Effectiveness of strategies to suppress antibodies to infliximab in pediatric inflammatory bowel disease
Abstract
Objectives
Antibodies to infliximab (ATIs) are associated with loss of response in children with inflammatory bowel disease (IBD). We aimed to describe the effectiveness of strategies for treatment modification following ATI development in pediatric IBD: (1) treatment escalation; and (2) switching to another anti-TNF agent.
Methods
This multicenter retrospective study included children with IBD (4–18 years) on infliximab. Therapeutic drug monitoring (TDM) < 6 months and corticosteroid-free remission following each strategy were evaluated for low ATI titers (≤30 AU/mL) and high ATI titers (>30 AU/mL).
Results
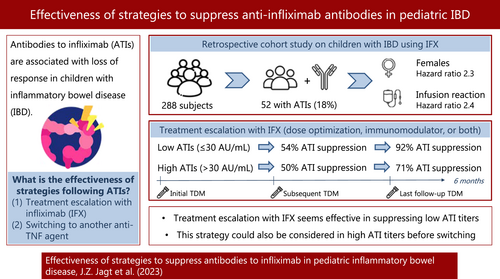
Anti-infliximab antibodies were detected in 52/288 patients (18%) after a median of 15.3 months. Three of 52 ATI-positive patients were excluded due to alternative treatments. Of the remaining 49 patients, 19 had low titers and 30 had high titers. Of 19 low-ATIs, 16 (84%) underwent treatment escalation with infliximab (IFX). Of 13 patients with TDM available, seven (54%) achieved ATI suppression at subsequent TDM and 12 (92%) at any time point. Among 30 patients with high-ATIs, 17 (57%) continued with IFX; immunomodulators were started in seven patients. Of 14 patients with TDM, seven (50%) achieved ATI suppression at subsequent TDM and 10 (71%) at any time point. At 24 months of follow-up, 73% of low-ATI patients and 50% of high-ATI patients could continue with IFX without steroids. Thirteen of 30 high-ATI patients (43%) switched to another anti-TNF agent, of whom 54% and 46% had clinical response at 6 and 24 months, respectively.
Conclusions
Dose optimization and/or adding an immunomodulator seem effective in suppressing low ATI titers. This strategy could also be considered in high ATI titers before switching.
Graphical Abstract
The development of antibodies to infliximab (ATIs) commonly causes a loss of response in children with inflammatory bowel disease. This retrospective cohort study showed that treatment escalation with infliximab (dose optimization and/or adding an immunomodulator) seems to be an effective strategy to suppress ATIs in patients with low ATI titers (≤30 AU/mL). For high ATI titers, this strategy could also be considered before switching.
Highlights
What is Known
-
Pharmacokinetic loss of response to infliximab in pediatric inflammatory bowel disease (IBD) is commonly caused by anti-infliximab antibodies (ATIs).
What is New
-
Treatment escalation (dose optimization and/or adding an immunomodulator) resulted in 92% and 71% ATI suppression, at any time point, in low-ATIs (≤30 AU/mL) and high-ATIs (>30 AU/mL), respectively.
-
Long-term follow-up showed that 73% of low-ATI patients and 50% of high-ATI patients continued with IFX without steroids at 24 months following treatment escalation with IFX.
-
Even though ECCO-ESPGHAN guidelines recommend switching to another anti-TNF when high ATI titers are detected, dose optimization and/or adding an immunomodulator could be considered in these patients.
1 INTRODUCTION
Infliximab (IFX) is a chimeric monoclonal antibody to tumor necrosis factor-alpha (TNF-α), which is effective in inducing and maintaining remission in children with inflammatory bowel disease (IBD).1, 2 However, primary nonresponse affects up to 40% of adults with IBD, while secondary nonresponse occurs in 20%–37%.3, 4 Although index studies in pediatric IBD have reported a lower primary nonresponse rate, recent data have shown this is likely due to more stringent inclusion criteria in clinical trials compared with the use of these drugs in real-world patients.5 A common cause of pharmacokinetic loss of response is the formation of antibodies to IFX (ATIs), which is associated with lower trough levels and the occurrence of infusion reactions.6 Immunogenicity-related factors include IFX therapy without a concomitant immunomodulator (IM),7-10 suboptimal drug concentrations,4 and carriage of the HLA-DQA1*05 allele.11 Since other options for biologic therapies in children with IFX failure remain limited, developing adequate strategies for retaining therapeutic effect is essential.
Strategies for suppressing ATIs include dose optimization (increasing the dose and/or shortening the interval), the addition of an IM, and switching to an alternative anti-TNF agent or another class of biologics. Studies in children with IBD have shown that dose optimization and/or adding an IM could suppress ATIs and recapture the clinical response in 38% and 54% of cases, respectively.12-14 However, data on the optimal strategy remain limited, especially in relation to ATI titers.13 The ECCO-ESPGHAN guideline15 recommends performing dose optimization in patients with low ATI titers, yet it is unknown whether this strategy could also be effective in high ATI titers. This study aimed to describe the effectiveness of commonly applied strategies for ATI suppression relative to ATI titers. The secondary aim was to investigate predictive factors for ATI development in pediatric IBD.
2 METHODS
2.1 Study design and patient population
This retrospective, multicenter cohort study was performed at the Amsterdam University Medical Center, locations VU University Medical Center (VUmc) and Academic Medical Center (AMC), the Netherlands. Children aged 4–18 years diagnosed with IBD16 who were prescribed IFX between January 2006 and November 2021 were eligible to participate. The exclusion criterion was participation in the TISKids study.17 This study was approved by the medical ethical committee of the Amsterdam University Medical Center.
2.2 IFX treatment and anti-IFX antibodies
IFX was prescribed according to the ECCO-ESPGHAN guidelines.15, 18 Concomitant IMs, including thiopurines and methotrexate, were prescribed as standard care for approximately 6–12 months following the start of IFX.15, 18 Intensified dosing of IFX was based on disease activity and therapeutic drug monitoring (TDM).15, 18 Since 2019, proactive TDM has been implemented, mainly performed during maintenance but increasingly at the end of induction and at start of maintenance.19, 20 Before 2019, the assessment of trough levels and/or ATIs was based on the treating physician's judgment since no standardized protocol was available. Serum trough levels and ATI titers were analyzed at a central lab (Sanquin, Amsterdam). A validated enzyme-linked immunosorbent assay (ELISA) technique was used to determine serum trough levels, whereas ATIs were measured using a radioimmunoassay (RIA) antigen-binding test (expressed as arbitrary units per milliliter [AU/mL]).21, 22 This is a drug-sensitive assay, which means that ATIs could not be detected when IFX trough levels are higher than 1 μg/mL.21, 23 Hence, according to the manufacturer's instructions, ATIs were assessed in a standardized manner if IFX trough levels were <1 μg/mL. Antibodies were detected from 12 AU/mL and quantifiable from 30 AU/mL. ATI positivity was defined as titers >12 AU/mL.
2.3 Data collection and outcomes
The following data were collected: sex, age at diagnosis and start of IFX, IBD phenotype,24 IFX dose, IM and premedication use, and initial serum trough and ATI levels. Infusion reactions were documented.25 Patients with ATIs were divided into three groups according to the type of strategy: (1) continuation of IFX by dose optimization (either by increasing the dose or shortening the interval, or both) and/or the addition of an IM, or watchful waiting; (2) the switch to an alternative anti-TNF agent; and (3) an alternative treatment, including the switch to another class of biologics or surgery. The effectiveness of strategies in groups 1 and 2 was evaluated. The primary outcomes were subsequent serum trough levels and ATI titers within 6 months following the strategy. Suppression of ATIs was defined as undetectable ATI titers (<12 AU/mL). If no subsequent ATI assessment was performed but only serum trough levels were measured, we defined IFX trough levels >3 μg/mL as ATI clearance based on previous literature.26-29 Since the decision to perform TDM following the strategy was based on the treating physician's judgment, the time points of subsequent TDM were variable within these 6 months. Clinical response was defined as the continuation of IFX without the need for steroids, assessed at 6, 12, and 24 months following the initiation of the strategy. The loss of follow-up was documented. Secondary outcomes were predictive factors for ATI development, which included the patients' and IFX characteristics.
2.4 Statistical analyses
The Statistical Package for the Social Sciences (SPSS, IBM; v26) was used. p-Values < 0.05 were considered to be statistically significant. Medians and interquartile ranges (IQR) were calculated for nonnormally distributed data. The Mann–Whitney U was performed to compare nonparametric continuous data, whereas categorical variables were compared using the Chi-square test or Fisher's exact. To determine the ATI-free survival in this cohort, the Kaplan–Meier method was used. The survival probabilities were compared using the log-rank test. Furthermore, Cox regression analysis was performed to study potential predictive factors for ATI development. All variables with a p < 0.20 in the univariate analysis were included in the multivariate Cox regression. The results were expressed as hazard ratios (HR) with 95% confidence intervals (CIs). To assess the effectiveness of the applied strategies to suppress ATIs, the study cohort was divided into two groups based on the ATI titers: ≤30 AU/mL was defined as low ATI titers, whereas >30 AU/mL was defined as high ATI titers. This cut-off value was based on previous literature showing that ATI titers >30 AU/mL (using the same assay) were consistently associated with undetectable IFX levels.30 Receiver operator curve (ROC) analysis was performed to investigate predictive cut-off values for ATI suppression.
3 RESULTS
3.1 Baseline characteristics
In total, 288 children with IBD (209 Crohn's disease [CD], 68 ulcerative colitis [UC], and 11 IBD-unclassified) on IFX were included (Figure S1), with a total follow-up of 857 years. The group of ATI-positive patients (n = 52) consisted of significantly more CD patients, females, patients with a younger age at diagnosis, and patients with infusion reactions compared with ATI-negative children (Table 1).
| Patients with ATIs (n = 52) | Patients without ATIs (n = 236) | p-Value | |
|---|---|---|---|
| Median age at diagnosis, years (interquartile ranges [IQR]) | 12.1 (10.8–15.1) | 14.0 (11.4–15.5) | 0.03 |
| Male, n (%) | 19 (36.5) | 129 (54.7) | 0.02 |
| Type of disease, n (%) | 0.01* | ||
| CD | 46 (88.5) | 163 (69.1) | |
| UC | 6 (11.5) | 62 (26.3) | |
| IBD-U | 0 (0) | 11 (4.7) | |
| Paris location of CD patients, n (%) | 0.78 | ||
| L1: ileal | 8 (17.4) | 28 (17.2) | |
| L2: colonic | 15 (32.6) | 45 (27.6) | |
| L3: ileocolonic | 23 (50.0) | 90 (55.2) | |
| Upper GI involvement: L4a, L4b | 22 (47.8), 1 (2.2) | 62 (38.0), 17 (10.4) | |
| Paris behavior of CD patients, n (%) | n.a. | ||
| B1: nonstricturing, nonpenetrating | 42 (91.3) | 133 (81.6) | |
| B2: stricturing | 4 (8.7) | 17 (10.4) | |
| B3: penetrating | 0 (0) | 10 (6.1) | |
| B2B3: both penetrating and stricturing | 0 (0) | 3 (1.8) | |
| P: perianal disease modifier, n (%) | 16 (34.8) | 49 (30.1) | |
| Paris location of UC patients, n (%) | n.a. | ||
| E1: ulcerative proctitis | 0 (0) | 5 (8.1) | |
| E2: left sided UC (distal splenic flexure) | 2 (33.3) | 7 (11.3) | |
| E3: extensive (hepatic flexure distally) | 0 (0) | 5 (8.1) | |
| E4: pancolitis (proximal hepatic flexure) | 4 (66.7) | 45 (72.6) | |
| Median infliximab dose, mg/kg (IQR) | 5.2 (4.9–5.8) | 5.2 (5.0–5.8) | 0.77 |
| Median time to start infliximab, months [IQR] | 9.9 (3.9–26.5) | 9.7 (3.2–22.9) | 0.67 |
| Infusion reaction, n (%) | 11 (21.2) | 25 (10.6) | 0.04 |
| Premedication with hydrocortisone, n (%) | 21 (40.4) | 114 (48.3) | 0.30 |
| Use of IM concomitant with infliximab, n (%) | 43 (82.7) | 193 (81.8) | 1.0 |
| Azathioprine | 34 (79.1) | 154 (79.8) | |
| Mercaptopurine | 1 (2.3) | 6 (3.1) | |
| Methotrexate | 8 (18.6) | 33 (17.1) | |
| Use of steroids <4 weeks of start infliximab, n (%) | 21 (40.4) | 107 (45.3) | 0.52 |
| Infliximab as induction treatment (top-down), n (%) | 7 (13.5) | 22 (9.3) | 0.44 |
- Note: Age, sex, type of disease, and Paris classification were collected at diagnosis. At initiation of infliximab, the infliximab dose, premedication with hydrocortisone, use of concomitant immunomodulators, use of steroids, and a top-down treatment strategy were collected.
- Abbreviations: ATIs, antibodies to infliximab; CD, Crohn's disease; IBD-U, inflammatory bowel disease-unclassified; IM, immunomodulator; UC, ulcerative colitis.
- * Post-hoc analysis showed that the group of patients with anti-infliximab antibodies consisted of significantly more children with CD (p-value = 0.02) than children with UC.
3.2 Incidence of anti-IFX antibodies and associated factors
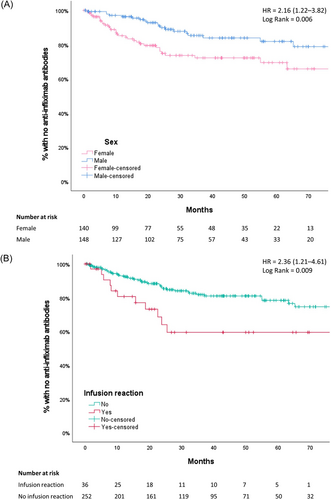
Fifty-two patients (18.1%) developed ATIs that were detected after a median of 15.3 months (IQR: 7.0–24.1) following IFX initiation. Within the first 12 months, 9% of patients developed ATIs, and 21% within 36 months (Kaplan–Meier). The incidence rate of ATIs was 6.1 per 100 years of follow-up. At ATI detection, 22/52 patients (42.3%) used an IM, including thiopurines (n = 17) and methotrexate (n = 5). In a multivariate model, the rate of immunogenicity was higher in females compared with males (HR: 2.31; 95% CI: 1.29–4.14; p < 0.01) and in patients with (n = 36) versus without (n = 252) infusion reactions (HR: 2.38; 95% CI: 1.21–4.69; p = 0.01), as shown in Figure 1 and Table S1. ATI development was negatively associated with increasing age at diagnosis (HR: 0.91; 95% CI: 0.83–0.99; p = 0.03). Concomitant IM use was not associated with immunogenicity.
3.3 Strategies to suppress anti-IFX antibodies
IFX therapy was continued in 33/52 ATI-positive patients (63.5%) by dose optimization (n = 20), the addition of an IM (n = 3), or both (n = 8). Two patients had no treatment changes (watchful waiting). Sixteen patients (30.8%) switched to an alternative anti-TNF agent. These patients had significantly higher initial ATI titers (median titer 160 AU/mL, IQR: 50–468, p = 0.01) and lower trough levels (median level 0.03 μg/mL, IQR: 0.03–0.03, p = 0.02) compared with dose-optimized patients (median ATI titer 31 AU/mL, IQR: 24–94; median trough level 0.04 μg/mL, IQR: 0.03–0.28). Three patients discontinued IFX upon the detection of ATIs, of whom one continued with oral mesalazine and topical steroids, one started with nutritional therapy, and one switched out of class. These patients were excluded from the analyses on the effectiveness of the strategies applied to suppress ATIs.
3.4 Strategies in low anti-IFX antibody titer group
Among the 49 ATI-positive patients, 19 had low ATI titers of ≤30 AU/mL (38.8%), of whom 16 underwent treatment escalation with IFX (84.2%), whereas three patients switched to an alternative anti-TNF agent (15.8%).
3.4.1 Continuation of IFX
Sixteen patients continued with IFX through dose optimization (n = 10), addition of IM (n = 1), both (n = 3), or watchful waiting (n = 2). Of these 16 ATI-positive patients, 13 underwent a subsequent TDM within 6 months following the strategy. Seven of 13 (54%) had ATI suppression at subsequent TDM, accompanied by a median rise in serum trough levels of 4.80 μg/mL (IQR: 3.15–5.30). These patients had a median initial ATI titer of 19 AU/mL (range: 14–30 AU/mL) and trough level of 0.09 μg/mL (range: 0.03–0.70 μg/mL), whereas patients without ATI suppression had a median ATI titer of 29 AU/mL (range: 20–30 AU/mL, p = 0.07) and trough level of 0.35 μg/mL (range: 0.04–0.80 μg/mL, p = 0.45). The clinical outcomes at 6 and 24 months are shown in Figure 2.
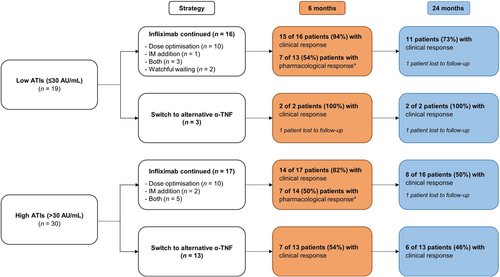
Of the 10 dose-optimized patients, five (50.0%) had ATI suppression at subsequent TDM with a median increase in trough levels of 3.30 μg/mL (IQR: 1.66–4.98). Among the five patients without ATI suppression at subsequent TDM, four achieved ATI suppression and could continue with IFX without steroids at 12 months of follow-up following additional IM addition (n = 1), further dose optimization (n = 1), or without further intervention (n = 2). Hence, overall, 9/10 patients (90%) achieved ATI suppression following dose optimization after a median time of 3.2 months (IQR: 2.5–5.7).
In one patient, an IM (thioguanine) was initiated following ATI development. No TDM was available within 6 months following the strategy. Clinical outcomes showed that this patient achieved steroid-free remission at 6, 12, and 24 months of follow-up. In three patients, both interventions (dose optimization and IM addition) were performed, of whom two achieved ATI suppression (67%) at subsequent TDM (Table 2). The other patient with persistent ATIs had a second TDM performed within 6 months following the intervention, showing ATI clearance based on the IFX trough level. Out of overall 13 patients with TDM (at any time point), 12 (92%) achieved ATI suppression following treatment escalation with IFX (either dose optimization, IM addition or both). Two patients underwent watchful waiting without subsequent TDM. One achieved steroid-free remission, while the other switched to an alternative anti-TNF agent at 24 months of follow-up.
| High versus low ATI titer group | Type of intervention | Median ATI titer, AU/mL (range) | Median TL, μg/mL (range) | TDM after interventiona, n | Median time subsequent TDM, days (range) | ATIs suppressed at subsequent TDM, n (%) | ATIs suppressed at last follow-up TDM, n (%) |
|---|---|---|---|---|---|---|---|
| Low ATIs (≤30 AU/mL) | Continuation of infliximab (n = 16) | 24 (14–30) | 0.20 (0.03–0.80) | 13 | 84 (21–160) | 7 (54) | 12 (92) |
|
25 (14–30) | 0.25 (0.03–0.70) | 10 | 84 (21–160) | 5 (50) | 9 (90) | |
|
30 | 0.20 | 0 | n.a. | n.a. | n.a. | |
|
20 (19–24) | 0.40 (0.09–0.80) | 3 | 66 (54–125) | 2 (67) | 3 (100) | |
|
(23–26) | (0.03–0.03) | 0 | n.a. | n.a. | n.a. | |
| High ATIs (>30 AU/mL) | Continuation of infliximab (n = 17) | 96 (31–540) | 0.03 (0.03–0.20) | 14 | 70 (22–171) | 7 (50) | 10 (71) |
|
92 (31–370) | 0.03 (0.03–0.20) | 8 | 77 (22–157) | 3 (38) | 6 (75) | |
|
(40–150) | (0.03–0.05) | 2 | (56–156) | 2 (100) | 2 (100) | |
|
110 (31–540) | 0.03 (0.03–0.03) | 4 | 70 (53–171) | 2 (50) | 2 (50) |
- Abbreviations: ATIs, antibodies to infliximab; IM, immunomodulator; TDM, therapeutic drug monitoring; TL, trough level.
- a Therapeutic drug monitoring results >6 months following the intervention were excluded from analysis.
3.4.2 Switch to an alternative anti-TNF agent
Three patients with low ATI titers (range: 19–22 AU/mL; range trough level 0.03–3.0 μg/mL) switched to adalimumab. TDM was performed in one of these patients, who did not develop antibodies to adalimumab. Of the other two patients, one continued with adalimumab until 24 months of follow-up, while the other was lost to follow-up (Figure 2).
3.5 Strategies in high anti-IFX antibody titer group
Of 49 ATI-positive patients, 30 had high ATI titers (>30 AU/mL). Of these patients, 17 continued with IFX, whereas 13 switched to an alternative anti-TNF agent.
3.5.1 Continuation of IFX
IFX was continued in 17 patients by applying the following strategies: dose optimization (n = 10), addition of an IM (n = 2), or combination of both strategies (n = 5). In 14 patients, TDM was performed following the strategy, of whom seven (50%) had ATI suppression (Table 2). These patients had a median rise in serum trough level of 6.67 μg/mL (range: 0.17–32.97). Patients with versus without ATI suppression had a median initial ATI titer of 96 AU/mL (range: 37–190) and 87 AU/mL (range: 31–540, p = 1.0), respectively. Clinical response at 6 and 24 months is shown in Figure 2.
Ten patients with high ATI titers underwent dose optimization by increasing the dose (n = 3), shortening the interval (n = 3), or both (n = 4). Of eight patients with subsequent TDM, three had undetectable ATIs and a rise in IFX trough levels (range: 0.17–32.97 μg/mL). These patients with ATI suppression received an increased dose of 10 mg/kg, and two also had a shortened interval. Of five patients without ATI suppression at subsequent TDM, three achieved ATI suppression and continued with IFX at 12 months of follow-up following additional IM addition (n = 1), further shortening of the IFX interval (n = 1), or without further intervention (n = 1). Thus, six of overall eight patients (75%) achieved ATI suppression after dose optimization (median time of 3.9 months, IQR: 1.6–5.6). The two patients without TDM switched within 3 months to adalimumab: one due to intolerance (initial ATI titer 370 AU/mL) and one due to nonresponse (initial ATI titer 200 AU/mL).
In two patients, an IM (azathioprine and methotrexate) was initiated next to IFX, leading to ATI suppression at the following TDM and steroid-free remission after 6, 12, and 24 months of follow-up. In five patients, dose optimization and IM addition were performed. Among four patients with TDM, two achieved ATI suppression (initial ATI titers: 110 and 190 AU/mL) and still had clinical response at 24 months of follow-up. Out of overall 14 patients with subsequent TDM (at any time point), 10 (71%) achieved ATI suppression after treatment escalation with IFX (either dose optimization, IM addition or both).
3.5.2 Switch to an alternative anti-TNF agent
Thirteen patients with high ATI titers (median ATI titer 220 AU/mL, range: 35–880; median trough level 0.03 μg/mL, range: 0.00–0.03) switched to an alternative anti-TNF agent: 12 to adalimumab and one to golimumab. Four patients underwent TDM within 6 months following the switch, of whom one developed antibodies to adalimumab. Two other patients developed anti-drug antibodies (ADA) within 1 year of adalimumab therapy. Two of three patients with ADA against adalimumab used an IM after the switch. The long-term outcomes are visualized in Figure 2.
3.6 Factors associated with anti-IFX antibody suppression
Using ROC analysis, we investigated cut-off values for ATI titers and trough levels associated with ATI suppression in the patients who underwent treatment escalation with IFX (27 patients with available TDM). Suppression of ATIs could not be predicted (Figure S2). Regarding the high-ATI group, the characteristics of the patients with and without ATI suppression following continuation of IFX are described in Table S2. No predictive factors for ATI suppression were identified.
4 DISCUSSION
This real-life cohort study described the effectiveness of commonly applied strategies to suppress ATIs in pediatric IBD. The majority of low-ATI patients continued with IFX through treatment escalation, of whom 54% had suppressed ATIs at subsequent TDM (92% at any time point); 73% were still on IFX therapy without steroids at 24 months. Less patients (57%) continued IFX in the high-ATI group. Nonetheless, ATI suppression at subsequent TDM was observed in 50% of patients (71% at any time point). At 24 months of follow-up, half of patients could continue with IFX without steroids. Although ECCO-ESPGHAN guidelines recommend switching to an alternative anti-TNF agent in the case of high ATI titers, dose optimization and/or adding an IM could be considered to avoid too hasty cycling through the limited options for biologic therapies in children with IBD.
Dose optimization might be more effective in suppressing ATIs among patients with low (50%) versus high ATI titers (38%). In adults with IBD, Vande Casteele and colleagues31 showed that higher levels of ATIs (>9.1 U/mL) at time of loss of response resulted in a likelihood ratio of 3.6 for an unsuccessful IFX dose adjustment. A retrospective cohort study,32 including 58 ADA-positive children with IBD, observed that 54% demonstrated ADA suppression following dose optimization, according to a protocol that recommended intensifying dose if ADA titers were <10 U/mL. This percentage was comparable to our results when evaluating the effectiveness of dose optimization on ATI suppression in children with low ATI titers.
Even though the numbers of patients per predefined strategy were small in the high-ATI group, both IM addition and dose optimization seem more successful in ATI suppression compared with dose optimization alone. A study in adults with IBD observed that adding an IM cleared ADA to anti-TNF agents in 77% of ADA-positive patients.33 A retrospective study, including 89 ADA-positive children with IBD, showed that the addition of an IM next to anti-TNF is effective to suppress ADA and recapture clinical response.12 They identified that ADA levels >329 ng/mL were predictive of an unsuccessful response to dose optimization alone.12 The different ADA assays hamper comparing the cut-off value with the ATI titers in the present study. We observed that in some children without ATI suppression after dose optimization, ATIs could be suppressed through additional interventions (IM addition and further dose optimization) within 6 months following ATI detection. This illustrates that it might be beneficial to perform these additional strategies, at least for 6 months, before changing to a different biologic treatment.
Overall, 18% of patients developed ATIs, which is comparable to previous retrospective studies in pediatric IBD.32, 34 Two prospective studies observed higher rates (up to 68%) in pediatric IBD,4, 14 which might be attributed to the drug-tolerant assay that was used. Remarkably, females had a twofold higher risk of developing ATIs compared with males. To our knowledge, this has not been reported previously in pediatric IBD. Female sex as a risk factor for discontinuing anti-TNF therapy without ADA measurement has been identified in adults with IBD,35-37 rheumatoid arthritis, and psoriasis.38, 39 Specifically, side effects of anti-TNF agents were significantly more prevalent in females versus males.37, 40 In pediatric CD, female gender was associated with IFX-related infusion reactions.41 Sex differences in pharmacokinetics, such as higher body fat percentage and hepatic clearance in females versus males, and the influence of sex hormones might be related to the higher risk of ATI development.42, 43 However, further research is needed to identify sex-specific differences in immunogenicity, which could possibly result in developing sex-specific regimens to prevent ATIs.
Consistent with previous adult and pediatric IBD studies,6, 25, 44, 45 ATIs were positively associated with infusion reactions. The hypothesis is that ATIs-IFX immune complexes are formed, which activate complement, resulting in infusion reactions.46 Although the use of combination therapy of IFX with IM has been demonstrated to reduce the risk of immunogenicity,7-10 we could not replicate this finding. This is probably due to the small number of patients receiving IFX monotherapy (18%). Younger age at diagnosis as predictive factor for ATI-development has also been observed in a retrospective study including 215 children with IBD, possibly caused by higher disease activity scores or subtherapeutic trough levels.20
The strengths of this study are the multicenter design, the relatively large number of children and years of IFX exposure, and the 24-month follow-up duration after the initiation of the different treatment strategies in ATI-positive patients. The main limitation is the retrospective study design, which results in a lack of standardization of collected data. Especially, TDM following the strategies was not performed in a standardized manner, leading to missing data and differences in the time points of subsequent TDM. Additionally, before 2019, the decision to assess trough levels and/or ATIs was largely based on the treating physician's judgment. This has contributed to the heterogeneity of the reported data and could have introduced selection bias. Adjustments to the dose of IFX and IM and the exact duration of IM use were not recorded. The drug-sensitive assay that was used could have possibly underestimated ATI formation. Future prospective studies comprising larger sample sizes per strategy are recommended to clearly define ATI titers/trough levels cut-off values (preferably using a drug-tolerant assay) and identify clinical or pharmacokinetic factors that could predict ATI suppression. This can aid in clinical decision-making when ATIs are detected.
In conclusion, 18% of pediatric IBD patients developed ATIs. Overall, ATI suppression (at any time point) was achieved in 92% and 71% of patients with low and high ATIs following treatment escalation with IFX, respectively. This illustrates that dose optimization and/or IM addition for low-ATIs seemed to be an effective intervention to suppress ATIs and maintain steroid-free remission on IFX. Our data suggest that this strategy could also be considered even in cases of high-ATIs, with clinical response at 24 months in 50% of cases. The predominance of female sex in children with ATIs needs to be explored in future studies since this finding could result in sex-specific strategies to prevent ATI development.
ACKNOWLEDGMENTS
This work was supported by the “Right on Time” grant with number WO19–25, from The Dutch Digestive Foundation (MLDS).
CONFLICT OF INTEREST STATEMENT
Nanne K. H. de Boer has served as a speaker for AbbVie and MSD and has served as a consultant and principal investigator for TEVA Pharma BV and Takeda. He has received a (unrestricted) research grant from Dr. Falk, TEVA Pharma BV, MLDS, and Takeda. Johan E. Van Limbergen reports consulting, travel, and/or speaker fees and research support from AbbVie, Janssen, Nestlé Health Science, Novalac, Pfizer, Merck, P&G, GSK, Illumina, Otsuka. The other authors declare no conflict of interest.