Copper and temperature modify microbial communities, ammonium and sulfate release in soil
Abstract
Recent studies suggest an important role of thermophilic bacterial communities of the Phylum Firmicutes on soil C, N and S cycling, and a positive effect on crop productivity through the production of sulfate (SO
 ) and ammonium (NH
) and ammonium (NH
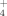 ), essential plant nutrients. Copper (Cu) is commonly supplemented to soils as a fungicide in phytosanitary treatments although its consequences to the bacterial communities is frequently overlooked. Herein, we report on the influence of temperature and Cu on the microbial communities, namely those of the Phylum Firmicutes, from a soil collected at an olive orchard in S Portugal. Community fingerprints and band identification through sequencing was combined with measurement of SO
), essential plant nutrients. Copper (Cu) is commonly supplemented to soils as a fungicide in phytosanitary treatments although its consequences to the bacterial communities is frequently overlooked. Herein, we report on the influence of temperature and Cu on the microbial communities, namely those of the Phylum Firmicutes, from a soil collected at an olive orchard in S Portugal. Community fingerprints and band identification through sequencing was combined with measurement of SO
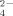 and NH
and NH
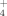 production at different supplemented amounts of Cu and at moderate and high temperatures (30°C and 50°C, respectively). Both temperature and Cu induced changes in these communities, selecting for specific bacteria. Temperature induced the dominance of Brevibacillus, and Cu addition to soil caused a reduction of SO
production at different supplemented amounts of Cu and at moderate and high temperatures (30°C and 50°C, respectively). Both temperature and Cu induced changes in these communities, selecting for specific bacteria. Temperature induced the dominance of Brevibacillus, and Cu addition to soil caused a reduction of SO
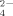 release by soil bacteria. Ammonium production during bacterial growth at moderate and high temperatures was not affected by Cu addition. A Cu-tolerant thermophilic isolate, belonging to the Bacillus genus, showed significant inhibition by high Cu concentrations and a reduction of NH
release by soil bacteria. Ammonium production during bacterial growth at moderate and high temperatures was not affected by Cu addition. A Cu-tolerant thermophilic isolate, belonging to the Bacillus genus, showed significant inhibition by high Cu concentrations and a reduction of NH
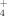 release during growth; genera Brevibacillus and Bacillus have been previously reported as high NH
release during growth; genera Brevibacillus and Bacillus have been previously reported as high NH
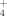 and SO
and SO
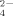 producers of the Firmicutes phylum. Results indicate that Cu treatments select specific tolerant bacterial strains which could influence natural soil fertilization in Cu-treated orchards.
producers of the Firmicutes phylum. Results indicate that Cu treatments select specific tolerant bacterial strains which could influence natural soil fertilization in Cu-treated orchards.
1 Introduction
Soils are complex systems, whose characteristics depend on the interaction of both biological and physico-chemical factors. Among the biological factors, the influence of microorganisms is of maximum importance and contributes decisively to soil health and maintenance.
The role of microbial diversity in soils is essential to a variety of metabolisms, functionalities, and mechanisms of action covering all imaginable scenarios, including responses to natural and artificial alterations as well as extreme events.
Reports by Marchant et al. (2002, 2008) suggested the omnipresence of thermophilic bacteria in soils of the temperate zone. Among these thermophiles, the Phylum Firmicutes includes most representative bacteria with the genus Geobacillus as the typical example (Zeigler, 2014). However, their role has remained to be explained until Portillo et al. (2012) proposed an environmental role for these soil thermophiles. Portillo et al. (2012) showed that at high temperatures for temperate soils (i.e., 50–70°C), these thermophiles, including the genera Geobacillus and Brevibacillus, were able to mineralize organic matter (OM) releasing ammonium (NH
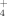 ) and sulfate (SO
) and sulfate (SO
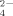 ), thus representing a missing link in the C, N and S cycling in soils of the temperate zone. Firmicutes thermophiles are particularly relevant in organic S-mineralization; indeed, in spite of a large pool of organic S in soils under temperate conditions around 20°C, organic S-mineralization to SO
), thus representing a missing link in the C, N and S cycling in soils of the temperate zone. Firmicutes thermophiles are particularly relevant in organic S-mineralization; indeed, in spite of a large pool of organic S in soils under temperate conditions around 20°C, organic S-mineralization to SO
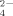 has been reported to be very limited (Ghani et al., 1993; Eriksen, 1996). On the other hand, Firmicutes genera Geobacillus, Ureibacillus and Brevibacillus release significant quantities of SO
has been reported to be very limited (Ghani et al., 1993; Eriksen, 1996). On the other hand, Firmicutes genera Geobacillus, Ureibacillus and Brevibacillus release significant quantities of SO
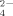 under high temperature conditions as a metabolic product (Portillo et al., 2012; Santana et al., 2013). Santana et al. (2015) proposed a thermophilic C dissimilatory organic-S mineralization characteristic pathway for the release of SO
under high temperature conditions as a metabolic product (Portillo et al., 2012; Santana et al., 2013). Santana et al. (2015) proposed a thermophilic C dissimilatory organic-S mineralization characteristic pathway for the release of SO
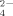 and NH
and NH
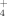 depending on the decomposition of proteinaceous substrates. The temperatures, considered to be necessary for these thermophiles to provide significant activity, have been shown to be highly frequent at medium and low latitudes (Gonzalez et al., 2015) and during daily cycles (Portillo et al., 2012); for instance, temperature measurements during summer days showed values > 40°C in topsoils from S Spain for more than 10 h a day (Portillo et al., 2012). This high temperature niche observed in soils offers these thermophiles their own window of opportunity to develop a significant role in the terrestrial environments. Besides, Santana et al. (2013) suggested these thermophiles to be used in soil nutrient supplementation for plant growth, since the medium where these thermophiles grew induced important plant growth enhancing properties. Thus, soil treatments and processes should be analyzed on the effect of aerobic thermophilic Firmicutes communities and their contribution to soil nutrient turnover in relationship to the maintenance of soil properties and use for agricultural purposes.
depending on the decomposition of proteinaceous substrates. The temperatures, considered to be necessary for these thermophiles to provide significant activity, have been shown to be highly frequent at medium and low latitudes (Gonzalez et al., 2015) and during daily cycles (Portillo et al., 2012); for instance, temperature measurements during summer days showed values > 40°C in topsoils from S Spain for more than 10 h a day (Portillo et al., 2012). This high temperature niche observed in soils offers these thermophiles their own window of opportunity to develop a significant role in the terrestrial environments. Besides, Santana et al. (2013) suggested these thermophiles to be used in soil nutrient supplementation for plant growth, since the medium where these thermophiles grew induced important plant growth enhancing properties. Thus, soil treatments and processes should be analyzed on the effect of aerobic thermophilic Firmicutes communities and their contribution to soil nutrient turnover in relationship to the maintenance of soil properties and use for agricultural purposes.
Among the different possible treatments to be carried out in soils to preserve agricultural profits, copper (Cu) has been used frequently as fungicide. Copper is a micronutrient required for multiple metal-dependent enzymes although at higher concentrations it presents toxicity (Macomber and Imlay, 2009; Solioz et al., 2010) and fungi present high sensitivity to this metal. Copper treatments in soils can present different drawbacks, for example, changes in the bacterial communities. Specifically, the effect of Cu treatments on aerobic thermophilic Firmicutes remains to be studied.
In this study, the effects of temperature and Cu on bacterial communities and specifically on the thermophilic Firmicutes were analyzed. We aimed to determine the influence of these two factors on the active role of this bacterial group as major contributors to the cycling of NH
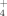 and SO
and SO
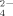 in soils.
in soils.
2 Material and methods
2.1 Sampling and enrichment cultures
Soil samples were collected in an olive orchard of “Cobrançosa” variety, in the parish of São Manços, municipality of Évora in Alentejo, S Portugal (38°29′54.1′′ N; 7°45′38.5′′ W). The soil sampling site has been classified as Haplic Luvisol (IUSS Working Group, 2006), and characterized to have moderate drainage, low erosion, and low topsoil organic C content (ca. 0.75%) (Monteiro and Alexandre, 2008). The olive orchard had been partially submitted to phytosanitary treatment with Cuprital® (Cu-oxychloride, Cl2Cu4H6O6) made by aspersion on the tree crowns, one month before collection, which was performed in November 2011 (average air temperature 12°C, 100 mm precipitation).
Composite samples, each resulting from three adjacent soil cores pooled together, were collected aseptically from topsoil (3.5 to 7.5 cm depth, soil temperature at the point of collection was ca. 10°C). Two composite samples were collected at soil areas treated in October 2011 (T0) and treated before that month, at different periods T6 and T20 (6 months and 20 months, respectively, before collection), either between the tree crowns crop line (EC) or below tree crowns (DC). Analysis of physicochemical parameters was performed following standard procedures (L.Q.A.R.S., 2006) (Table 1).
| Samples | T0-DC | T0-EC | T6-DC | T20-EC |
|---|---|---|---|---|
| Phosphorus (P2O5) / mg kg−1 soil | 104.00 | 78.00 | 88.00 | 42.00 |
| Copper (Cu) / mg kg−1 soil | 104.50 | 13.90 | 96.50 | 3.50 |
| Potassium (K2O) / mg kg−1 soil | 470.00 | 176.00 | 188.00 | 106.00 |
| Nitrate (NO3) / mg kg−1 soil | 4.50 | 4.50 | 7.00 | 6.50 |
| Total N / % | 0.076 | 0.061 | 0.120 | 0.041 |
| pH (H2O) | 6.80 | 6.60 | 7.30 | 7.50 |
| Texture | Sandy loam | Sandy loam | Sandy loam | Sandy loam |
Six grams aliquots of the composite sample T20-EC (Table 1) were added to 100 mL flasks containing 15 mL of Nutrient Broth (Oxoid, pH 7.0) under sterile conditions. Unsupplemented media, 100 µM and 500 µM CuSO4 supplemented flasks were incubated. The soil cultures were incubated at 30°C or 50°C, with shaking (180 rpm) for 24 h, an analogous procedure to the one reported in Portillo et al. (2012). After this period, serial dilutions were plated in NBA (Nutrient Broth plus 15% w/v Agar) at 50°C for 24 h and the colony-forming units (CFU) mL−1 for each enrichment were determined.
2.2 Isolation and identification of a copper-resistant thermophilic bacteria
Bacteria were isolated from the enrichments on agar Petri dishes with NBA supplemented with copper at 50°C. Colonies were picked from the plates and reinoculated to obtain monospecific cultures of these bacteria by repeated isolation. Isolates were tested for growth at 50°C in NBA containing 100 μM and 500 μM Cu and maintained in NB with glycerol 15% ((v/v)) at –70°C for long-term conservation.
Bacterial growth was monitored by optical density at 600 nm on a Cecil CF 1021 spectrophotometer. Bacteria were identified by PCR amplification of their 16S rRNA genes and subsequent sequencing. PCR amplification of the 16S rRNA genes was performed using primer 341F (Muyzer et al., 1993) and reverse primer 907R (Muyzer et al., 1998). Reactions were performed in a total volume of 50 µL, containing 1× PCR buffer, 0.25 mM dNTPs, 0.4 µM of each primer, 0.25 µL i-Max II DNA polymerase (inTRON Biotechnology Inc, Korea), and 5 µL of DNA extracted from an isolated colony suspension subjected to heat and cold alternation for cell lysis. PCR cycling was performed in a Bio-Rad MyCycler apparatus. The PCR-cycling reactions included an initial denaturation step at 95°C for 2 min, followed by 30 cycles at 95°C for 30 s, annealing at 54°C for 30 s, extension at 72°C for 60 s. The last cycle was followed by 5 min at 72°C. The fragments of approx. 600 bp, generated by the above PCR amplification, were purified with DNA Clean & Concentrator (Zymo Research, USA) and sequenced on both strands at Macrogen sequencing services (Macrogen Inc., Korea). Sequences were identified using Blast at the National Center for Biotechnology Information (NCBI; available at: http://www.ncbi.nlm.nih.gov/Blast.cgi).
2.3 Measurement of sulfate and ammonium production
Sulfate was determined by the turbidimetric assay described by Kolmert et al. (2000). One milliliter aliquot of each culture was centrifuged, the supernatant was taken and mixed with 1 mL of conditioning reagent (150 g NaCl, 100 mL glycerol, 60 mL concentrated HCl and 200 mL 95% ethanol, made up to 1 L with deionized water). Crystals of BaCl-dihydrate, approx. 60 mg, were added and the solution was vortexed for 30 s at a constant speed. The resulting suspension was immediately transferred to a cuvette and the turbidity was read at 320 nm using a NanoDrop® 2000c (ThermoScientific, EUA). A reaction mix using 1 mL of the respective non-inoculated sterile culture medium served as blank. Each sample was analyzed in triplicate. Concentration values were determined using a calibration curve for 0–5 mM K2SO4 solutions.
The quantification of the NH
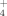 was made as described in Taylor et al. (1974), with minor modifications. Forty microliters of sample were mixed to 760 μL Reagent A: 0.54% w/v ortho-phthalaldehyde, 0.05% (v/v) β-mercaptoethanol and 10% (v/v) ethanol in 400 mM K-phosphate buffer (pH 7.3). After 20 min at room temperature, 200 μL were transferred to a microplate and the absorbance was read at 415 nm with a Bio-Rad Model 680 Microplate Reader. A reaction mix with 40 μL of sterile non-inoculated medium served as blank. Samples were analyzed in triplicate. Concentration values were determined using a calibration curve for 0–5 mM NH4Cl solutions.
was made as described in Taylor et al. (1974), with minor modifications. Forty microliters of sample were mixed to 760 μL Reagent A: 0.54% w/v ortho-phthalaldehyde, 0.05% (v/v) β-mercaptoethanol and 10% (v/v) ethanol in 400 mM K-phosphate buffer (pH 7.3). After 20 min at room temperature, 200 μL were transferred to a microplate and the absorbance was read at 415 nm with a Bio-Rad Model 680 Microplate Reader. A reaction mix with 40 μL of sterile non-inoculated medium served as blank. Samples were analyzed in triplicate. Concentration values were determined using a calibration curve for 0–5 mM NH4Cl solutions.
2.4 DNA extraction and quantification
DNA was extracted from the enrichments to amplify their 16S rRNA genes and perform microbial community analysis. DNA extraction was carried out with DNA Isolation kit PowerSoil® (MoBio Laboratories Inc, USA) following the manufacturer's instructions.
DNA integrity was evaluated by staining with ethidium bromide after electrophoresis on a 0.8% agarose gel in 1 × TAE buffer. Quality and quantity of DNA was determined by absorbance at 260 nm and 280 nm using a NanoDrop® 2000c (Thermo Scientific, EUA).
2.5 RNA extraction and quantification
RNA was extracted from bacterial enrichments to evaluate the activity of specific bacterial groups. The amount of RNA per bacterial cell is proportional to its metabolic activity (Molin and Givskov, 1999), unlike DNA that indicates presence or abundance of bacterial cells in a community. RNA was used to quantify targeted bacterial groups through quantitative real-time reverse transcription PCR. RNA was extracted from the selective enrichments at 30°C and 50°C without and with added Cu at 500 μM. Approx. 0.25 g of each culture pellet, collected by centrifugation at 12,000 × g during 5 min, was added to a ZR BashingBead™ Lysis Tube (from ZR Soil/Fecal RNA MicroPrep kit), and the extraction followed the manufacturer's protocol.
RNA is prone to degradation due to the omnipresence of RNases, so RNA integrity was checked by electrophoresis on a 1% agarose gel in 0.5 × TBE buffer made in DEPC-treated water. Before gel deposition, samples were mixed with 2 × RNA loading dye solution (Fermentas), heated at 70°C for 10 min and immediately chilled on ice until loading. The concentration and purity of the RNA was determined by reading of the absorbance at 260 nm and 280 nm using the NanoDrop® 2000 c (Thermo Scientific, EUA).
2.6 DGGE analyses
Microbial community fingerprints were obtained by using DGGE (Denaturing Gradient Gel Electrophoresis) which separates amplified DNA products through a chemical gradient of DNA denaturing agents (urea and formamide) as previously described (Muyzer et al., 1993). Separation of DNA bands can differentiate up to a single nucleotide difference in the DNA fragments loaded in this electrophoretic analysis. The genes targeted for DGGE analyses were the 16S rRNA genes which are generally used to identify bacteria through the sequence of this gene. We used the primer pair 341F-GC and 518R (Muyzer et al., 1993) to amplify a fragment of the bacterial 16S rRNA genes in the studied communities. To amplify Firmicutes 16S rRNA genes we performed a nested-PCR which consisted in successive PCR amplifications using different primer pairs to increase specificity. A first PCR, using the primer pair 9bfm and 1512uR (Mühling et al., 2008), was followed by subsequent nested reactions using the primer pair Firm 350F and Firm 814R (Mühling et al., 2008) and the primer pair 518F-GC and 785R (Table 2). To analyze the presence in these experiments of bacteria related to the most frequently detected genera (Brevibacillus), we proposed a primer pair aiming to preferentially amplify the 16S rDNA genes within the family Paenibacillaceae. These primers were PanA (5′GRG YCY GCG TYY CAT TAG C) and PanB (SYC CGR CAY CTA GYR TYC ATC) (Table 2), the PCR with these primers was followed by a nested-PCR with the primers 341F-GC and 518R. Amplification reactions with the first primer pair were made on a total volume of 50 μL containing 1 × PCR Buffer, 0.2 mM dNTPs, 0.4 μM of each primer, 0.2 μL of FideliTaq DNA polymerase 5U/µl (USB®, USA) and 10 ng of genomic DNA. Subsequent nested amplifications utilized 2 μL of the previous PCR product. PCR reactions were as follows: after an initial denaturation at 95°C for 2 min, 35 PCR cycles were performed including a denaturing step at 95°C for 1 min, followed by annealing at the appropriate temperature (see Table 2) for 1 min, and extension at 68°C for 1 min (for the primer 9bfm/1512 Ur, the extension was of 90 s). The last cycle was followed by an extension step at 68°C for 5 min. The last PCR reactions, preceding the DGGE gel deposition, were also carried out in a total volume of 50 μL, containing 1 × PCR buffer, dNTPs 0.2 mM, 0.5 μM of each primer, and 0.3 μL DreamTaq DNA polymerase 5U/µl. Amplification cycling reactions comprised an initial denaturation step at 95°C for 2 min, followed by 25 cycles each with a denaturation at 95°C for 30 s, annealing at the appropriate temperature (see Table 2) for 30 s, and extension at 72°C for 30 s. The last cycle was followed by a last extension step at 72°C for 5 min. All PCR reactions were performed in a Bio-Rad MyCycler apparatus.
| Primer | Target group | E. coli position | Annealing temperature / °C | Reference |
|---|---|---|---|---|
| 9 bfm | Bacteria | 9–27 | 52 | Mühling et al. (2008) |
| 1512 uR | Bacteria and Archaea | 1492–1512 | ||
| Firm 350F | Firmicutes | 350–369 | 57 | Mühling et al. (2008) |
| Firm 814R | Firmicutes | 814–833 | ||
| 518F GC | Bacteria | 518–534 | 56 | Mühling et al. (2008) |
| 785R | Bacteria | 785–803 | ||
| PanA | Paenibacillaceae | 222–240 | 57 | this work |
| PanB | Paenibacillaceae | 810–830 | ||
| 341F GC | Universal | 341–357 | 56 | Muyzer et al. (1993) |
| 518R | Bacteria | 518–534 |
DGGE was performed following Muyzer et al. (1993). PCR amplified 16S rRNA gene fragments from four bacterial species were used as DGGE migration marker. The fragments corresponded to the amplification product obtained with the primer pair 341F GC-518R from the following microorganisms (from top to bottom of a DGGE gel): Pseudomonas aeruginosa PAO1, Escherichia coli K12 CECT 433, Paenibacillus sp. DSM 34, Streptomyces caviscabies ATCC 21619. After the run, each gel was stained with ethidium bromide (0.5 mg L−1), visualized on a transilluminator, and photographed with Kodak software Logic Gel 200 (Kodak, USA). The ImageJ program (Schneider et al., 2012) at NIH (National Institute of Health, USA) was used to evaluate the relative intensity of the distinct bands.
The major DGGE bands were selected and excised from the gels, reamplified, purified, and cloned in pCR2.1-TOPO vector from TOPO TA Cloning Kit (Invitrogen). Selected clones were sequenced at Macrogen® sequencing services (Macrogen Inc., Korea). Sequences were identified using Blast at NCBI.
2.7 Real-time quantitative reverse transcription PCR
Extracted RNA was used as template for quantitative reverse transcription PCR amplifications to quantify the activity of bacterial targets. Power cDNA synthesis kit (iNtRON Biotechnology, Inc.) was used to synthesize cDNA from 500 ng of RNA samples (cf section 2.5). The manufacturer's protocol was followed. Primer 518R (Table 2) and BrevR1 (5′AGC TGC GGC ACT RAG GGT ATT G-3′) were used for the synthesis of bacterial and Brevibacillus 16S rDNA, respectively. Reverse transcription was made by incubation of each mix reaction at 42°C for 5 min, then at 50°C for 1 h, and terminated by incubation at 70°C for 5 min. A control reaction mixture for each RNA sample was also made with no addition of reverse transcriptase.
Two microliters of 1/50 dilutions of the above reactions were used as template in a total 20 μL volume, containing iTaq Universal SYBR Green supermix 1 × (Bio-Rad) and forward and reverse primers for qPCR assays with SYBR Green detection. Primers 341F and 518R (Table 2) were used for the amplification of 16S rDNA from bacteria, whereas BrevF (5′YGT AAA GTT CTG TTG TYA GGG A-3′) and BrevR1 where used for the amplification of 16S rDNA from Brevibacillus genus; primer pair BrevF/R1 hybridize to regions 430–451/846–867 of Brevibacillus sp. 16S rDNA. Three replicates of each reaction were loaded in a MicroAmp Optical 96-well reaction plate. Thermal cycler Applied Biosystem 7500 was programmed with the following protocol: an initial denaturation cycle at 95°C for 1 min, 40 cycles consisting of a denaturing step at 95°C for 15 s, and annealing and extension at 56°C (for 341F/518R primers) or 60°C (for Brev primers) for 60 s.
Quantification was performed following the sigmoidal curve-fitting procedure described by Rutledge (2004) with Sigmaplot 8.02 (Systat Software, Inc., London, UK).
2.8 Statistic analyses
ANOVA analysis (Sokal and Rohlf, 1981) was performed with MedCalc software version 12.3.0 to compare significant differences (P < 0.05) among treatments at the end of the experiments when variances were normally distributed. Significance of microbial community profiles by DGGE was statistically compared according to Portillo and Gonzalez (2008).
3 Results and discussion
3.1 Effect of copper and temperature on community abundance
Table 1 shows some characteristics of the soil samples collected during this study. The olive tree soil under study had neutral pH, which contributes to Cu retention in the topsoil. On the other hand, soil texture is sandy loam as previously characterized, with moderate drainage capacity due to the presence of a clay-enriched subsoil and low C content soil (Monteiro and Alexandre, 2008), factors leading to Cu run-off. Nevertheless, Cu levels reached 104.50 mg kg−1; this last value is ca. 7-fold the maximum reference value (15 mg kg−1) used to classify a soil as being of “high Cu content” (L.Q.A.R.S., 2006). T6-DC samples showed a high copper content, similar to T0-DC, likely due to the previous treatment 6 months earlier suggesting a potential for Cu accumulation in the soil.
Community fingerprinting showed an influence of Cu on the bacterial community at 30°C with notorious changes in the banding profile observed at the highest Cu concentration tested (500 μM) (Fig. 1). Comparing the enrichments at 30°C and 50°C, very different banding patterns were observed (Fig. 1) suggesting that a higher effect on the microbial community can be induced by temperature than by supplementation with Cu. The bacterial fingerprint from the 50°C bacterial enrichment at 500 μM Cu showed large differences with the community fingerprint at 50°C and 100 μM Cu; the dominant phylotypes were different at 500 μM than at 100 μM Cu or without Cu supplementation (Fig. 1). These results indicate that high concentration of Cu (in the 500 μM range) was required for significant changes to be observed in the bacterial community of the studied soils.
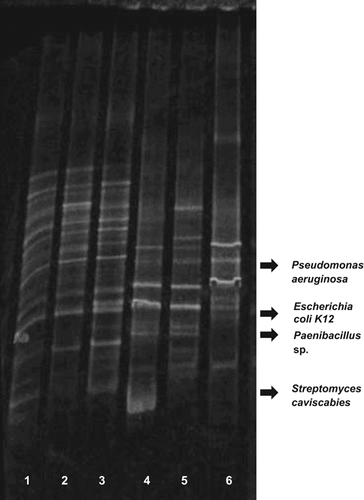
Bacterial community fingerprints by DGGE of the enriched samples at 30°C and 50°C with no Cu-treatment and supplemented with Cu at 100 μM and 500 μM. (1) 30°C; (2) 30°C and supplemented with 100 μM copper; (3) 30°C and supplemented with 500 μM Cu; (4) 50°C; (5) 50°C and supplemented with 100 μM Cu; (6) 50°C and supplemented with 500 μM Cu.
A selection of bacteria, belonging to the Phylum Firmicutes, was also inspected between 30°C and 50°C incubations and at different levels of Cu addition (Fig. 2A). At 30°C, similar bands were observed without and with Cu treatment although the proportion of the different bands showed differences in the community. For example, band 3.1, corresponding to a Bacillus sp., dominated the enrichment supplemented with 500 µM Cu, while at 100 μM copper band 2.1, corresponding to Lysinibacillus, was the more intense one in this fingerprint. Other identified members of the Firmicutes community developed at 30°C were Planococcus and other representatives of the genus Bacillus (Table 3). Relatively similar intensity was observed between the bands in the profile of the sample without copper addition suggesting that this sample maintained a higher number of phylotypes, while the addition of copper shifted the community towards the dominance of specific phylotypes, likely Cu-tolerant phylotypes. At 50°C, all bands identified in the profiles corresponded to the genus Brevibacillus, although different strains dominated the thermophilic communities in the 500 µM Cu-supplemented community than in the 100 µM Cu and unsupplemented communities (Fig. 2A). These results suggest that high Cu concentrations (i.e., 500 μM) are required to induce significant effects on the thermophilic Firmicutes community at 50°C. These high concentrations of Cu are equivalent to the high soil levels found after the use of fungicide at the studied olive tree field and the results indicated that Cu tolerance is a selective factor for specific Brevibacillus strains inhabiting soils.
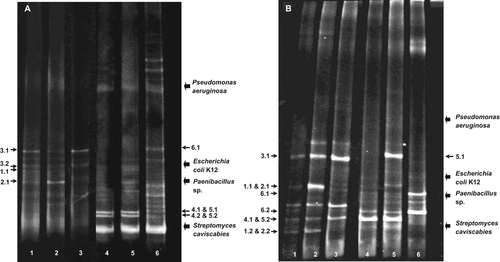
Bacterial community fingerprints by DGGE of the Firmicutes (A) and Paenibacillaceae (B) communities enriched at 30°C and 50°C with and without Cu treatment. (1) 30°C; (2) 30°C and supplemented with 100 μM Cu; (3) 30°C and supplemented with 500 μM Cu; (4) 50°C; (5) 50°C and supplemented with 100 μM Cu; (6) 50°C and supplemented with 500 μM Cu.
| Band IDa | Closest homologue (accession number) | Similarity (E score / %) |
|---|---|---|
| 1.1/2A | Planococcus rifietoensis strain LH-T4 (KF876875.1) | 3e−145; 99% |
| Planomicrobium okeanokoites strain WB-231 (KF749414.1) | 3e−145; 99% | |
| 2.1/2A | Lysinibacillus fusiformis strain S-1 (KF648506.1) | 6e−147; 100% |
| 3.1/2A | Bacillus sp. SGE129 (HM566657.1) | 6e−147; 100% |
| 3.2/2A | Uncultured bacterium clone RTKG73AG08 (KC993402.1) | 3e−145; 99% |
| Bacillus weihenstephanensis strain 23A (KC329820.1) | 1e−143; 99% | |
| 4.1 & 5.1/2A | Brevibacillus sp. THG-d53 (KF999709.1) | 2e−146; 100% |
| 4.2 & 5.2/2A | Brevibacillus limnophilus strain AG-42 (KF817656.1) | 2e−146; 100% |
| 6.1/2A | Brevibacillus limnophilus strain AG-42 (KF817656.1) | 5e−143; 99% |
| 1.1 & 2.1/2B | Brevibacillus reuszeri strain JN71 (KF687026.1) | 2e−96; 100% |
| 1.2 & 2.2/2B | Paenibacillus sp. Bac246W4 (KF496156.1) | 6e−96; 100% |
| 3.1/2B | Paenibacillus sp. AHM23 (KF371538.1) | 6e−96; 100% |
| 4.1 & 5.2/2B | Brevibacillus borstelensis strain GE8-2 (KF030754.1) | 2e−96; 100% |
| 5.1/2B | Paenibacillus residui strain R8-313 (JQ659929.1) | 3e−94; 99% |
| 6.1/2B | Uncultured bacterium clone ncd957e10c1 (HM329234.1) | 2e−96; 100% |
| Brevibacillus sp. FBl11 (JX897911.1) | 3e−93; 99% | |
| 6.2/2B | Brevibacillus limnophilus strain AG-42 (KF817656.1) | 2e−96; 100% |
- aFirst number in the band nomenclature indicates the gel lane.
When using primers PanA and PanB, dominant members of the genus Paenibacillus were detected in addition to Brevibacillus (Fig. 2B, Table 3). In the mesophilic assemblages, Paenibacillus members were sequenced; for instance, the sequence showing closest homology to Paenibacillus sp. AHM23 (band 3.1) was present in all the mesophilic assemblages corresponding to a copper resistant member, while a sequence homologue to Paenibacillus sp. Bac246W4 (bands 1.2 and 2.2) was absent at 500 µM Cu. The 16S rDNA sequence of band 2.1 was identical to Brevibacillus reuszeri, a species belonging to the B. brevis cluster as defined by Shida et al. (1996). From the thermophilic assemblages, only band 5.1 corresponded to a strain of the genus Paenibacillus, a dominant member at 50°C and 100 μM Cu, whose 16S rDNA sequence was similar to a bacterium isolated from urban waste compost (Vaz-Moreira et al., 2010). In general, the results registered for the Firmicutes primer pair (Fig. 2A), concerning the differences between the 500 μM Cu-supplemented and the 100 µM Cu and unsupplemented communities, were also observed for the PanA/PanB primer pair (Fig. 2B).
qPCR was used to quantify 16S rRNA gene levels from the frequently detected Brevibacillus members in the enrichments at 50°C without and with 500 µM Cu. The results are depicted in Fig. 3 and show that Brevibacillus population at 50°C was about 6-fold the one at 30°C, both for unsupplemented and 500 μM Cu-supplemented enrichments. The results also indicated a ca. twofold decrease of this population with Cu addition, which corroborates the DGGE fingerprinting pattern change at 500 μM Cu with bands corresponding to fewer distinct Brevibacillus Cu-tolerant stains (Fig. 2B).
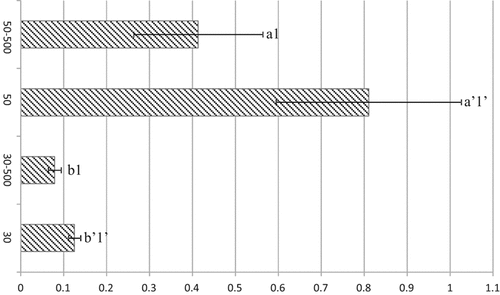
Ratios of normalized expression levels of 16S rRNA genes (query/bacteria 16S marker) for Brevibacillus in 30°C and 50°C enrichments unsupplemented and supplemented with 500 μM Cu. Data represent the average of two independent experiments with three replicates each. Statistically significant differences are represented by different letters (a, b) for temperature (at each copper concentration) and different numerals (1, 2) for Cu concentration (at each temperature).
3.2 Effect of copper and temperature on ammonium and sulfate release
Changes in the microbial communities due to specific agricultural treatments could lead to functional variations in the metabolic products generated by these communities. In this case, we monitored the release of NH
 and SO
and SO
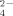 by these enrichments as a response to temperature and the addition of copper. During the growth of the bacterial community at 30°C similar NH
by these enrichments as a response to temperature and the addition of copper. During the growth of the bacterial community at 30°C similar NH
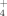 levels were measured in the three experimental cases (addition of 100 μM and 500 μM Cu; no addition; Fig. 4A). Also, similar production of NH
levels were measured in the three experimental cases (addition of 100 μM and 500 μM Cu; no addition; Fig. 4A). Also, similar production of NH
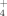 was observed by the community developed at 50°C both with and without addition of Cu. These results indicate that the soil bacterial enrichments responded similarly to changes in temperature and Cu supplementation with respect to their function releasing ammonium, in spite of structural changes in these communities.
was observed by the community developed at 50°C both with and without addition of Cu. These results indicate that the soil bacterial enrichments responded similarly to changes in temperature and Cu supplementation with respect to their function releasing ammonium, in spite of structural changes in these communities.
Measurements of SO
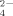 released during growth of the soil bacterial communities resulted in higher SO
released during growth of the soil bacterial communities resulted in higher SO
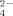 production at the highest temperature (50°C) than at 30°C, confirming the previous results that thermophilic bacteria produced higher SO
production at the highest temperature (50°C) than at 30°C, confirming the previous results that thermophilic bacteria produced higher SO
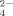 concentrations than mesophilic bacteria (Portillo et al., 2012) (Fig. 4B). In addition, SO
concentrations than mesophilic bacteria (Portillo et al., 2012) (Fig. 4B). In addition, SO
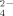 production decreased at increasing the amount of Cu supplementation of the enrichments both at 30°C and 50°C. Similar decreasing rate versus copper increase was observed at both temperatures being always lower the level of SO
production decreased at increasing the amount of Cu supplementation of the enrichments both at 30°C and 50°C. Similar decreasing rate versus copper increase was observed at both temperatures being always lower the level of SO
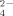 released at 30°C than at 50°C. These results indicate a significant negative effect of Cu on the potential cycling of S and the supply of SO
released at 30°C than at 50°C. These results indicate a significant negative effect of Cu on the potential cycling of S and the supply of SO
 to be used, for example, for plant growth. The addition of Cu in phythosanitary treatments of soil might require an increment of fertilizers to compensate for the potential decrease of SO
to be used, for example, for plant growth. The addition of Cu in phythosanitary treatments of soil might require an increment of fertilizers to compensate for the potential decrease of SO
 recycled in Cu-amended soils.
recycled in Cu-amended soils.
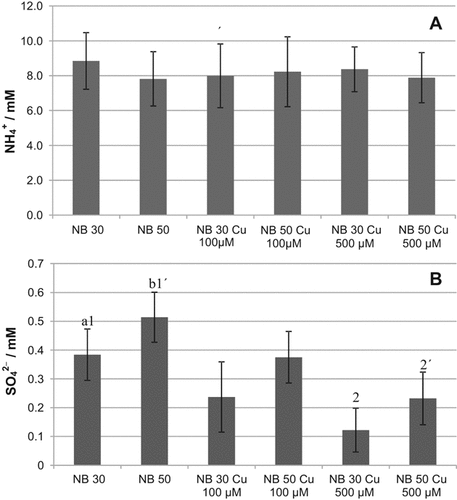
Ammonium (A) and sulfate (B) production during growth of enriched bacterial communities at 30°C and 50°C with no copper treatment and supplemented with Cu at 100 μM and 500 μM. Data represent the average of two independent experiments performed in three to four replicates. Only statistically significant differences are represented by different letters (a, b) for temperature (at each Cu concentration) and different numerals (1, 2; 1′, 2′) for Cu concentration (at each temperature).
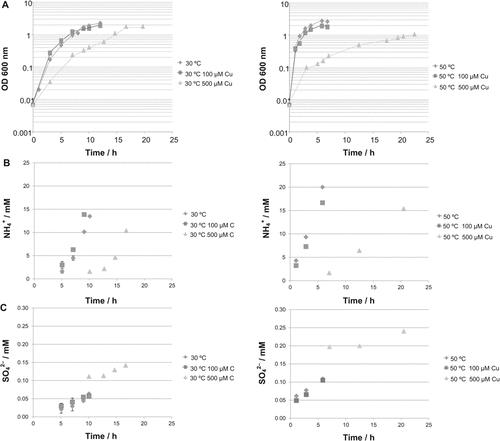
Growth curves (A), ammonium (B) and sulfate (C) production at 30°C and 50°C without and with Cu supplementation for a soil thermophilic isolate, strain 1BL, belonging to the Bacillus genus. Values are the average of three independent experiments with two replicates each.
3.3 Study of 1BL isolate
An isolate, strain 1BL (partial 16S rDNA sequence accession number KP271988) belonging to the thermophilic Firmicutes community, was obtained as described in section 2.2 and used to corroborate the results from the mix bacterial communities. Strain 1BL was identified as belonging to the genus Bacillus and was grown under 500 μM Cu concentration, which indicates that it is a Cu-tolerant strain. This isolate showed faster growth at 50°C than at 30°C (Fig. 5A), which confirms that this bacterium is a moderate thermophile.
Supplementing the cultures with 100 μM Cu at both tested temperatures (30°C and 50°C) had no significant effect on growth of the isolate 1BL (Fig. 5A). The isolate generated NH
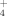 faster at 50°C than at 30°C without supplementing Cu (Fig. 5B) likely due to a faster growth as previously demonstrated by Portillo et al. (2012). No significant differences were observed for NH
faster at 50°C than at 30°C without supplementing Cu (Fig. 5B) likely due to a faster growth as previously demonstrated by Portillo et al. (2012). No significant differences were observed for NH
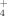 production of strain 1BL between 100 μM Cu-supplemented and unsupplemented cultures. Nevertheless, in 500 μM Cu-supplemented cultures the production of NH
production of strain 1BL between 100 μM Cu-supplemented and unsupplemented cultures. Nevertheless, in 500 μM Cu-supplemented cultures the production of NH
 by strain 1BL was much reduced than in no supplemented or 100 μM supplemented cultures (Fig. 5B).
by strain 1BL was much reduced than in no supplemented or 100 μM supplemented cultures (Fig. 5B).
Much more SO
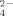 was released during the growth of strain 1BL at 50°C than at 30°C (Fig. 5C) in agreement to the observations above and to previous results both with cultures and natural enriched bacterial assemblages (Portillo et al., 2012; Santana et al., 2013). Similar amounts of SO
was released during the growth of strain 1BL at 50°C than at 30°C (Fig. 5C) in agreement to the observations above and to previous results both with cultures and natural enriched bacterial assemblages (Portillo et al., 2012; Santana et al., 2013). Similar amounts of SO
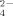 were produced under no addition and addition of 100 μM Cu, however, at 500 μM Cu the release of SO
were produced under no addition and addition of 100 μM Cu, however, at 500 μM Cu the release of SO
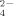 was higher than at the unsupplemented and 100 μM Cu supplemented cultures (Fig. 5C). This last observation for strain 1BL, suggesting increased SO
was higher than at the unsupplemented and 100 μM Cu supplemented cultures (Fig. 5C). This last observation for strain 1BL, suggesting increased SO
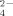 produced at the highest concentration Cu treatment, is in disagreement to the result of enriched bacterial communities mentioned above, i.e., a decrease of SO
produced at the highest concentration Cu treatment, is in disagreement to the result of enriched bacterial communities mentioned above, i.e., a decrease of SO
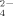 released with increasing concentration of supplemented Cu (Fig. 4B). One explanation for this controversy is that specific strains could show different behavior according to their tolerance to Cu, and this could explain the dynamics of the thermophilic Firmicutes phylotypes as an effect on the bacterial communities in Cu-supplemented soils.
released with increasing concentration of supplemented Cu (Fig. 4B). One explanation for this controversy is that specific strains could show different behavior according to their tolerance to Cu, and this could explain the dynamics of the thermophilic Firmicutes phylotypes as an effect on the bacterial communities in Cu-supplemented soils.
4 Conclusions
This study shows that both temperature and Cu-supplementation have decisive influence on the selection of specific soil bacteria. This effect could have important consequences on nutrient cycling in soils and consequently on plant growth. Copper addition should be minimized due to its long-lasting persistence in soils and its effects on bacterial communities. Specifically, Cu interferes with the dynamics of thermophilic bacteria which have been identified as major N and S cyclers under aerobic conditions in temperate soils during high temperature seasonal periods. It is herein demonstrated that members of these bacteria are able to thrive at 500 µM Cu, a value of the magnitude of those found in T0-EC soil sections (Table 1). Nevertheless, the overall SO
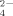 production by the soil thermophilic bacteria is reduced with the different phylotype dominance and with the reduction of their biomass under excess Cu.
production by the soil thermophilic bacteria is reduced with the different phylotype dominance and with the reduction of their biomass under excess Cu.
Some guidelines for future olive orchard management can be inferred from this work. Olive orchards are usually treated with Cu before the first rains in autumn and after every period of fog or rain to prevent fungal diseases. As aforementioned, Cu addition should be minimized to one preventive treatment at late autumn and treatments after large-scale rain periods, followed by monitoring of Cu concentration in the soil. The amendment of lyophilized thermophilic bacteria to crops can also be envisaged in the near future.
Acknowledgements
This work was funded by FEDER Funds through the Operational Program for Competitiveness Factors (COMPETE) and National Funds through FCT (Foundation for Science and Technology) under the Strategic Project PEst-C/AGR/UI0115/2011. We thank Eng. G. Pinheiro from Eugénio Almeida Foundation (FEA) for providing access to an FEA olive orchard and Professor C. Alexandre for guidance in soil parameters evaluation.




