Effect of soil drying on mucilage exudation and its water repellency: a new method to collect mucilage
Abstract
Despite the importance of mucilage for soil–plant relations, little is known about the effect of soil drying on mucilage exudation. We introduce a method to collect mucilage from maize growing in wet and dry soils. Mucilage was collected from brace roots. The amount of mucilage exuded did not change with soil water content and transpiration rate. Mucilage exuded in dry soils had a higher degree of hydrophobicity, suggesting that the wetting properties of mucilage change in response to soil drying.
1 Introduction
Roots actively modify the rhizosphere through production of root exudates. In particular, the presence of mucilage, a polymeric gel that is exuded by most plant roots, is believed to be one of the main factors influencing the physical and chemical properties of the root–soil interface. Mucilage is mainly composed of carbohydrates, amino acids, and organic acids, in addition to a smaller quantity of glycolipids and other phospholipids (Chaboud, 1983; Read et al., 2003). A variety of functions have been attributed to mucilage, including maintenance of good contact between roots and soil particles, reduction of friction during root growth, facilitation of root water uptake by increasing the rhizosphere water content, and selective absorption and storage of ions (McCully, 1999; Carminati et al., 2010; Carminati and Vetterlein, 2013; Ahmed et al., 2014).
Most of the data regarding the physical and chemical properties of mucilage refer to young roots (age ≤ 7 d) grown in axenic hydroponic culture (Chaboud, 1983; Read et al., 2003; Sobolev et al., 2006). Although these investigations have substantially enhanced our knowledge of the physical and chemical properties of mucilage, it is not clear if these results can be generalized to mature roots growing in different soils and moisture conditions.
An alternative method is the collection of mucilage from the brace roots of maize growing in the field after cutting the roots and immersing them in water for mucilage hydration (Morel et al., 1986; McCully and Boyer, 1997). Other methods allow quantitative visualization of mucilage but are not suitable for the collection and characterization of mucilage (e.g., Iijima et al., 2003). The advantage of the method proposed by Morel et al. (1986) is that high amounts of mucilage can be collected from the thick brace roots. According to McCully and Boyer (1997), mucilage from these roots should be similar to that exuded into soil.
We modified the method of Morel et al. (1986) by collecting mucilage from growing roots without detaching them. The main advantage of our modified method is that, by leaving the root intact, we were able to monitor mucilage exudation in real time, when plants transpired and the environmental conditions varied. Furthermore, this method can be used to study the effect of different treatments (e.g., nutrient supply, transpiration demand, and soil water content) on mucilage quantity and quality.
Additionally, it has been speculated that mucilage reduces the rewetting rate of the rhizosphere upon drying (Carminati et al., 2010) and it consequently limits root water uptake after soil drying and subsequent irrigation (Zarebanadkouki and Carminati, 2014). Moradi et al. (2012) measured distinct water repellency in the vicinity of roots of lupines in sandy soils. Carminati (2013) observed higher degrees of water repellency in the rhizosphere of lupines that were subjected to severe soil drying compared to the rhizosphere of lupines that were well irrigated. These studies suggest an important role of mucilage on rhizosphere rewetting kinetics during drying and wetting cycles. However, to the best of our knowledge, we are not aware of direct measurements of the contact angle of mucilage collected from plants growing in soils with varying water content.
The objectives of this study were: (1) to introduce a new method to collect mucilage from maize plants growing in soils with different water contents, and (2) to measure the quantity, water content, and contact angle of mucilage collected from plants growing in dry and wet soils.
2 Material and methods
2.1 Soil and plant preparation
We used a sandy soil collected in Göttingen, Germany. The soil was sieved to a particle size < 2 mm and then filled into 10 containers. Maize seeds were soaked in 10% H2O2 solution for 1 min and then germinated on moist filter paper for 48 h. The seedlings were then planted at a depth of 0.5 cm into the containers (one seed per container). The upper soil layers were covered with a 1 cm layer of quartz gravel (3 mm diameter) to reduce evaporation. The plants were grown with a daily cycle of 14 h light and 10 h darkness, with a light intensity of 250 µmol m−2 s−1, day/night temperatures of 22/19°C, and relative humidity of 60%. During the first two weeks, the plants were kept at a soil water content of around 0.20–0.25 cm3 cm−3. Afterwards, the plants were divided into two groups: five plants were kept at a soil water content of ca. 0.20 cm3 cm−3 and five plants were kept at a soil water content of ca. 0.06 cm3 cm−3. Transpiration was calculated by weighing samples at intervals of 3 h. The average daytime transpiration of 5-week-old plants with a soil water content of 0.20 and 0.06 cm3 cm−3 was 3.4 ± 0.21 g h−1 (n = 5) and 1.9 ± 0.15 g h−1 (n = 5), respectively.
2.2 Mucilage collection
Mucilage was collected from the emerging brace roots of five-week-old maize plants. The brace roots had a diameter of 3.5–4 mm. The brace roots were immersed in plastic cylinders filled with water for 24 h (Fig. 1). The tubes were connected in the morning and the roots were left in water for the whole day and night. The next morning the cylinders were removed and a blob of mucilage covered the root segment immersed in water (Fig. 1). Mucilage was removed from the roots using a syringe. After this first round, the roots were immersed in new water-filled cylinders for 1 d. The mucilage collected in the next morning was considered as the mucilage exuded in 24 h. The wet mucilage was weighed. Then it was let dry and weighted again. Using this information, we calculated the amount of mucilage exuded per root tip and the water content of the mucilage collected from plants grown in dry and wet soils.
Note that the extraction of mucilage occurred under identical local conditions in the two treatments—i.e., the water potential of the root tips immersed in water for 24 h was null. Nevertheless, the average root water potential of the plants growing in dry conditions was more negative than the water potential of the plants in wet soils. Our hypothesis was that, even if the tips of some roots were immersed in water during extraction, the stress felt by the other roots growing in dry soils affects mucilage exudation.

Left: Brace roots of a five-week-old maize plant. Roots were kept in plastic tubes filled with water for 24 h to keep mucilage fully hydrated. Right: Close up of one brace root after removing the plastic tube.
2.3 Contact angle measurements
The contact angle (CA) of mucilage was measured for varying mucilage concentrations (gram of dry mucilage per unit surface area). The collected mucilage from plants growing in dry and wet soils was spread on glass slides having a surface area of 4 cm2. Then mucilage was let dry on the glass slides.
We measured CA for four different mucilage concentrations. CA was determined with the sessile drop method by monitoring the placement and subsequent behavior of a drop of deionized water (sessile drop method, SDM; Goebel et al., 2013) using a CCD-equipped CA microscope (OCA 15, DataPhysics, Filderstadt, Germany). Evaluation of the recorded images was performed with the software SCA 20 (DataPhysics, Filderstadt, Germany) by drop shape analysis (ellipsoidal fit), and fitting tangents to both sides of the drop at the 3-phase boundary. Besides the initial CA (30 ms after placement), CA additionally was determined after 5,000 ms as an estimate of CA stability. Drop volume was 1 µL and the recording frequency 30 s−1. CA is given as the mean of the left and the right side in the images.
3 Results and discussion
3.1 Mucilage quantification
Table 1 shows the wet and dry weight of mucilage collected from plants growing in dry and wet soils. The results of each treatment are the average of 17 roots. The weight of wet mucilage collected per root tip was higher for the plants growing in wet soil compared to the plants growing in dry soil. In contrast, the dry weight of mucilage was slightly lower for plants growing in wet soil (Table 1). The mucilage water content was calculated by dividing the mucilage wet and dry weights. We found that the water content of mucilage collected from plants growing in wet soil was 21% higher than the one from plants growing in dry soil (Table 1). However, the t-test revealed that these differences are not statistically significant, mainly because of the relatively high standard deviation of our measurements. Note that this water content refers to the saturated water content of the mucilage. In fact, as mucilage was let in the water-filled cylinders for 24 h, we expect that mucilage was in equilibrium with the water in the cylinder and had therefore a water potential close to zero. However, the average soil water potential felt by the plant was different in the two treatments. In this sense we investigated the effect of an “average” global stress on mucilage exudation. Indeed, we found that mucilage exuded in the two treatments differed in water content and contact angle (cf chapter 3.2), despite the fact that the local water potential of the root tips immersed in water was identical (water potential equal to zero) for 24 h.
| Wet mucilage per root / mg d−1 | Dry mucilage per root / mg d−1 | Mucilage water content / – | |
|---|---|---|---|
| Wet soil | 107.83 ± 37 | 0.61 ± 0.12 | 179.2 ± 45 |
| Dry soil | 96.69 ± 29 | 0.69 ± 0.17 | 147.8 ± 38 |
Table 1 shows that the plants growing in dry soils exuded as much as the plants growing in wet soils. Considering that the transpiration rate was smaller in dry soils than in wet soils (transpiration rate was 1.9 ± 0.15 g h−1 in dry soils and 3.4 ± 0.21 g h−1 in wet soils) and that, consequently, also photosynthesis was likely to be smaller (as the stressed plants had a slightly lower biomass compared to the well-watered plants), we conclude that the ratio between mucilage exudation and photosynthesis increases as the soil dries. Consistently with our results, studies investigating C allocation found that under water limited conditions a relatively larger proportion of the assimilated C was transported to the roots compared to water sufficient conditions (Palta and Gregory, 1997; Sanaullah et al., 2012) and that drought stress increased total organic C exuded per gram of dry plant (Henry et al., 2007). In the same line, we could show for the first time that mucilage exudation relative to transpiration increases under water limiting conditions.
3.2 Contact angle
Figure 2 shows the contact angle (CA) of dry mucilage collected from plants growing in dry and wet soils. For both treatments, mucilage coverage on the glass slide was hydrophobic (CA > 90°) at concentrations above 0.07 mg cm−2. CA decreased for lower mucilage concentrations. For concentrations < 0.07 mg cm−2 mucilage from plants growing in dry soil had a higher CA and therefore had a higher degree of water repellency. Contact angles were quite stable up to 5,000 ms (Fig. 2b).
The higher water repellency of mucilage exuded in dry soils is probably the result of changes in the mucilage chemical composition. One possibility is that this mucilage contains more phospholipids compared to the mucilage exuded in wet soils. But why should plants exude a more water repellent mucilage under water-limited conditions?
One explanation is that the higher content of phospholipids decreases the mucilage surface tension, as shown by Read et al. (2003), and limits the capillary forces that would suck mucilage far from the root surface. In fact, the efficiency of mucilage in shaping the rhizosphere and maintaining it wet would be lost if the mucilage could easily spread far from the root surface into the bulk soil. A lower surface tension would maintain the mucilage near the root surface, particularly in dry soils, when the importance of mucilage for water and nutrient uptake is highest (Carminati and Vetterlein, 2013; Ahmed et al., 2014). Following a similar argumentation, the lower water content of the mucilage exuded in dry soils (Table 1) would also limit the spreading of mucilage into the bulk soil and it would keep it closer to the root surface. However, further studies are needed to confirm these speculations.
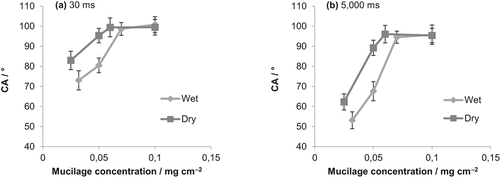
Contact angle at varying mucilage concentrations. Mucilage was collected from plants growing in dry and wet soil. (a) Contact angle after 30 ms. (b) Contact angle after 5,000 ms.
4 Conclusions
We introduced a new method to collect mucilage from maize plants growing in soils with varying water content. The method can be used to study the effect of different treatments (e.g., nutrient supply, transpiration demand, and soil water content) on mucilage quantity and quality. The amount of mucilage exuded did not change with changing soil water content despite the significant decrease in transpiration of the plants growing in dry soils. Upon drying mucilage became water repellent. Mucilage contact angle (CA) increased with mucilage concentrations (gram of dry mucilage per unit surface area). At concentration > 0.07 mg cm−2 mucilage was hydrophobic (CA > 90°).The contact angle of mucilage from plants growing in dry soil was higher than the one from plants growing in wet soils.
Acknowledgements
The doctoral position of Mutez A. Ahmed was funded by the German Academic Exchange Service (DAAD), Bonn, Germany. The maize seeds were provided by the seed company KWS (Kleinwanzlebener Saatzucht), Einbeck, Germany.




