Linking atomic force microscopy with nanothermal analysis to assess microspatial distribution of material characteristics in young soils†
Focus Issue “Soil processes regulation at ‘hot spots'”. Selected papers presented on a workshop organized by the German Soil Science Society (DBG), Commissions II, III, and VII held in Freising-Weihenstephan (Germany), May 4–6, 2014 (Conveners: Carsten W. Muller, Thilo Eickhorst, Markus Steffens, and Sven Marhan).
Abstract
Coupling of atomic force microscopy (AFM) with nanothermal analysis (nTA) has the potential to assess material characteristics in soils on the lower µm-scale, but has been shown to require additional characteristics for clear distinction of materials. The objective of this study was to evaluate to which extent the combination of AFM-nTA with AFM adhesion force analysis and structural features allows distinction of organic materials in soils. Using soil samples from a chronosequence from the Damma Glacier forefield, Switzerland, as example, we tested a grid analysis approach for assessing distribution of adhesion forces and nanothermal characteristics. This approach was compared with an approach involving pre-selection of structural features of interest via morphological criteria. Only three types of nanothermograms were distinguished in the soil samples based on different thermal expansion-compression characteristics and phase transition temperatures. Combined evaluation of nanothermal characteristics, adhesion forces and morphological characteristics allowed distinction of a larger set of materials than using nanothermal analysis, adhesion force distribution or morphological characteristics separately. Part of the analyzed features showed a combination of characteristics similar to that of fresh bacterial cells which we analyzed as a potential reference material. Their stronger occurrence in the regions of interest of older samples than in those of younger samples may underline their relevance in soil development. Achieving the long-term objective of identification of materials still requires more information on reference materials, understanding the impact of mixed layering of materials on thermal profiles and the assessment of variability of the characteristics within and between different material groups.
1 Introduction
Structural heterogeneity and complexity are intrinsic features of soils (Odum, 1969), in particular of soil organic matter (SOM) which originates from degradation products of plant materials (Kögel-Knabner, 2002) and from microbial cell residues (Kögel-Knabner, 2002; Miltner et al., 2009; Miltner et al., 2012). Microscale spatial organization of SOM materials in the highly heterogeneous soil samples has far-reaching consequences for the physical and chemical quality of SOM (Nunan et al., 2001; Young and Crawford, 2004). Therefore, the microscale architecture in soil samples and in SOM requires further attention.
The greatest challenge for microscale and nanoscale techniques lies in finding suitable methods to determine chemical and material properties with high spatial resolution and without disturbing the sample structure (Herrmann et al., 2007; Totsche et al., 2010), and to analyze representative parts of the sample. Among currently available high-resolution methods, atomic force microscopy (AFM) is capable to achieve highest resolution in the range of a few nanometers. It reveals material properties such as the very weak interaction forces with the tip material (Cheng et al., 2009). Atomic force microscopy, acquiring sample-tip interaction forces when the tip approaches the sample, is thus able to provide material specific information on intermolecular interactions at the nanometer scale (Leite and Herrmann, 2005). Its applicability in soil science has been proven in studies, e.g., on surface hydrophobicity (Cheng et al., 2009) and surface chemistry (Hoffman et al., 2014), dissolution and precipitation of minerals (Li et al., 2014; Wang et al., 2015), interaction between microbial species and natural organic matter (NOM) (Abu-Lail et al., 2007), morphological characteristics of humic substances and clays (Dias et al., 2013; Yu et al., 2013), and characterization of various mineral surfaces (Eastman and Zhu, 1996; Leite et al., 2003). Introduction of peak force quantitative nanomechanical mapping (PFQNM) allows for extraction of more detailed information on mechanical force distributions on surfaces. This mode of AFM is meanwhile commonly applied in material science (e.g., Tielemans et al., 2012) and in cell biology (e.g., Heu et al., 2012). The challenge for its application to soil is the strong heterogeneity in and on soil particles. Moreover, although nanomechanical and physicochemical characteristics already give some information on surface chemistry, different materials with similar chemical composition, but of different origin, cannot easily be distinguished without investigation of additional material characteristics.
Localized thermal analysis at the nanoscale (nanothermal analysis, AFM-nTA) is another promising AFM application, using a heatable tip (Chiou et al., 2006). The measurement is the nanoscale analogue of thermomechanical analysis which investigates sample softening and expansion as function of temperature. AFM-nTA has only previously been applied for various purposes in material and pharmaceutical research. For example, Dai et al. (2009) investigated the phase transition behavior of lactose with AFM-nTA in order to identify crystalline and amorphous nanoregions in the sample. In a similar manner, microparticles of binary polymer mixtures were characterized (Meeus et al., 2012), and crystallinity in drug products (Nakamoto et al., 2013) and phase separation in thin films (Qi et al., 2013) were explored using nanothermal analysis. Only recently, researchers started using the heatable tip in combination with localized mass spectrometric analysis (e.g., Tanji et al., 2013; Ovchinnikova et al., 2014; Owens et al., 2014; Ovchinnikova et al., 2015), which demonstrates the wide spectrum of application of this new analysis method. AFM-nTA was only recently applied to soils to reveal thermomechanical properties at the nanometer and micrometer scales (Schaumann and Kunhi Mouvenchery, 2012). In that pilot study, various material types separated by nanometer and sub-micrometer distances were distinguished and their spatial distribution was explored. However, only a low number of different thermal profiles was observed (Schaumann and Kunhi Mouvenchery, 2012) despite the expected complexity of soils. This suggests that a number of different materials reveal similar thermal profiles such that it becomes clear that nanothermal characteristics alone will not be sufficient to unambiguously identify certain materials in soil samples. The same is true when only assessing adhesion force characteristics, but until now it remains unclear to which extent a combination of these two characteristics may help to better distinguish material types in soils.
Therefore, the first objective of this study was to evaluate the potential of combining AFM-nTA with AFM force mapping in order to improve the distinction of structural features in soils in the micrometer and upper nanometer scale. For this explorative study, we selected young soils from a glacier forefield along a 150-y chronosequence as an example, where heterogeneity was expected to be still low compared to mature soils. In these samples, the abundance of 200 nm to 500 nm sized flat organic fragments increased with increasing soil age; they were hypothesized to be microbial cell envelope fragments contributing to SOM genesis (Miltner et al., 2012; Schurig et al., 2013) and cell wall polysaccharides tend to accumulate with soil depth (Kögel-Knabner, 2002).
We used this explorative study in our second objective also to perform one step towards material identification by evaluating to which extent material characteristics of two biological reference materials (bacterial cells from a fresh culture and a plant root material obtained from one of the soil samples) can be located in real soil samples. Furthermore, we tested to which extent additional characteristics (i.e., morphological characteristics) will help to further narrow down the set of candidates resembling the test materials. Bacterial cells were selected as organic reference material in order to locate material characteristics similar to those of fresh bacterial cells in the soil sample. Comparing these results with findings of a study by Miltner et al. (2012), suggesting that cell envelopes or their fragments contribute to a significant extent to SOM, we also aimed at evaluating the question to which extent these structures may represent cells which were fragmented only recently and are not yet significantly degraded. The selection of the second organic test material (plant root material) was made to locate material characteristics related to plant-derived materials. Inorganic materials were not included in this exploratory study because pronounced thermal events are absent in the temperature range applicable for AFM-nTA as shown in previous studies (Schaumann and Kunhi Mouvenchery, 2012; Plante et al., 2009).
In order to reach the long-term-objective of applying AFM-nTA for identification of materials and their distribution in soil samples, a next step would be the comprehensive comparison of material characteristics in soils with those of reference materials. For this, the set of reference materials will have to cover the spectrum of materials expected in the soils, and for each material, the variability of the characteristics, depending on their expected multitude of degradation states, needs to be known. Furthermore, statistical assessment will be required in order to find out the minimal number of replicates and regions of interest (ROI) within samples and references required for giving information on the quantitative composition and distribution of materials in the soil. Therefore, comparison of AFM-nTA characteristics of soil samples to those of a larger number of carefully selected reference materials and upscaling of the results for characterization of soil samples will be the next steps which could not be covered by the present study.
2 Material and methods
2.1 Materials
The sampled chronosequence is located in the Damma glacier forefield in Switzerland (46°38' N, 8°27' E; about 2000 m asl). Details on the Damma glacier forefield have been described previously (e.g., Bernasconi, 2008; Bernasconi et al., 2011; Temme and Lange, 2014; Rime et al., 2015). Historical records report a recession of the Damma glacier since 1850 (Bernasconi et al., 2011). This recession was interrupted by two re-advances (1920–1928 and 1970–1992), resulting in two small end moraines in the forefield and a division of the forefield into three areas of different soil age (Bernasconi et al., 2011). The bedrock material of the forefield is granite throughout the chronosequence (Bernasconi et al., 2011). The soils were already chemically characterized for a number of soil parameters (Miniaci et al., 2007; Töwe et al., 2010; Bernasconi et al., 2011; Dümig et al., 2011; Smittenberg et al., 2012; Sigler et al., 2002; Sigler and Zeyer, 2002; Schurig et al., 2013). Soil samples were taken in August 2009 at 8 sampling sites as described by Schurig et al. (2013). Approximate soil ages range from 0 to 120 y. Briefly, at each sampling site ten subsamples were taken from below the root layer of the surface vegetation (< 5 cm) in a 5 m circumference around the sampling sites DG0–DG18 (Schurig et al., 2013). In the sample designations, increasing numbers indicate an increasing distance from the glacier and thus increasing time after deglaciation and can be grouped as follows according to the re-advances mentioned above: group 1: DG0 (0 y), DG2 (7 y), DG4 (15 y); group 2: DG6 (65 y), DG10 (70 y), DG13 (80 y); and group 3: DG17 (110 y) and DG18 (120 y) (see Schurig et al., 2013). The subsamples from each site were pooled and sieved immediately by passing through a 2-mm sieve. All samples were kept on ice during transport to the laboratory and stored at –18°C until air-drying prior to the AFM-nTA measurement.
In order to investigate to which extent material characteristics of selected test materials can be located in soil samples, we investigated two defined test materials as starting point. One is a pure culture of Bacillus subtilis ssp. spizenii (DSM 347) cells grown on mineral medium with glucose. The cells were harvested by centrifugation and washed with distilled water. This strain is a typical Gram-positive soil bacterium. These cells thus represent a material with surface characteristics similar to those of intact cells of soil microbes. Even though the composition and thermal behavior of these fresh cells might differ from those of cell envelope fragments (Claessens et al., 2006), the temperature ranges and sequence of thermal events characteristic for certain processes may be similar, in particular for cells which died recently. The cell surfaces are assumed to consist mainly of peptidoglycane, lipids, and proteins (Madigan et al., 2014). The second reference is a long fibrous piece of plant root material of 0.5 mm thickness and 3 mm length, picked manually with a pair of forceps from soil DG0. This material was selected because it is expected to represent partially degraded plant material as it is present in soils. Care was taken to select ROI representing the plant material surface without any fungal hyphae and with as few mineral particles as possible.
2.2 AFM–force mapping and nanothermal analysis
2.2.1 Sample preparation
Air-dried soil samples and the plant material were fixed on glass slides using double-sided adhesive tape. The particles were gently spread on the tape with a spatula in order to fix them sufficiently to withstand the AFM analysis without movement, and loosely-fitting particles were removed by a stream of pressurized air. This procedure for AFM sample preparation is suitable for relatively large soil particles (Schaumann and Kunhi Mouvenchery, 2012). Similarly, the plant root piece was fixed on a glass slide with double-sided adhesive tape. The bacterial culture was centrifuged and a small amount of the washed pellet was spread on a glass slide and air-dried overnight before AFM analysis.
2.2.2 Selection of regions of interest
ROI suitable for AFM analysis were selected on each sample using an environmental scanning electron microscope (ESEM Quanta 250 from FEI Company, Frankfurt/Main, Germany) in low vacuum mode. Image acquisition parameters (acceleration voltage 5–8 kV, pressure 90 Pa) were adapted to ensure non-destructive imaging (Stokes, 2008). ROI were randomly selected among relatively flat regions, assessed by appearance of the micrographs, considering that rough or inclined surfaces are not suitable for AFM analysis. For that, regions on 100–200 µm large soil grains were considered in order to be able to relocate the ROI under AFM and to engage the probe without damage. In this explorative study, at least four ROI were selected for AFM analysis in each soil sample. The selected ROI were relocated under AFM by navigating over the surface using the built-in light microscope of the AFM instrument. The low number of ROI and the arbitrary selection of ROI will therefore allow for exploration of the material distribution in the ROI, but this distribution is not necessarily representative with respect to quantification in whole soil, which was not in the focus of this study.
2.2.3 AFM setup and quality assurance.
The selected ROI were re-located in the AFM (Dimension ICON, Veeco, NY, USA) which was coupled to a thermal analytical unit (VITA, Veeco, NY, USA). PFQNM and nanothermal analysis were performed using a thermal probe (VITA-DM-NANOTA–200; tip radius 31 nm) made of doped Si provided by Bruker (Germany). Following the current practice (Anasys Instruments, 2012), the tip deflection will be shown in the unit of V in this publication. Temperature calibration over a range of 30°C to 300°C of the probe was done using polycaprolactone, polyethylene terephthalate, high density polyethylene and metallic gallium. All AFM measurements were performed at 22 ± 2°C and at a relative humidity of 30 ± 2%.
To ensure tip quality and to be sure that no sample material was attached to the tip during analysis, the drive frequency of the tip was checked before and after every imaging, which always stayed constant (66 ± 1 kHz). Drive frequency of the tip is expected to be altered by contaminants that stick on it (Veeco Instruments, 2010). Further, the image quality and reproducibility were checked by imaging the same ROI at different scan angles. The closest possible contact of the tip was always maintained by fixing the tip z-range such that further downward movement would have caused the risk of tip damage.
2.2.4 AFM imaging and force mapping.
In each ROI, one image was acquired over an area of 2 x 2 µm at a scan rate of 0.5 Hz. Data were captured along 512 lines with 512 points on each line. Height, adhesion, and peak force images were recorded in each channel. Currently available nanothermal probes have spring constants below 3 Nm−1. With this, they are too soft to obtain reliable Derjaguin–Muller–Toporov modulus of elastic contact and deformation data on hard soil surfaces (Veeco Instruments, 2010). Height images were analyzed using the Nanoscope analysis software (Veeco, New York/USA). Adhesion histograms were generated on each image with a bin size of 5 nN. As all measurements were conducted at the same relative humidity (30 ± 2%), the influence of capillary forces due to surface hydration could be assumed to be similar in all samples and no difference is expected to arise due to this effect (Pelin et al., 2012).
2.2.5 AFM-nanothermal Analysis
The spatial resolution of AFM-nTA is lower than that of the adhesion force mapping. Therefore, we evaluated two approaches to select the positions of the nanothermal analysis in the ROI, a grid approach and a morphology-directed approach, the latter using additionally morphological information for selection of the points for nTA analysis.
Grid analysis approach. After imaging in PFQNM mode, 16 points on each image were selected along a 4 ×4 grid and thermal analysis was performed heating the tip from 30°C to 300°C at a rate of 1 K s−1. Cantilever deflection, which reflects the dimensional change in z direction (expansion or compression) at the sample surface by heating, was recorded in units of voltage (V) as a function of temperature in the thermogram. The grid of size 4 ×4 with 16 data acquisition points was selected after ensuring that these points are far enough from each other to avoid analyzing areas which may have been affected by heat during prior analysis of points in the immediate vicinity. This was done by comparing images before and after heating, based on alterations in morphological features.
Morphology-directed approach. In order to be able to focus more closely on a selected material group in this approach, despite lower spatial resolution of nTA than PFQNM, we identified 8 to 10 structural features of 200–500 nm size in each image which, based on their morphology, were suggested to represent potential candidates for cell envelope fragments according to Schurig et al. (2013) and Miltner et al. (2012). The question was to which extent these structures reveal characteristics of fresh bacterial cell envelopes, i.e., representing cells only recently fragmented and not yet significantly degraded. Their selection was made after verifying that they lie far enough from previously heated regions and from each other to avoid artifacts. Nanothermal analysis was performed also on such features and their average adhesion force values were extracted from the adhesive force images. In this strategy, particle size will form the basic criterion to which information on thermal and mechanical properties are added in further steps in order to achieve better selectivity for different materials. The same cantilever tip was used for both imaging and nanothermal analysis.
2.2.6 Evaluation of nanothermograms
Nanothermograms were analyzed using the software Origin 7.5 by OriginLab. Thermograms were grouped with respect to thermogram shape as follows: each thermogram was divided into sub-regions indicating thermal expansion or compression. Their slopes on basis of linear regression characterize the apparent linear expansion coefficient (α) or the apparent linear compression coefficient (κ) in units of mV °C−1. The points of intersection of two linear components correspond to the respective inflection temperatures and represent phase transition temperatures. Grouping was performed based on the intersection temperature, on the direction and magnitude of slopes and their changes at intersection temperatures. This procedure provides unique and unambiguous results and does not require any statistical analysis in the present stage of a pioneering study.
3 Results and discussion
3.1 Surface heterogeneity
ESEM images, showing ROI as well as AFM height, and adhesion force images of one representative image for three selected soil samples [DG0 (recently deglaciated), DG10 (69 y), and DG18 (119 y) (Schurig et al., 2013)] and of 2 x 2 µm subsections of the bacterial cells and the plant root are shown in Fig. 1. The soils and the plant material revealed smooth, layered or fluffy surfaces. A comparison of all recorded images show that differences in frequency of occurrence, size and shape of morphological structures were observed between the soil samples but also between replicates. Spherical structures with sizes between 200 nm and 500 nm were notably detected on each soil sample in varying abundance and size distribution.
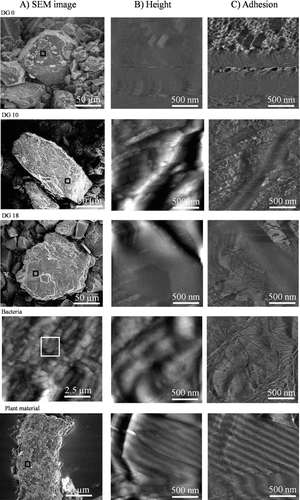
Micrographs showing ROI of Damma Glacier soil samples DG0, DG10, and DG18, and of bacterial cells and a plant tissue (A) identified using AFM for bacterial cells and using ESEM for all other materials; (B) AFM height and (C) adhesion images for all the samples. The large scale height image of bacteria in panel A shows a cloud of several cells, the marked area of which was scanned for a closer view, as shown in the other two panels. Color scale: in height images, darkest and lightest points correspond to a depth of 80 nm and a height of 80 nm, respectively, from the mean plane. For adhesion images, darkest and lightest points respectively correspond to forces of 20 nN and 40 nN for bacteria, and 0 nN and 50 nN for plant material and soil samples. Reproducibility of the linear pattern on the surface of plant material was confirmed by testing at a different scan angle (45°).
3.2 Adhesive force distribution
Adhesive interactions between the AFM tip and sample are characteristics of surface morphology as well as the material of tip and sample (Eastman and Zhu, 1996). In our experiment, we used the same tip to image all the samples, and the tip radius did not change between the measurements. Therefore, the tip properties are the same for all measurements and their effect can thus be neglected for the comparison of the samples. Tip-surface adhesive interactions indicating the stickiness between tip and surface (Pelin et al., 2012) were profiled in adhesion images (Fig. 1C). Figure 2 shows histograms of adhesion forces up to 50 nN for the ROI of each soil sample as an average of all imaged regions with the error bars showing standard deviation between imaged micro-regions within each sample. Adhesive forces dominate between 0 nN and 50 nN in all images. A very small fraction (< 1%) of adhesion forces were in the range 50–80 nN.
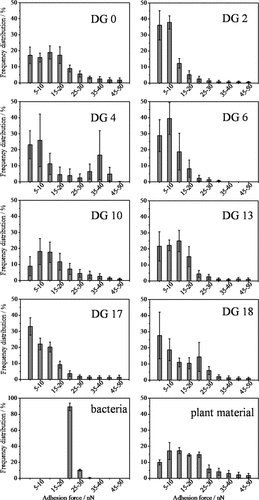
Sample-tip adhesion force histogram showing similar distribution in plant materials and soils. The narrower distribution in bacteria shows higher homogeneity of the surface. The adhesion force range with maximum abundance varies between samples (total number of force curves per histogram is 262144).
The ROI of the bacterial cells showed a comparably narrow range of adhesive force distribution averaging at 20 nN with most abundant forces ranging between 20 and 25 nN and a standard deviation of 3 nN. This is in line with the expectedly lower heterogeneity for bacterial cells than for soil particles. For the ROI of the plant material, 81% of the data points revealed forces of 0–25 nN with an average of 18 nN (standard deviation: 17 nN). The broader force distribution shown by the plant-originated material than that of the bacterial surfaces underlines the wider range of different cell structures and constituents in the plant material.
A broad distribution was observed in the ROI of the soil samples (Fig. 2), in which the most abundant adhesive force values also differed between the individual samples and in some cases bimodal distributions were found (e.g., DG04, DG18). Average adhesion forces ranged between 8 and 16 nN with standard deviations between 6 and 14 nN. There is no clear trend for the average adhesion force with increasing soil age. All this underlines the huge heterogeneity of the soil samples. The soil samples are expected to contain organic matter (OM) with different degrees of degradation including OM coatings, root exudates, extracellular polymeric substances or other organic compounds, which can explain the broad distribution of adhesive forces within the ROI in soils. Furthermore, it cannot be excluded that a part of the regions contains very small clay particles and Fe-oxides which contribute to the width of the distribution. Particulate OM is also expected to consist of more than one material; therefore we expect a wide range of adhesion forces also for particulate OM.
The lack of a clear trend for the shape of force distribution or the average adhesion force with increasing soil age or for the standard deviation could be due to the limited representativeness of the quantitative results due to the small number of ROI chosen for this explorative study, which does not allow to transfer the distribution data to bulk soil composition.
However, each individual AFM adhesion image shows clear domains and structural units exhibiting characteristic adhesion forces (see Figs. 1C and 2), indicating nanoscale material heterogeneity. Variation in occurrence of peak positions and the size of error bars in the histograms between ROI indicate substantial sample-to-sample variability.
3.3 Nanothermal analysis
Assessment of localized thermal characteristics in addition to force distribution data will be helpful for more detailed and less ambiguous characterization of materials on the micro- and nanoscale. The samples revealed five different types of thermal profiles which are distinguished by the number and slopes of expansion or compression phases and their intersection temperatures (Fig. 3).
3.3.1 Thermogram types found in soil samples and reference materials
Type I thermograms revealed one steep expansion phase throughout the investigated temperature range. In few thermograms of this type, expansion stopped around 250°C. This thermogram type resembles type E in the previous study (Schaumann and Kunhi Mouvenchery, 2012) and, accordingly, we preliminarily attributed it to inorganic materials. This is in line with the fact that for inorganic surfaces most thermal events occur above 300°C (see Plante et al., 2009), although a compression phase starting at 120°C was observed for ground quartz (Schaumann and Kunhi Mouvenchery, 2012). General verification of this assignment in the current set of samples would require larger datasets including various mineral materials.
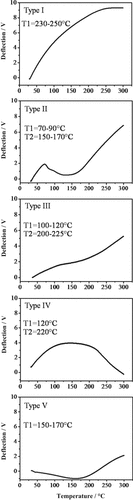
Different types of thermograms revealed by different samples. Thermograms detected in the samples are grouped into five individual types with respect to the intersection temperatures (referred to as T1, T2 in the figures) between the thermal events (for details please refer to the text).
Type II. This type of thermograms consisted of three phases, namely two expansion phases which are clearly separated by a compression phase starting at 70–90°C and lasting until 150–170°C. Most of these thermograms were characterized by a steep compression (κ ≈ 0.023 V °C −1) in the second sub-region, whereas few others expressed only a slight compression (κ ≈ 0.0031 V °C −1) in this sub-region. This difference is most probably due to a fast-expanding layer present underneath in the latter case, which masks the intrinsic compression phase of the surface layer, which is under direct influence of the heated tip. This pattern of thermal events was not observed before, but the presence of the compression phase suggests OM (see below and Schaumann and Kunhi Mouvenchery, 2012). It might represent the melting or thermal degradation of SOM constituents (Schaumann and Kunhi Mouvenchery, 2012). Differences in the extent of compression and expansion might indicate different thicknesses of organic layers on mineral material (for a more detailed discussion, see section 3.3.2).
Type III thermograms consist of two steep expansion phases interrupted by a compression phase or a mild expansion regime, depending on the location. The transitions between the phases occur at 100–120°C and 200–225°C and, thus, are higher than in type II thermograms. Similar thermal profiles, but with different slopes, were observed previously in samples of charcoal, manure, and peat, and were attributed to organic domains, especially due to the presence of a compression phase (Schaumann and Kunhi Mouvenchery, 2012). The current thermograms differ in intensity of the compression phase in the second sub-region (between 100–120°C and 200–225°C), which could either be due to several various types of OM and/or to the effect of underneath layers.
Type IV. The bacterial cells showed Type IV thermograms, consisting of an initial steep expansion up to 120°C followed by a slight compression until 220°C and a steeper compression phase afterwards. The positive slope was between 30°C and 120°C with an expansion coefficient of α = 0.046 V °C −1, while the other two had negative slopes with compression coefficients of κ1 = 0.0030 V °C −1 (120°C to 220°C) and κ2 = 0.046 V °C −1 (220°C to 300°C). This corresponds to an expansion–slight compression–strong compression sequence. The expansion can be due to thermal expansion of cell envelope material at relatively low temperatures. The two compression phases are most probably due to heat-induced softening and thermal degradation of the cells, respectively, and the one at low temperature could be attributed to the melting of lipid structures as suggested by Kučerik et al. (2014).
Type V thermograms were revealed by the fibrous plant root material and are entirely different from those of the bacteria, probably due to chemically more complex supporting tissue in plants than in bacterial cell envelopes. It consists of an initial steep compression phase from 30°C to 170°C (κ = 0.011 V °C −1) followed by an expansion phase (α = 0.029 V °C −1). The initial compression can be due to temperature-induced softening of the material, which may be overlaid by thermal expansion of material underneath that becomes effective after completion of the compression phase of the top layer. All measurements of the plant material showed the same type of thermogram, which indicates similar OM without significant presence of bare mineral surfaces. Presence of fungi on partly degraded plant material cannot fully be excluded for our test material. However, according to SEM images, there were no intact fungal hyphae close to our ROI. Smaller fragments of fungal hyphae, which might be present but not visible in the SEM images, were considered part of this reference material.
Bacterial cells and plant material showed distinct differences in thermal behavior reflecting differences in their thermomechanical stability. Plants contain more complex and harder materials such as lignin, cellulose, and suberin; the former two are characterized by glass transitions at 72°C (LeBoeuf and Weber, 1998) and 225°C (Akim, 1978), respectively, and the latter one shows melting around 40°C (Cordeiro et al., 1998). The bacterial cells mainly contain peptidoglycanes, lipids, and proteins in their cell envelopes. Proteins (Welzel, 2002) and bacterial polysaccharide (Villain-Simonnet et al., 2000) undergo conformational changes above 50°C and 65°C, respectively. Nanothermograms of neither plant material nor bacteria show any of these thermal events. This might be caused by overlap of more than one process due to the complexity in cell and tissue structures and chemical composition. This points to an intrinsic weakness of the nanothermal analysis due to the significant reach of the thermal field around the tip and will be discussed in 3.3.2. Clear understanding of the thermograms and how materials of deeper layers contribute to the overall thermograms is still missing. If possible at all, a deconvolution of the thermograms and the assignment of the identified thermal events to particular processes or materials requires targeted experiments with a huge set of samples with well-known thermal properties as well as the targeted investigation of layered materials, which was beyond the scope of this pioneering study. However, it needs to be done in future for exploring additional utilities of AFM-nTA. Even without this knowledge, however, we can use the contrasting thermal behavior of bacterial cells and plant material to estimate contributions of OM with thermal properties similar to those of the reference materials based on similarity of the thermograms.
3.3.2 Comparison of nanothermograms
When interpreting nTA thermograms, it is important to consider that the effective thermal expansion or compression will always be a result of all materials reached by the heat of the tip. According to the manufacturer, the vertical resolution of the tip, i.e., the immersion depth of the heat, is in the range of 1 µm. Within this range, it can be expected that more than one material is layered in the soil sample. Each individual layer will contribute to the overall thermal expansion or compression. Therefore, a compression of the top layer can be masked by a parallel expansion of the layers beneath. The thermal reaction of the deeper layers is delayed compared to the top most one because they are only indirectly heated by heat transfer. The extent of contribution will depend on the thermal conductivity and capacity of the materials along the vertical dimension. Similar effects have been demonstrated for polymeric materials (Duvigneau et al., 2008). Moreover, the overlay of thermal events in layered materials could result in a shift in the exact temperature ranges of thermal events but will not change the sequence of the events.
The attribution of thermogram types was generally clear and unambiguous; thermograms within each group varied only with respect to total expansion, degree of expression of maxima, minima and inflections and their exact positions, indicating further sub-types of materials or combinations of materials in layered structures. In the samples investigated in this study, the thermograms of Type II and III could stand for organic materials as they are characterized by compression regions similar to those found for bacteria and plant fibers and as no inorganic materials are known revealing thermal compression in the respective temperature range (Schaumann and Kunhi Mouvenchery, 2012).
Even though Type II and Type V thermograms do not resemble each other at first glance with respect to their shapes, some important similarities can be found. Both types reveal a compression phase with comparable coefficients (κ ≈ 0.023–0.0031 V °C −1 for type II and κ ≈ 0.0011 V °C −1 for Type V). Also, the maximum temperature of the compression phase is the same for both thermograms (150–170°C). Therefore, regions with Type II thermograms could be candidates for degraded plant material in soils. However, the presence of an initial expansion phase in Type II was not observed in Type V and suggests that the thermogram is an overlay resulting from thermal behavior of a thin organic layer (most likely plant tissue, Type IV) on inorganic material (Type I). In addition, it is highly probable that a larger set of materials is responsible for these thermograms.
In contrast, Type III thermograms are more similar to Type IV thermograms. In particular the temperature ranges of the characteristic compression phases in the second sub-region (100–120°C to 200–225°C for Type III and 120–220°C for Type IV) reveal significant similarities. Thermograms differ from each other in the slope of the compression phase and in development of the third phase. The lack of an effective compression above 220°C again hints to the overlay of inorganic (Type I) and OM (most likely bacterial-derived residues, Type IV) responses. The difference in the slope of the compression and expansion phases may also partly be due to differences between the thermal behavior of fresh (intact) bacterial cells in the reference sample compared to cell envelope fragments (necromass) in the soil. The melting temperature was slightly lower and the thermal expansion and compression coefficients potentially differed. This can be due to either a partial degradation of the lipid structures in the cell envelopes after cell death or due to the presence of bacteria other than the Bacillus subtilis ssp. spizenii used as reference material in this study.
For a quantitative analysis on the basis of slopes of the compression phase, i.e., thermal expansion coefficients and compression coefficients, it will be necessary to characterize the nanothermal and nanomechanical properties of a larger set of bacteria and their fragments in future studies. We also will need to understand how and in which manner the overlay of different materials will affect the expansion signal and how the expansion coefficient of the top-layer material can be determined from the slope of the overall expansion signal.
3.4 Distribution of thermograms within the ROI of the soil samples
The frequency of occurrence of the thermogram types in the ROIs of each soil sample, collected from 16 points in each AFM image along a 4 ×4 grid, is shown in the rows denoted with “total” in Fig. 4A. The majority of points (47–75%) revealed Type I thermograms in the samples DG0–DG10, whereas in the older soil samples thermogram Type III was dominating (44–51%), followed by Type I (37–41%). Although the distribution pattern varied between replicates, the heterogeneity was lower than observed in the sandy soil investigated in a previous study (Schaumann and Kunhi Mouvenchery, 2012), owing to the lower complexity of the very young soils in the glacier forefield. Thus, the results suggest a trend of decreasing abundance of inorganic surfaces (Type I thermograms) (P < 0.0002) and increasing abundance of Type III thermograms with increasing distance from the glacier (P < 0.0001), but no change in the abundance of Type II thermograms. This trend goes along with increasing total organic C and fatty acid contents of the soil samples with increasing distance from the glacier (Bernasconi et al., 2011; Schurig et al., 2013). Although this quantitative assessment needs to be taken with care due to the low number of ROI, it does not contradict the expectations that with increasing soil age increasingly more inorganic surfaces are covered by OM, in particular those having Type III thermograms. For a verification of this assumption, more ROI have to be investigated and additional evidence from other properties and materials with significantly more AFM images are needed, in particular for quantification.

Abundance of the thermogram types–adhesion force range combinations in soil samples, collected (A) along a 4 × 4 grid on each AFM image and (B) on 8–10 selected structural features of 200–500 nm size per ROI. White: abundance < 10%; grey: abundance 10–19%; black: abundance ≥ 20%. The frames indicate the combination expected for bacterial cells.
3.5 Combining adhesive force information and thermal characteristics
3.5.1 Grid analysis approach
Aiming to attain more reliable discrimination of the types of materials revealed by nTA or adhesion force assessment alone, thermal characteristics were combined with the subset of adhesion force data at the points at which nTA was conducted. Figure 4A shows the distribution of the thermogram types among the adhesion force ranges in each soil sample. The numbers give the abundance of each adhesion force-thermogram type combination along the 4 × 4 grid on the AFM images. The distribution patterns were unique for each soil. However, the following generalization is possible: thermogram Type I surfaces in the very young samples (DG0, DG2, and DG4) reveal adhesion forces in the lower force range (0–20 nN). Intermediate samples (DG6 and DG10) were dominated by Type I and Type III thermograms with uniformly distributed forces in the range up to 40 nN. DG13 can also be grouped along with them apart from the exceptional notion that a strong dominance of Type I thermogram in combination with very low adhesion forces (0–10 nN) was found. The most developed soils (DG17 and DG18) were dominated by Type III thermograms with an adhesion force range up to 30 nN but differed with respect to the dominating adhesion force. The co-occurrence of Type III with the adhesion force range 20–30 nN (framed cells in Fig. 4A), which would be the combination expected for bacterial cells, increased with increasing soil age from 3% (DG0) to 26% (DG17). However, for DG18 no point of the 4 ×4 grid of the AFM images showed this combination.
This underlines the potential weakness of the grid analysis approach for locating materials with selected nanothermal characteristics due to the limited number of points which can be evaluated by AFM-nTA in each ROI. Even if such structures are present in the ROI, they will be detected only if they close enough to one of the grid points. A larger number of ROI could help avoiding this problem. This, however, results in substantially increased analysis effort.
3.5.2 Morphology-directed approach
The potential weakness of AFM-nTA, discussed above, might at least partly be resolved by pre-selecting points of interest in each ROI which fulfill additional characteristics representative for certain material types. This allows for characterization of material properties of morphologically similar soil constituents. In order to test this, we selected spots in the AFM images indicating features with a size of 200–500 nm, which have been hypothesized by Schurig et al. (2013) to represent microbial cell envelope fragments. The abundance of these features in the ROI tends to increase from 5% to 55% with soil age, which is in line with Schurig et al. (2013) for the same soil samples analyzed by SEM. For the analysis, 8 to 10 potential candidates for 200–500 nm large structures per image were selected. Nanothermal analysis was conducted on each of these candidates and then combined with the measured adhesion force data (Fig. 4B). Similar to the grid analysis, 50–75% of the thermograms were of Type I (rows denoted with “total” in Fig. 4B), suggesting that a large part of the structural features of size 200–500 nm represents inorganic materials. This is supported by the expectation that clay particles are within this size range as demonstrated by an AFM study on organic-free clay minerals by Liu et al. (2003), which revealed fluffy features larger than 200 nm for this type of material. However, also Type III thermograms were detected and their abundance increased steadily from 13% (DG0) to 50% (DG18), suggesting that with increasing soil age an increasing percentage of the selected features may be of organic nature.
Tip-surface adhesive forces of the selected features of 200–500 nm ranged between 0 and 30 nN (Fig. 4B). The most frequent adhesion force range within thermogram Type III was 20–30 nN, the abundance of which increased from 10% (DG0) to 41% (DG18). This combination is highlighted in Fig. 4B and matches with the bacterial cell characteristics. The data suggest that, based on the ROI investigated, the older soil samples contain more materials with characteristics similar to bacterial cells than young soils, which is underlined by the observation that the relative abundance of these structures increased significantly (p < 0.05) in the order: group 1 (0–15 y) < group 2 (65–70 y) < group 3 (80–120 y). Under the assumption that at least the younger part of such cell envelope fragments has characteristics similar to those of fresh bacterial cells, this development would be qualitatively in accordance with a previous study suggesting an increasing abundance of cell envelope fragments with increasing soil age (Schurig et al., 2013). For older cell envelope fragments, characteristics might differ from those of the fresh cell envelope fragments (Claessens et al., 2006) and their abundance remains unresolved. However, from our results it cannot be excluded that the abundances given by Schurig et al. (2013) based solely on size and shape may be overestimated. This underlines the need for further characterization of these morphological features.
4 Conclusions
The current study demonstrates for the first time that the combination of the two independent AFM tools (force mapping and thermal analysis) allows for localization and distinction of structural features in soils on small scales and with a higher degree of certainty than obtained either by PFQNM or by nTA alone. The clarity of discrimination is further increased by combination with morphological characteristics, e.g., the size, as done in our study. By this approach, the bias resulting from overlapping of adhesion force ranges between several materials is overcome. Therefore, AFM-nTA combined with PFQNM can be used to determine a set of material characteristics of selected points of interest, to identify materials by similarity analysis. Alternatively, it can be used to exclude certain spots from a large set of candidates identified solely by morphological characteristics (e.g., by electron microscopy) based on dissimilarity of the nanomechanical and nanothermal properties at these spots from those of the target materials. The qualitative characteristics alone will already help to distinguish various soil constituents on material basis and may help to understand the significance of the contribution of selected materials to SOM development.
The current challenges of AFM-nTA for material identification are the unknown impact of layered materials on the overall thermograms, and the very limited availability of reference thermograms for the multitude of materials expected in soils as well as the knowledge of the variability of the thermogram within material groups due to different states of degradation. If quantification of composition is required, a sufficiently large set of representative ROI has to be chosen. For further exploration of the potential of AFM-nTA, the currently existing database needs to be extended by a sufficiently large set of carefully selected representative reference materials and more complex and developed soil systems should be included in the investigation. The current approach could be further improved by additional use of chemical force microscopy (Alsteens et al., 2007), allowing for an even clearer discrimination between materials. At the time being, AFM-nTA is a relatively young and unexplored methodology, but further development of AFM-nTA and the combination with other AFM-related analyses can be expected to increase the potential to provide quantitative results.
Acknowledgements
We acknowledge Ms. Priya Mary Abraham for fruitful discussions. We thank Rienk Smittenberg for granting access to the BigLink sites in the Damma glacier forefield. This study has been supported by the Deutsche Forschungsgemeinschaft (DFG), projects SCHA849/8 and MI 598/2 within the priority program SPP 1315 ‘Biogeochemical Interfaces in Soil' and by the European Commission in the framework of the IP ModelPROBE (contract number 213161).




