized in 12 out of 17 children with PSC who received oral vancomycin for a minimum of 3 months. Moreover, a significant portion of these children also achieved biochemical endoscopic and histologic remission of colitis.62 A article from the Pediatric PSC Consortium that was recently published is worth mentioning in this context as well.63 A group of 70 children with PSC–IBD, who in addition to conventional IBD medication also received oral vancomycin for at least 3 months, was matched with a group of 210 children with PSC–IBD who were not exposed to vancomycin. The probability of reaching IBD clinical remission was higher in the vancomycin-treated subgroup.
Two small prospective studies (respectively 45 and 14 participants) showed significant changes in liver biochemistries in the majority of children enrolled.64, 65 In the largest retrospective case-control study so far, 264 children diagnosed with PSC were matched 1:1:1 to those treated with vancomycin, UDCA, or observed without therapy.66 After 1 year, no discernible improvement in outcomes was observed across the treatment groups. Specifically, GGT levels normalized in 53%, 49%, and 52%, while the liver fibrosis stage improved in 20%, 13%, and 18% among the vancomycin, UDCA, and observation-only groups, respectively. However, liver fibrosis worsened in 11%, 29%, and 18%, respectively. Furthermore, the 5-year likelihood of liver transplant listing stood at 21%, 10%, and 12%, respectively. Notably, spontaneous normalization of liver biochemistry was frequently observed in children who did not receive therapy, particularly those with a mild phenotype and minimal fibrosis.
6.2.2 Practical guidance
There are many uncertainties regarding the use of oral vancomycin in patients with PSC-IBD, including optimal dosage, efficacy across different stages of fibrosis and the length of safe treatment. In an open-label prospective clinical trial children with PSC were treated at a dose of 50 mg/kg/day divided into three times per day if weight was <30 kg, and at a dose of 500 mg three times a day if weight was ≥30 kg.64 Until more data is available, we recommend judicious use of the drug. Patients who experience a rapid reduction and subsequent normalization of GGT within 12 weeks may be considered for longer-term therapy with periodic evaluation for vancomycin-resistant enterococcus.60
6.3 Corticosteroids and antimetabolites
Position statement 8
In children with PSC–IBD and biochemical, serological, and histological features of AIH, the use of corticosteroids and antimetabolites may suppress immune-mediated hepatitis.
In the absence of convincing AIH features, the use of corticosteroids and antimetabolites is not indicated to manage PSC.
Low certainty | Agreement: 95% | Weak recommendation
6.3.1 Evidence
Some studies address long-term use of corticosteroids and antimetabolites to control autoimmunity in patients with PSC and features of AIH based on biochemical improvement or reduction of parenchymal inflammation in the liver. However, the bile duct disease progresses in about 50% of cases, leading to end-stage liver disease requiring transplantation more frequently than in isolated AIH.21
Maintenance therapy with corticosteroids, usually between 5 and 7.5 mg per day,21 daily, may cause adrenal suppression.67 Also, the effect of corticosteroids on growth are well documented. However, a recent retrospective study including 74 children with autoimmune liver disease (PSC not mentioned separately) treated with a daily maintenance dose of steroids for a median duration of 11 years (interquartile range 8.4–13.8) showed mean z-scores for weight, height, and body mass index within the normal range.68
6.3.2 Practical guidance
There are no convincing data that use of corticosteroids and antimetabolites improves long-term outcomes in children with PSC and overlapping features of AIH. However, in children with biochemical, serological, as well as histological features of AIH it is reasonable to trial corticosteroids and antimetabolites using a similar approach as in AIH.21 The prednisolone starting dose is weight-dependent and should be tapered once aminotransferases start to decrease, but not later than 4 weeks after initiation. Frequently, a low-dose prednisolone maintenance dose (2.5–5 mg daily) is necessary to keep aminotransferase levels in range.
A steroid-sparing antimetabolite such as azathioprine or mercaptopurine is usually introduced a few weeks after the initiation of prednisolone, or when aminotransferases stop decreasing. A recent adult study on the use of mycophenolate mofetil with prednisolone as induction therapy for AIH showed better outcomes than the combination prednisolone–azathioprine.69 However, patients with PSC and IBD were excluded from participating in this study. Currently, there is insufficient evidence to recommend the use of mycophenolate mofetil in children with PSC–IBD and convincing AIH features. There may be a role for repeated liver biopsy in the patient with PSC–AIH who continues to display elevated liver enzymes to help clarify whether there is ongoing inflammation in the liver parenchyma, or only biliary damage. In the latter situation withdrawal of corticosteroids and antimetabolites rather than long-term use could be considered. Immunosuppressive therapy is not recommended for PSC without AIH features.
6.4 Biologicals
In a retrospective analysis of 141 adults with PSC–IBD, antitumor necrosis factor-α (anti-TNF-⍺) agents were primarily prescribed for the IBD indication. Anti-TNF-⍺ medication was effective in reducing intestinal inflammation, although not as effective as in patients with non-PSC IBD, with no specific safety signals.70 One double-blind, randomized controlled study in adult patients with UC and PSC was specifically designed to evaluate the efficacy of infliximab on PSC symptom relief, liver biochemistry and histology. When an interim analysis revealed that infliximab did not provide a significant treatment benefit on PSC symptoms and liver histology, the study was halted prematurely, due to the disproportionately high risk to participants (resulting from liver biopsies at baseline and at 26 weeks).71
In a spin-off study of the Pediatric PSC Consortium, a retrospective multicenter research registry, 37 children with PSC–IBD on vedolizumab therapy were followed for a year. IBD activity improved on vedolizumab at rates similar to non-PSC IBD, but hepatobiliary outcomes did not improve.72
Currently, there is insufficient evidence to recommend one biological treatment over another to stop progression of PSC. For decisions around IBD treatment, we refer to the latest ECCO–ESPGHAN treatment guidelines for Crohn's disease and UC.73, 74 The effect of IBD activity on PSC progression is unclear. However, as in non-PSC IBD, mucosal healing should be the treatment goal in PSC–IBD, as it improves long-term outcomes of IBD and possibly mitigates the increased risk of CRC.
6.5 ERCP and strictures
Practice point
Children with PSC, relevant bile-duct strictures and cholestatic symptoms should be assessed for liver transplantation. When their symptoms are likely to improve following biliary intervention, ERCP can be considered. Referral to a centre with expertise in pediatric interventional ERCP is highly recommended.
As discussed in Section 4.3 in connection with diagnostic imaging, MRCP has supplanted the role of ERCP to identify focal narrowing and ballooning of bile ducts. ERCP is now mainly a means to manage dominant biliary strictures, or to sample tissue for suspected CCA.8, 12
The possible presence of a dominant stricture in the bile ducts should be investigated when a patient with known PSC-IBD suddenly develops cholestatic symptoms (including jaundice, bacterial cholangitis, itching, or pain in the right upper abdomen) and a cholestatic biochemical profile. When narrowing in the extrahepatic or first-order intrahepatic ducts is observed on MRCP, with or without upstream dilatation, there is an indication for ERCP. When ERCP confirms a dominant stricture, primary treatment should consist of balloon dilation. Dilation followed by stent placement does not lead to better outcomes but is associated with significant adverse events, such as bacterial cholangitis and pancreatitis.75
Based on the invasiveness of the procedure, the considerable exposure to radiation and its potential for severe complications, ERCP in children is only justified in the situation of a favorable benefit-risk ratio, such as a high suspicion of a dominant stricture or malignancy on MRCP.76 Referral to a centre with expertise in pediatric interventional ERCP is highly recommended.8
6.6 Colectomy
Colectomy in adults with IBD, specifically UC, alongside PSC, presents a complex clinical scenario. There are data supporting that IBD (and its inflammatory activity) is important for PSC progression. Transplant- and cancer-free survival is poorer in patients with UC when compared to patients with no IBD or Crohn's disease.47 Furthermore, the longer time-to-transplantation in adults who underwent colectomy before diagnosis of PSC compared to those with their colon in situ supports the notion that gut inflammation may affect PSC progression.77 In adults with UC and PSC, the contemplation of colectomy often arises due to the heightened risk of CRC associated with PSC. The decision to proceed with colectomy depends on various factors, including disease severity, response to medical treatments, and the presence of dysplasia or cancer. While evidence indicates that colectomy can reduce the risk of CRC, it does not stop PSC progression, and individuals may still require liver transplantation in the future. Colectomy may also offer relief from UC-related symptoms, but this surgical intervention carries inherent risks and significant lifestyle implications.
The use of colectomy in adults with IBD and PSC has been debated as a pre-emptive measure to reduce the risk of recurrent PSC after liver transplantation. Currently, the European Association for the Study of the Liver advises against performing a pre-emptive colectomy before liver transplantation in adults with PSC-IBD. However, in refractory cases, colectomy should be considered at a slightly lower threshold than in individuals with PSC who have not yet undergone a liver transplant.8
In children with IBD and PSC, colectomy is less frequently considered compared to adults.33 The appropriateness of colectomy in children with PSC–IBD requires individualized assessment taking into account their growth, development and overall quality of life. Decisions regarding colectomy should be made in consultation with a multidisciplinary team.
7 CONCLUSION
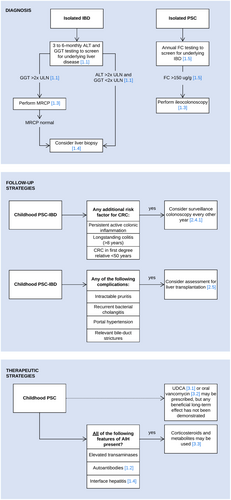
The aim of this ESPGHAN position paper is to guide clinicians' decisions with the best evidence available to establish the right diagnosis, follow-up and treatment strategies for children with IBD-associated PSC. Summary flowcharts are shown in Figure 6. It is up to every clinician to make adaptations to local regulations and to the patient's individual characteristics and needs.
8 KNOWLEDGE GAPS
The number of patients with PSC is increasing along with the substantial increase in the incidence of IBD and autoimmune diseases in general during the past decade.78 However, the absolute number of patients with PSC in each pediatric center is still low pointing out the need for multicenter prospective studies to increase knowledge of the pediatric phenotype of PSC. To date, we lack the means to identify the young patients at risk for the development of end-stage liver disease and CCA, and we do not have medications to stop disease progression. UDCA is widely prescribed, and it may have an effect in normalizing liver biochemistry, but there is no evidence that it indeed improves the outcomes of the patients. This is true for other drugs with some reported benefit on the levels of liver enzymes as well, such as vancomycin. Also, it is an unanswered question whether PSC with features of AIH comprises a disease subtype of its own with different outcomes or, as recently suggested, a stage on the disease continuum between AIH and PSC. As most children with PSC have concomitant IBD, they are at risk for developing CRC. Reassuringly, the absolute risk for colon cancer in PSC is low at a young age. Unfortunately, we do not know how to stratify those at substantial risk. Adult guidelines on PSC–IBD recommend annual surveillance endoscopies that seem overcautious for young patients and there is a need for guidelines adjusting the recommendations according to the age and duration of disease history in childhood-onset PSC. Disease progression in childhood-onset PSC is individual and the optimal follow-up with MRCP is to be defined. Follow-up is needed for timely detection of disease progression which may be an indication for ERCP and brush cytology to diagnose dysplasia that precedes the development of cancer. Patient selection for ERCP remains a challenge. Future research will hopefully identify novel prognostic biomarkers to better characterize the subtype of childhood-onset PSC. Such markers would aid in focusing the most intensive follow-up on those patients with the highest risk for adverse outcomes and spare the other patients from invasive investigations (Box 3).
Box 3.. Suggestions for future studies based on current knowledge gaps
-
Prospective international registry using agreed clinical definitions, combined with the collection of biological samples (including −80°C frozen stool, urine, blood, and DNA) to assess the natural history of PSC–IBD and explore new biomarkers.
-
Nested diagnostic study to predict the presence of colonic inflammation in children diagnosed with isolated PSC.
-
Controlled study of vancomycin use to evaluate short and medium-term outcomes.
ACKNOWLEDGMENTS
We thank Hanna van Rheenen for creating the illustration in Figure 4. This project was funded by the European Society for Pediatric Gastroenterology Hepatology and Nutrition.
CONFLICT OF INTEREST STATEMENT
Patrick F. van Rheenen: Research Grant—European Crohn & Colitis Organisation. Leadership Role—Chair of Dutch PIBD Guideline (2022-2024). Receipt of Donated Equipment—BÜHLMANN Laboratories AG. Kaija-Leena Kohlo: Grants or Contracts: Helsinki University Hospital Research Fund—ongoing research projects on fecal microbiome; Pediatric Research Foundation (Finland)—ongoing research project on pediatric IBD Consultancy fees: Abbvie; Blocodex. Richard K. Russell: Grant/Contract: Nestle Health Science—ongoing; Ferring—past. Consultancy fees: Celtrion and Lily. Participated in Data Monitoring Committee: OCEAN Trial. Medical Writing: Pfizer. Honoraria Lecture: Janssen. Payment for Meeting attendance: Abbvie and Celltrion. Marina Aloi: Honoraria for Lectures—Takeda, Nestle. Safety Monitoring Board—Pfizer. Annamaria Deganelloe: none. Séamus Hussey: Research Grant—Janssen. Norman Jung: Speaker at annual meeting/HEPCOM member—ESPGHAN. Data Monitoring Committee—ERN Rare Liver member for Rare Liver Registry. Leadership Roles—ERN Rare Liver, ERN Transplant Child. Jan De Laffolie: Consulting Fees—Mirum, Takeda, Sanofi. Lecture Honoraria—Mirum, Baxter, Sanofi. Safety monitoring board member—Sanofi. Advisor to patient organizations—DCCV, DZG. Mark R. Deneaui: none. Emer Fitzpatrick: Grant/Contract—Health Research Board, Ireland (author's institution). Board member—ESPGHAN. Anne M. Griffiths: Grants/Research Support—Abbvie. Member of advisory board—Abbvie, Janssen, Lily. Honoraria for Lectures—Abbvie, Janssen, Nestle. Honoraria for Consultation—Abbvie, Amgen, Bristol Meyers Squib, Pfizer, Merck, Janssen, Lily. Iva Hojsak: Consultancy Fees—Abbot and Biocodex. Honoraria for Lectures—Sandoz, Nestle, BioGaia, Hipp, GM Pharma. Emanuele Nicastro: Served as member on advisory board—Mirum Pharmaceuticals. Andreia Nita: none. Mikko Pakarinen: none Amanda Ricciuto: none Lissy de Ridder: Collaboration (such as involved in industry-sponsored studies, investigator-initiated study, consultancy) with Abbvie, Eli Lilly, Takeda, Janssen, Medtronic and Pfizer. Aurelio Sonzogni: none Andrea Tenca: none Marianne Samyn: none Giuseppe Indolfi: Payment/honorarium Kedrion Pharma 2020.