Measurement practices of alanine aminotransferase in children: Temporal changes and etiology for increased values
Abstract
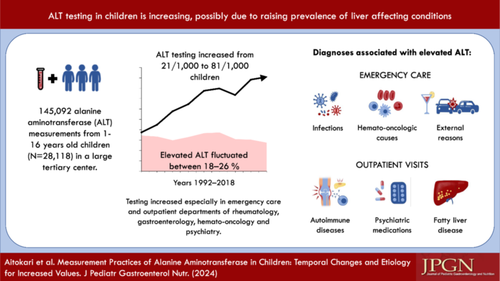
Data on alanine aminotransferase (ALT) measurement practices and diagnoses associated with increased values are limited. We evaluated these issues by collecting ALT measurements from 1- to 16-year-old patients investigated in 1992–2018 in a tertiary center. Diagnoses were gathered in 2008–2018. Altogether 145,092 measurements from 28,118 children were taken 42% undergoing repeated testing. Testing increased from 21/1000 to 81/1000 children and the prevalence of elevated values fluctuated between 18% and 26%. An increase was seen especially in emergency care and departments of rheumatology, gastroenterology, hemato-oncology, and psychiatry. Common acute causes associated with elevated ALT were infections (45%), hemato-oncologic conditions (17%), and external reasons (13%), whereas autoimmune diseases (28%), psychiatric conditions (14%), and metabolic-dysfunction associated steatotic liver disease (10%) were common chronic causes. In conclusion, ALT testing increased 3.9-fold while the proportion of increased values remained stable, indicating that increased testing was justified. However, in some departments the testing efficiency was low.
Graphical Abstract
Highlights
What is Known
-
Excessive laboratory testing may cause unnecessary downstream evaluations and burden for patients and healthcare.
-
Real-life data on the alanine aminotransferase (ALT) testing practices, efficiency, temporal changes, and the diagnoses associated with increased values in children are lacking.
What is New
-
ALT testing increased 3.9-fold 1992–2018 in a tertiary center, whereas the prevalence of abnormal results fluctuated 18%–26%.
-
The testing was most frequently and increasingly ordered by emergency care and outpatient departments of rheumatology, gastroenterology, hemato-oncology, and psychiatry.
-
The most common acute diagnoses associated with elevated ALT were infections, hemato-oncologic diseases, and external reasons, while common chronic causes were autoimmune and psychiatric conditions and metabolic dysfunction-associated steatotic liver disease.
1 INTRODUCTION
Alanine aminotransferase (ALT) is among the most frequently used laboratory measurements in clinical routine, the utilization of which has risen over the last decades.1, 2 At the same time, recommendations for liver value measurement in children remain limited, and are mainly focused on fatty liver disease suspicion or differential diagnostics for elevated values in asymptomatic children.3-6 This increases the risk for inconsistent measurement approaches and overutilization of the tests, and subsequently expensive and invasive downstream evaluations causing unnecessary burden on patients and healthcare.7
The rapid increase of obesity has also resulted in higher prevalence of metabolic dysfunction-associated steatotic liver disease (MASLD), while autoimmune diseases potentially affecting the liver and the use of various hepatotoxic medications may also be increasing.8-11 However, the influence of these changes on measurement practices and prevalence of increased liver values has scarcely been studied.12 Furthermore, the few studies so far have focused on rather narrow patient groups, such as children with fatty-liver disease or those attending gastroenterology outpatient clinics.13, 14
We aimed to (1) evaluate the long-term trends in measurement practices and prevalence of increased ALT values 1992–2018 and (2) to ascertain diagnoses associated with elevated values.
2 MATERIALS AND METHODS
The study was conducted at Tampere University and Tampere University Hospital. All ALT measurements taken 1992–2018 from 1 to 16-year-old children were identified from the hospital register. The hospital served as a secondary and tertiary referral center for approximately 90,000 same-aged children in the former Pirkanmaa Hospital District. Data collected included demographics, ALT results, and the hospital department ordering the testing.
Annual number of measurements was adjusted by local population each year and thus reported as 1 per 1000 1- to 16-year-old individuals living in the area. In 2008–2018, ALT analyses were performed with ALTLP assay by Roche/Hitachi Cobas 6000 automatic analyzer, in 1993–2007 by INTEGRA ALTL 500/Integra 800 automatic analyzer and 1992 by Roche/Hitachi analyzer. Assays were based on the International Federation of Clinical Chemistry and Laboratory Medicine recommendations, included pyridoxal phosphate, and the results are comparable between the timepoints. ALT was defined as normal or elevated based on the local laboratory cutoffs applied during the study period (≥40 U/L). Supplementary analyses were conducted using cutoffs ≥22 U/L for girls and ≥26 U/L for boys as recently recommended by the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN).4, 15 Prevalence of increased values were reported as percentages of all measurements. Temporal changes in ALT testing and the prevalence of increased values were reported for all measurements with both ALT cutoffs and for different pediatric departments with local cutoff. Temporal trends were examined by calculating incidence rate ratios (IRR) with 95% confidence intervals (CIs) using negative binomial regression model for a nonnegative count-dependent variable. Each one-point increase in the coefficient reflects the change in the IRR for a 1-year change in time.
Conditions associated with elevated ALT were scrutinized in a subgroup of patients undergoing testing 2008–2018. Diagnosis codes documented within a week of the first elevated ALT were collected, and in approximately 60% of the cases, the information was further supplemented from medical records. χ2 test was used to compare the distribution of diagnoses between emergency and outpatient departments. Statistical significance was defined as p < 0.05. All analyses were performed using SPSS software, version 27.0 (IBM Corp.).
The study was conducted in accordance with the Helsinki Declaration. Data collection were approved by the Pirkanmaa Hospital District according to the Finnish national guidelines. Ethical committee consent was not needed as the study was register-based and no patients were contacted.
3 RESULTS
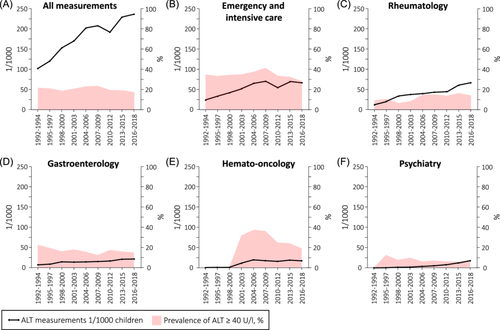
Overall, 145,092 ALT measurements from 28,118 children (mean age at first measurement 8.7 years, 48.7% girls) were recorded in 1992–2018. Altogether 42.1% of the children had undergone repeated testing (2–277 per child), which was most common in the outpatient departments of hematology (96.3% of all measurements were follow-up tests), rheumatology (95.1%), organ transplants (91.8%), and gastroenterology (84.6%). The children with repeated testing were more often girls (51.9% vs. 48.7%, p < 0.001), older (mean 9.6 vs. 7.9 years, p < 0.001) and had higher ALT levels (mean 49.8 vs. 20.5 U/L, p < 0.001) than those with a single testing, whereas the groups did not differ in the year of measurement. The number of ALT measurements increased significantly during the study period (IRR: 1.03 [95% CI: 1.02–1.04]), while the prevalence of elevated ALT values fluctuated between 18% and 26% (1.00 [0.99–1.00]) (Figure 1A).
ALT testing site was available for 98.8% of the measurements. Of all measurements, the testing proportions were in emergency (ECU) and intensive care unit (ICU) 29.3%, outpatient departments of rheumatology 22.0%, gastroenterology 7.8%, neurology 6.7%, general pediatrics 6.6%, hemato-oncology 6.2%, organ transplants 4.4%, endocrinology 3.7%, psychiatry 3.1%, dermatology 1.4%, internal medicine 0.8% and others combined 0.9%, and inpatient departments of internal medicine 3.5% and others combined 3.4%.
Testing increased significantly in ECU/ICU (IRR: 1.04 [95% CI: 1.03–1.05]), and outpatient departments of rheumatology (1.06 [1.05–1.07]), gastroenterology (1.05 [1.03–1.06]), hemato-oncology (1.27 [1.11–1.44]), psychiatry (1.21 [1.18–1.24]) (Figure 1B–F), and in dermatology (absolute change from 0.4 to 1.3/1000, IRR: 1.04 [1.03–1.6]), internal medicine (0.3–0.8/1000, 1.03 [1.01–1.04]), and other outpatient departments (0.1–0.7/1000, 1.11 [1.05–1.18]). Testing decreased in outpatient departments of neurology (4.2–2.9/1000, 0.98 [0.97–0.99]) and general pediatrics (4.6–3.6/1000, 0.97 [0.96–0.98]), and fluctuated non-significantly in departments of endocrinology (0.9–2.1/1000), organ transplants (2.3–2.4/1000), inpatient department of internal medicine (1.6–2.0/1000), and other inpatient departments (2.0–2.7/1000).
The pooled prevalence of elevated ALT was 20.8% in all departments and 34.5% in ECU and ICU, 29.6% in hemato-oncology, 15.5% in gastroenterology, 12.7% in rheumatology, and 6.3% in psychiatry when using the local cutoff (≥40 U/L). Elevated ALT increased in rheumatology (1.03 [1.02–1.05]), decreased in gastroenterology (0.99 [0.98–0.99]), and fluctuated nonsignificantly in ECU/ICU, hemato-oncology, and psychiatry (Figure 1B–F). In other departments, increased ALT (reported with IRR in case of significant changes) was seen in 5.3% (1.02 [1.01–1.03]) in neurology, 17.0% (0.94 [0.92–0.95]) in general pediatrics, 19.7% in organ transplant, 12.2% in endocrinology, 5.5% in dermatology, 18.5% in internal medicine, 5.7% in other departments, and in 26.9% (0.97 [0.96–0.97]) of patients in inpatient departments of internal medicine and 17.1% in other inpatient departments. In the supplementary analysis with lower ALT cutoffs,4, 15 the pooled prevalence of elevated ALT in all departments was 39.8%, and the prevalence fluctuated over time non-significantly between 33.3% and 46.0% (1.00 [1.00–1.01]).
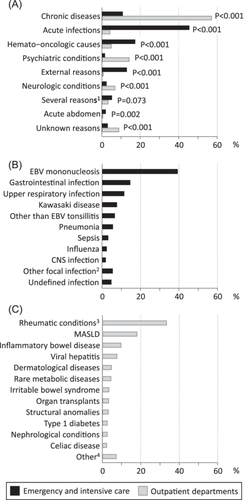
There was a significant difference in the distribution between the conditions associated with the increased ALT between ECU/ICU and outpatient departments. The most common conditions associated with increased values can be seen in Figure 2.
4 DISCUSSION
We observed a 3.9-fold increase in ALT testing in 1992–2018, especially due to rising use in ICU/ECU and outpatient departments of rheumatology, hemato-oncology, psychiatry, and gastroenterology, which were also the largest testing sites. To the best of our knowledge, no similar data have previously been reported. As an indirect comparison, laboratory testing as a whole increased 3.3-fold in 2000–2015 in UK primary care, with a 4.1-fold increase in liver tests,1 but the numbers were not given separately for specific tests or children. Moreover, ALT testing increased 22.1-fold in 2000–2017 among Welsh adults.2 The rising prevalence and improved awareness of MASLD, autoimmune, and psychiatric conditions may partially explain the increased testing, both in general and in the departments of rheumatology, gastroenterology, and psychiatry.8-11 ALT is used in the evaluation and follow-up of these conditions and their medications, but routine screening with ALT is not recommended in Finland. Other diagnostic indication for testing is unclear symptoms/findings. From this perspective, also changes in the general attitudes toward the use of laboratory tests could explain some of the change.
The prevalence of increased ALT in all children was 21% by local laboratory cutoff and 40% by NASPGHAN recommended, lower and gender-specific cutoffs,4, 15 and remained quite stable during the study period. Earlier studies have reported figures of 1.3%–30.4% depending on the population and cutoffs used.8, 16, 17 This highlights the significance of the used cutoffs on the number of children presenting with abnormal results and thus needing follow-up and downstream investigations. Additionally, age, puberty stage and ethnicity might affect the ALT levels and more studies on optimal references are needed. Welsh et al. found that proportion of elevated ALT increased from 3.9% to 10.7% by NASPGHAN cutoffs and from 0.8% to 2.7% by >40 U/L cutoffs within 20 years among US adolescents,8 whereas no change was seen in Korean adolescents within 5 years by applying gender specific ALT cutoffs 24 U/L for boys and 18 U/L for girls.17 One reason for this country difference could be the recent upsurge of MASLD in the United States.8 The high percentage of elevated ALT despite increased testing in our center implies a reasonable measurement strategy, that is, in the hemato-oncology and gastroenterology departments, whereas the low percentage in some other sites suggests overutilization. For example, rapidly increased testing in case of psychiatric conditions or medications could be questioned as, according to the low prevalence of increased values, testing efficacy was poor. Frequent testing in ICU/ECU could also be debatable, even if increased ALT was common. For example, testing in case of benign viral infections known to be associated with transient ALT increase may lead to unnecessary downstream evaluations and concerns.6 There is also evidence that liver tests are used increasingly in adults in ECU and during hospitalization, often without clear indication.7
The diagnoses associated with increased ALT were numerous and differed between patients in ECU/ICU and outpatient departments. The most common associated conditions in ECU/ICU were infections, hemato-oncologic conditions, and external reasons, whereas in outpatient departments autoimmune diseases and MASLD were particularly common. Earlier research has focused narrowly either on children with ALT elevation persisting 6–12 months and those attending a gastroenterology department,14, 18, 19 or mixed patients from ECU and outpatient departments.12, 20 Conditions associated with increased transaminases have varied markedly, MASLD explaining 10%–55%, infections 0%–58%, inherited metabolic disorders 1%–31%, drug toxicity 0%–19%, autoimmune diseases 3%–12%, and other/idiopathic reasons 17%–49% of increased transaminases.12, 14, 18-20 There are well-known country differences with higher prevalence of MASLD and autoimmune conditions in high income countries while viral infections, for example, are more common in countries with lower public hygiene levels and vaccination coverage. Overall, the high prevalence of MASLD, autoimmune conditions, and psychiatric diseases/medications seen here strengthens the notion of these diseases possibly explaining the increase in measurements, and the parallel increase in abnormal results.
The main strengths of our study were the large cohort of patients and use of systematically maintained medical records. Furthermore, public healthcare is easily accessible and free of charge for children in Finland, reducing the risk of selection bias due to socioeconomic status. The main limitation was the retrospective design. Moreover, we could not analyze separately patients with possible multifactorial etiology for ALT elevation (e.g., both a hepatotoxic disease and its medication) or consider the possible effects of growth or puberty on the results. The diagnoses were based on clinical decision-making and hepatotropic viruses or creatine phosphokinase, for example, were not tested systematically. Also, due to data quality issues, collection of the diagnoses was focused on those with elevated ALT based on the local laboratory cutoff during the most recent 10 years. Data about diagnoses was incomplete in individuals with normal ALT (available for <10% with diagnosis code search) and in those studied during earlier years. The study was conducted in a tertiary center and in population comprising homogenous ethnicity, with majority (>90%) being white Caucasian, which may limit the generalizability of the results to primary care settings and other geographical areas.
To conclude, ALT testing increased 3.9-fold in 1992–2018, particularly due to rising use in ICU/ECU and outpatient departments of rheumatology, hemato-oncology, psychiatry, and gastroenterology. Overall, the proportion of elevated values remained quite high and stable, indicating a simultaneous increase in or improved identification of liver-affecting diseases and medications. The testing strategies used thus appear to be appropriate at a general level, but in some departments testing efficiency was quite low. Frequent testing in case of psychiatric conditions/medications or temporary infections in particular could be questioned as overutilization may lead to patient and familial burden and unnecessary downstream investigations. This underlines the need for better recommendations regarding ALT measurements in children and adolescents.
ACKNOWLEDGMENTS
The Päivikki and Sakari Sohlberg Foundation, the Foundation for Pediatric Research, the Competitive State Research Financing of the Expert Area of Tampere University Hospital, The Juho Vainio Foundation, the Mary and Georg C. Ehrnrooth Foundation, the Paulo Foundation, the Emil Aaltonen Foundation, the Finnish-Norwegian Medical Foundation, and the Sigrid Jusélius Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or in the preparation of the present manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.