Labile organic matter fractions as early-season nitrogen supply indicators in manure-amended soils
Abstract
Soil test indicators are needed to predict the contribution of soil organic N to crop N requirements. Labile organic matter (OM) fractions containing C and N are readily metabolized by soil microorganisms, which leads to N mineralization and contributes to the soil N supply to crops. The objective of this study was to identify labile OM fractions that could be indicators of the soil N supply by evaluating the relationship between the soil N supply, the C and N concentrations, and C/N ratios of water extractable OM, hot-water extractable OM, particulate OM, microbial biomass, and salt extractable OM. Labile OM fractions were measured before planting spring wheat (Triticum aestivum L.) in fertilized soils and the soil N supply was determined from the wheat N uptake and soil mineral N concentration after 6 weeks. Prior to the study, fertilized sandy loam and silty clay soils received three annual applications of 90 kg available N (ha · y)−1 from mineral fertilizer, liquid dairy cattle manure, liquid swine manure or solid poultry litter, and there was a zero-N control. Water extractable organic N was the only labile OM fraction to be affected by fertilization in both soil types (P < 0.01). Across both test soils, the soil N supply was significantly correlated with the particulate OM N (r = 0.87, P < 0.001), the particulate OM C (r = 0.83, P < 0.001), and hot-water extractable organic N (r = 0.81, P < 0.001). We conclude that pre-planting concentrations of particulate OM and hot-water extractable organic N could be early season indicators of the soil N supply in fertilized soils of the Saint Lawrence River Lowlands in Quebec, Canada. The suitability of these pre-planting indicators to predict the soil N supply under field conditions and in fertilized soils from other regions remains to be determined.
1 Introduction
Accounting for the contribution of soil organic N to the soil N supply (SNS) is expected to lower N fertilizer inputs, resulting in more economic crop production and environmental protection, especially in manure-amended soils where organic matter inputs build the mineralizable organic N pool (Whalen et al., 2001; Gutser et al., 2005; Sharifi et al., 2011). The SNS for crops comes from NH
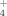 , which is mineralized from the soil organic N and then oxidized and nitrified to NO
, which is mineralized from the soil organic N and then oxidized and nitrified to NO
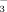 , another plant-available compound, collectively referred to as mineral N (NH
, another plant-available compound, collectively referred to as mineral N (NH
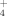 plus NO
plus NO
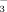 ). The SNS is derived from the mineralization of crop residues, soil organic matter (OM), and organic amendments during the current growing season, plus any residual mineral N from the previous growing season. Yet, the fraction of soil organic N that mineralizes during the growing season is not easily predicted, and laboratory-based methods may not reflect the process in situ. This means the contribution of the soil organic N fraction to the SNS is poorly quantified (Ros et al., 2011; St. Luce et al., 2014).
). The SNS is derived from the mineralization of crop residues, soil organic matter (OM), and organic amendments during the current growing season, plus any residual mineral N from the previous growing season. Yet, the fraction of soil organic N that mineralizes during the growing season is not easily predicted, and laboratory-based methods may not reflect the process in situ. This means the contribution of the soil organic N fraction to the SNS is poorly quantified (Ros et al., 2011; St. Luce et al., 2014).
The quantity of organic N that mineralizes during a growing season depends on microbial metabolism of C and N contained in labile OM fractions (Gutser et al., 2005), which include water extractable organic C and N (WEOC and WEON) and hot-water extractable organic C and N (hot-WEOC and hot-WEON) (Chantigny et al., 2010), particulate OM C and N (POMC and POMN), microbial biomass C and N (MBC and MBN) (Haynes, 2005), and salt extractable organic C and N (SEOC and SEON) (Zsolnay, 2003). The WEON represents about 0.75% of soil total N and the hot water extractable N is between 2.6 and 8.7% of total N (Curtin et al., 2006). Particulate OM N represented 18% of soil total N, on average, across 65 agricultural soils (Gregorich et al., 2006). The MBN is 3 to 5% of total N (Murphy et al., 2000). Most of these labile organic N fractions are larger than the mineral N pool, which usually represents 1% of soil total N (Jarvis et al., 1996). Although the labile organic N fractions collectively account for > 20% of soil total N, only 9–10% of total N is potentially mineralizable during a growing season (Sharifi et al., 2008, 2011, 2012). This implies that some labile organic N fractions are more susceptible to mineralization than others, and thus make a greater contribution to the SNS. Since organic N is always bound to organic C, the C and N concentrations and C/N ratios of labile OM fractions in the early growing season (e.g., pre-planting period) could be related to the SNS during vegetative growth, a period of high crop N uptake.
Water extractable OM (WEOM) merits evaluation as an indicator of soil N supply because it is a surrogate for in situ dissolved OM (Zsolnay, 2003), which is readily metabolized within hours to days. Ghani et al. (2003) found a strong positive correlation between hot-WEOC and anaerobically mineralizable N (R2 = 0.86; P < 0.001). Although the WEOC and WEON contain readily degradable forms of C and N that cycle rapidly through microbial biomass (Marschner and Bredow, 2002; Gregorich et al., 2003), the production of WEOC and WEON is proportional to the soil OM content in fertilized soils (Chantigny, 2003). This means that WEOC or WEON concentrations, or the WEOC:WEON ratio, may be an indicator of a soil's potential to mineralize N (Haney et al., 2012), at least during periods when equal quantities of these compounds are consumed and produced. The WEOC and WEON concentrations can be assessed by warm (20°C) or hot (50–80°C) water extraction of soil, but whether these methods are equally good indicators of the SNS is unclear. Salt extractable OM (SEOM) may represent the soil solution OM plus OM that is desorbed or dissolved during the extraction process (Zsolnay, 2003). Since the SEOM is an indicator of bioavailable organic N for microbial biomass (Murphy et al., 2000), it could be mineralized and contribute to the SNS, but it has not been evaluated as an indicator of SNS as far as we know.
Particulate organic matter C and N (POMC and POMN) are fragmented, partially decomposed crop residues left after harvest (Gregorich et al., 2006) and added in manure as partially digested and undigested residues from feces and animal bedding (Gosling et al., 2013). As a precursor to the WEOM and SEOM fractions, the POM fraction may be rapidly depleted and mineralized. For instance, St. Luce et al. (2014) reported 15N accumulation in the mineral N pool was related to a decrease in the 15N-POM fraction and this began 7 to 14 d after incubating soil amended with 15N-labeled crop residue. Given that manure application increased POMN concentrations (Griffin and Porter, 2004; Mallory et al., 2010; Nyiraneza et al., 2010) and that manure application produced a POM with a lower C/N ratio than POM originating from crop residues (Aoyama et al., 1999), it seems likely that POM would contribute to N mineralization and could be an indicator of SNS in manure-amended soils.
In most cases, manure application increases the soil microbial biomass (McGill et al., 1986; Rochette and Gregorich, 1998; Birkhofer et al., 2008; Jost et al., 2013) but not in all studies (Jensen et al., 2000). Soil microbial biomass is the only labile organic matter fraction with a metabolic function, making it a dynamic fraction that transforms organic N to mineral N. However, the MBN is considered the most labile organic N fraction in soil (Smith and Paul, 1990), and may act as both a source and sink of N (Brookes, 2001) depending on environmental conditions and the availability of C-rich substrates (Geisseler et al., 2010). Microbial biomass was suggested as an index for mineral N availability (Carter and MacLeod, 1987; Burton and McGill, 1992; Deng et al., 2000) due to its role mediating the decomposition and mineralization processes in soil, but whether it is an indicator of SNS remains to be seen.
The objectives of this study were to determine (1) how labile OM fractions were affected by manure amendments in soils with contrasting texture, and (2) which labile OM fractions were related to, and thus could be indicators of the SNS, in fertilized soils with contrasting texture. First, we measured the concentrations of WEOC and WEON, hot-WEOC and hot-WEON, POMC and POMN, SEOC and SEON, and MBC, and MBN in soils that received no N input [0-N control) or fertilizer (90 kg available N (ha · y)−1 from mineral fertilizer or manure]. The hypothesis was that the solid poultry litter-amended soils would contain the highest concentration of labile organic C and N because this manure had higher protein content than other manure sources and was mixed with woodchips. Second, the pre-planting concentrations of labile OM fractions were related to SNS, calculated as the wheat N uptake plus the soil mineral N concentration after 42 d. It was hypothesized that the WEOM and POM fractions would be better indicators of the SNS than whole soil or the microbial biomass fraction.
2 Material and methods
2.1 Soil sampling and amendment history
Soils were collected at the Laval University Experimental Farm near Saint-Augustin-de-Desmaures, Quebec, Canada (46°44′ N, 71°31′ W; 110 m asl). A poorly drained silty clay (432 g clay kg−1, 163 g sand kg−1, 35 g C kg−1, pH = 6.8) classified as a mixed frigid Dystric Eutrudept and a well-drained sandy loam (170 g clay kg−1, 680 g sand kg−1, 19 g C kg−1, pH = 7.0) classified as a mixed frigid Typic Dystrudept were selected for their contrasting properties. Experimental plots were established in May 2009 with spring wheat (Triticum aestivum L.) in a corn (Zea mays L.)–soybean (Glycine max L.)–spring wheat rotation. Above-ground crop residues were removed by hand at each harvest, and the remaining stubble was 10–15-cm above the soil surface. The contribution of crop residues to soil fertility during the crop rotation was not assessed, but should reflect the amendment history (i.e., plots with greater fertility produce higher yields and thus more crop residues).
Plots were 5 m × 7 m in size and arranged in a randomized complete block design on each soil texture with five experimental treatments [control (CTL), mineral NPK (NPK), liquid swine manure (LSM), liquid dairy cattle manure (LCM), and solid poultry litter (SPL)] replicated three times. Excluding the CTL, all plots received 90 kg available N ha−1 in spring of 2009, 2010, and 2011. For the organic N sources (LSM, LCM, SPL) 90% of total N is expected to be available in the year of application for LSM, and 70% of total N is expected to be available for LCM and SPL if applied in the spring and rapidly incorporated to minimize NH3 volatilization (CRAAQ, 2010). To simplify field work, an average coefficient of 0.8 was used for all manures, so the total N application rate for each manure was 112.5 kg total N ha−1. For the NPK treatment, the mineral N fertilizer was applied as Ca-NH4-nitrate at 90 kg N ha−1. The NPK and CTL treatments received P fertilizer applied as triple superphosphate (20 kg ha−1 P2O5 for the sandy loam; 30 kg P2O5 ha−1 for the silty clay), and K fertilizer applied as K-chloride (20 kg K2O ha−1 for both soil types), based on local recommendations (CRAAQ, 2010). There was no amendment applied in 2012. Eight soil samples (0- to 20-cm depth) were collected with a soil auger (10 cm in diameter) from each plot in spring 2012. Samples were composited, passed through a 4.75-mm sieve while field-moist, and stored at 4°C for 14 d until the start of the experiment. Total C and N of each soil were determined by dry combustion with a Flash EA 1112 series CN analyzer (ThermoFinnigan, Italy).
2.2 Manure analysis
Composite samples from each manure source were collected on the application date in 2009, 2010, and 2011, and refrigerated (4°C) until analysis (Table 1). The LCM and LSM were homogenized using a Polytron (Model PT 3100; Kinematica AG, Littau-Lucerne, Switzerland). Distilled water was added to the SPL (10:1, water/SPL ratio) and homogenized with the Polytron to make a slurry solution. The pH of each manure was then measured by direct reading with a glass electrode. Dry matter of the manure was determined as the mass of materials remaining after drying 100 mL of LCM and LSM and 50 g of fresh SPL for 96 h at 55°C. Total C in the SPL was measured by dry combustion and total C in the LSM and LCM was measured by injecting 50 μL of homogenized into an automated combustion C analyzer (Model Formacs; Skalar Analytical, De Breda, The Netherlands). Total N and P concentrations were determined by measuring NH
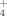 and PO
and PO
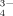 concentrations in Kjeldahl acid digests (Chantigny et al., 2007a) with an automated continuous-flow injection colorimeter (QuickChem 8000 FIA+; Lachat Instruments, Loveland, CO, USA). The mineral N concentration of the liquid manures and SPL slurry was determined by shaking 10 mL of the sample with 50 mL of 1 M KCl for 60 min. The extract was filtered through pre-washed (1 M KCl) Whatman #42 filter paper. The NH
concentrations in Kjeldahl acid digests (Chantigny et al., 2007a) with an automated continuous-flow injection colorimeter (QuickChem 8000 FIA+; Lachat Instruments, Loveland, CO, USA). The mineral N concentration of the liquid manures and SPL slurry was determined by shaking 10 mL of the sample with 50 mL of 1 M KCl for 60 min. The extract was filtered through pre-washed (1 M KCl) Whatman #42 filter paper. The NH
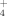 and NO
and NO
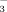 + NO
+ NO
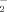 concentrations in the extracts were measured with the colorimeter described above.
concentrations in the extracts were measured with the colorimeter described above.
| Manurea | DMb | Total C | Total N | 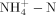
|
Total P | pH |
|---|---|---|---|---|---|---|
| / g L−1 | ||||||
| LSM | 71.9 ± 14.31c | 30.4 ± 6.40 | 7.4 ± 0.77 | 4.7 ± 0.65 | 1.4 ± 0.22 | 7.2 ± 0.05 |
| LCM | 54.3 ± 4.72 | 24.0 ± 1.44 | 2.7 ± 0.19 | 1.4 ± 0.23 | 0.5 ± 0.04 | 6.6 ± 0.09 |
| / g kg−1 | ||||||
| SPL | 742.2 ± 27.71 | 308.5 ± 12.57 | 27.8 ± 1.52 | 4.5 ± 0.28 | 10.1 ± 0.29 | 8.7 ± 0.09 |
- aLSM, liquid swine manure; LCM, liquid dairy cattle manure; SPL, solid poultry litter.
- bDM, dry matter.
- cNumbers following mean values represent SE.
2.3 Soil N supply determined from spring wheat N uptake
A short-term (42 d) plant growth study was conducted, similar to the procedure of Serna and Pomares (1991), in a controlled environment chamber (Conviron E15, Conviron Technology, Winnipeg, Canada). Day-length and temperature were set at 18 h and 18°C, and night temperature was set at 15°C. Standard 15-cm diameter pots were filled with sieved field-moist soil (850 g silty clay or 1000 g sandy loam per pot on dry soil basis) and placed on saucers. Six seeds of spring wheat (cv. A.C. Barrie) were sowed in each pot and were frequently misted with deionized water for one week until all seedlings emerged. The seedlings were then thinned to four plants per pot. Soil moisture was maintained at 55% water-filled pore space by mass measurement and watering daily. At day 14 after seedling emergence, a zero-N nutrient solution was applied based on a modified Hoagland solution (0.5 M K2SO4, 1 M MgSO4, 0.05 M CaH2PO4) to prevent nutrient deficiency. Upon the termination of the growth trial, plant above-ground biomass was cut at the soil surface and weighed before and after oven drying at 55°C for 48 h. The soil was separated from the plant roots by gently removing the roots by hand and sieving the soil at < 4.75 mm. Plant roots were discarded as their small biomass made them difficult to salvage. Wheat root N-uptake was 16% of crop N uptake in a 45-d study with a similar cultivar and growth conditions, but was not affected by N fertilizer treatments (Thomas, 2015). Thus, discarding the wheat roots was considered to be a small source of error in this experiment. Dry above-ground plant material was ground to pass through a 1-mm screen, and the total C and N concentrations were determined by dry combustion with a Flash EA 1112 series CN analyzer (ThermoFinnigan, Italy). At the beginning (Pre-Plant) and termination (Post-Harvest) of the growth study, a 5.0 g field-moist subsample of soil was extracted with 50 mL of 2 M KCl to determine the NH
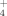 and NO
and NO
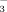 concentrations using the modified indophenol blue technique (Sims et al., 1995). Simultaneously, the gravimetric water content was calculated by drying 10 g of field-moist soil at 105°C for 24 h.
concentrations using the modified indophenol blue technique (Sims et al., 1995). Simultaneously, the gravimetric water content was calculated by drying 10 g of field-moist soil at 105°C for 24 h.
SNS = Post-Harvest Mineral N + Crop N uptake,
(1)where Post-Harvest Mineral N is the sum of NO
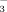 and NH
and NH
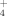 concentrations (mg N kg−1 dry soil) immediately following harvest; and Crop N uptake is the N accumulation in the above-ground biomass at harvest (mg plant N kg−1 dry soil). We assumed that gaseous N losses were negligible because maintenance of 55% water-filled pore space should maximize aerobic activity and minimize denitrification (Linn and Doran, 1984). Leaching N losses were controlled because water that passed through the pot was collected in a saucer underneath the pot and poured back onto the soil surface within 24 h.
concentrations (mg N kg−1 dry soil) immediately following harvest; and Crop N uptake is the N accumulation in the above-ground biomass at harvest (mg plant N kg−1 dry soil). We assumed that gaseous N losses were negligible because maintenance of 55% water-filled pore space should maximize aerobic activity and minimize denitrification (Linn and Doran, 1984). Leaching N losses were controlled because water that passed through the pot was collected in a saucer underneath the pot and poured back onto the soil surface within 24 h.
2.4 Extractable organic matter fractions
The WEOM and hot-WEOM extractions were based on the methods of Ghani et al. (2003) and Chantigny et al. (2010). Briefly, the field-moist composite soil from each experimental plot was air-dried and passed through a 4-mm sieve. A 5 g subsample was placed in a 50 mL polypropylene centrifuge tube and 30 mL of deionized water (20°C) was added. The tubes were shaken for 30 min on an oscillating shaker. The soil-water suspension was centrifuged for 20 min at 4,500 g for the sandy loam and at 10,000 g for the silty clay. The supernatant was decanted through a filter paper (Fisher Scientific Q5; pre-leached with 40 mL of deionized water) into an acid-washed Nalgene bottle. This filtered fraction was used to determine WEOC and WEON concentrations. The mass of the centrifuge tube plus the remaining wet soil was measured to calculate the entrained water volume to correct for dilution of the C and N concentrations. After filtering the supernatant, a 30 mL aliquot of deionized water (20°C) was added to each centrifuge tube and mixed thoroughly to re-suspend the soil. The tubes were tightly sealed and placed in a 50°C hot-water bath for 16 h, then tubes were centrifuged and the contents filtered again, as described above. This extraction was used to determine hot-WEOC and hot-WEON. The WEON and hot-WEON concentrations were determined after persulfate oxidation of 20°C water and 50°C hot-water extractions and were the difference between total N concentration after persulfate oxidation and the mineral N concentration of non-oxidized samples (Cabrera and Beare, 1993). The NO
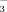 and NH
and NH
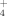 concentrations were determined colorimetrically using the modified indophenol blue technique (Sims et al., 1995). The organic C concentration in WEOC and hot-WEOC extracts was measured with a Sievers Innovox TOC analyzer (GE Analytical Instruments, Boulder, CO, USA). The C/N ratios of WEOM and hot-WEOM were calculated by dividing the organic C concentration by the organic N concentration (mg kg−1 dry soil) in each water extraction.
concentrations were determined colorimetrically using the modified indophenol blue technique (Sims et al., 1995). The organic C concentration in WEOC and hot-WEOC extracts was measured with a Sievers Innovox TOC analyzer (GE Analytical Instruments, Boulder, CO, USA). The C/N ratios of WEOM and hot-WEOM were calculated by dividing the organic C concentration by the organic N concentration (mg kg−1 dry soil) in each water extraction.
The POMC and POMN were measured following size fractionation (> 53 µm) based on Gregorich and Beare (2008). A 120 g subsample of field-moist soil was sieved < 2 mm and air-dried, then 25 g was dispersed in 100 mL of a weak Na-hexametaphosphate (5 g L−1) solution in a 250 mL Nalgene bottle by shaking bottles sideways on a reciprocating shaker for 16 h. The POM, including the sand remaining on the 53 µm sieve was air-dried overnight and then oven dried at 50°C for 24 h and finely ground to pass through a 250-µm sieve. The concentrations of POMC and POMN were determined by dry combustion using a Flash EA 1112 series CN analyzer (ThermoFinnigan, Italy).
The MBC and MBN were measured based on Voroney et al. (2008) and Horwath and Paul (1994) using chloroform fumigation-direct extraction. A 10.0 g field-moist soil sieved < 4.75 mm was fumigated with ethanol free chloroform for 24 h, the chloroform was then repeatedly evacuated using a 80 kPa pump, and then soils were directly extracted with 0.5 M K2SO4. Non-fumigated soil was simultaneously extracted with 0.5 M K2SO4. The MBC was calculated as total organic C in fumigated samples minus the total organic C in non-fumigated samples divided by a correction factor of 0.45 (Wu et al., 1990; Joergensen, 1996). The organic C concentrations were determined by a Sievers Innovox TOC analyzer (GE Analytical Instruments, Boulder, CO, USA). The MBN was calculated as total N in fumigated samples minus the total N in non-fumigated samples. For fumigated and non-fumigated soils, the MBN was determined by subtracting the NO
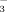 concentration of non-oxidized samples from the NO
concentration of non-oxidized samples from the NO
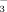 concentration of persulfate oxidized samples (Cabrera and Beare, 1993). The NO
concentration of persulfate oxidized samples (Cabrera and Beare, 1993). The NO
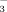 concentrations were determined colorimetrically using the modified indophenol blue technique (Sims et al., 1995). The MBN was then divided by a correction factor of 0.54 (Joergensen and Mueller, 1996). The concentration of organic C in the non-fumigated 0.5 M K2SO4 extract was considered to represent the SEOC. The SEON concentration was calculated as the difference in total NO
concentrations were determined colorimetrically using the modified indophenol blue technique (Sims et al., 1995). The MBN was then divided by a correction factor of 0.54 (Joergensen and Mueller, 1996). The concentration of organic C in the non-fumigated 0.5 M K2SO4 extract was considered to represent the SEOC. The SEON concentration was calculated as the difference in total NO
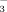 concentration of non-fumigated 0.5 M K2SO4 persulfate oxidized and non-oxidized samples (Chantigny et al., 2007b).
concentration of non-fumigated 0.5 M K2SO4 persulfate oxidized and non-oxidized samples (Chantigny et al., 2007b).
2.5 Statistical analysis
All data were first tested for normality using the Shapiro–Wilks W test, and log-transformed as required. The effect of fertilizer treatments on the WEOC, WEON, hot-WEOC, hot-WEON, POMC, POMN, MBC, MBN, SEOC, SEON concentrations, on the C/N ratio of each fraction, and SNS was analyzed by one-way ANOVA for each soil texture using the PROC GLM procedure of SAS 9.3 software (SAS Institute, 2011; Cary, NC, USA). When there was a significant fertilizer treatment effect (P < 0.05), differences among treatment means were evaluated using the least significant difference (LSD) test with TUKEYs adjustment at a P < 0.05 confidence level. Correlations among these parameters were determined with Pearson correlation coefficients (r) using the PROC CORR statement in SAS 9.3 software using all (n = 30) treatment replicates. In addition, simple linear regression analyses were performed for the POMN and POMC, and SNS for each treatment replicates (n = 30) using the PROC REG statement in SAS 9.3 software. Significant effects were accepted at a P < 0.05 confidence level.
3 Results
Fertilizer treatments did not affect the hot-WEOC, WEOC, POMC, POMN, MBC, MBN, or SEOC in the sandy loam or the silty clay soil, but significantly affected WEON concentrations in both sandy loam and silty clay soils (P < 0.01) and significantly affected the SEON and hot-WEON concentrations in the silty clay soil (P < 0.01 and P < 0.001, respectively; Table 2).
| Treatmenta (n = 3) | WEOCb | WEONb | Hot-WEOCc | Hot-WEONc | POMC | POMN | MBC | MBN | SEOC | SEON | SNSd |
|---|---|---|---|---|---|---|---|---|---|---|---|
| / mg kg−1 | |||||||||||
| Sandy loam | |||||||||||
| CTL | 305 ± 62.6e | 11.9 ± 1.3 cf | 458 ± 32.4 | 10.8 ± 2.2 | 2033 ± 323.6 | 76.4 ± 16.5 | 163 ± 5.0 | 29.0 ± 11.3 | 68.7 ± 6.7 | 12.8 ± 2.5 | 14.2 ± 0.5 |
| NPK | 239 ± 21.7 | 36.5 ± 3.0 ab | 527 ± 5.85 | 12.8 ± 1.4 | 1987 ± 262.4 | 63.0 ± 2.1 | 157 ± 26.1 | 9.53 ± 2.1 | 66.8 ± 8.8 | 15.5 ± 1.2 | 13.8 ± 2.9 |
| LSM | 258 ± 24.1 | 19.6 ± 5.0 bc | 489 ± 34.6 | 8.56 ± 1.0 | 2158 ± 457.8 | 70.2 ± 26.5 | 124 ± 24.4 | 24.8 ± 13.9 | 64.1 ± 13.3 | 14.3 ± 1.1 | 9.62 ± 0.7 |
| LCM | 257 ± 19.0 | 21.8 ± 2.6 abc | 549 ± 32.2 | 9.05 ± 1.6 | 1942 ± 170.6 | 68.2 ± 24.7 | 118 ± 38.5 | 36.4 ± 9.2 | 65.8 ± 5.1 | 12.9 ± 2.1 | 12.4 ± 1.7 |
| SPL | 261 ± 15.7 | 41.4 ± 6.7 a | 470 ± 26.0 | 9.38 ± 0.5 | 1766 ± 103.2 | 83.6 ± 13.2 | 155 ± 22.7 | 17.6 ± 0.6 | 59.6 ± 9.7 | 12.9 ± 1.3 | 14.4 ± 3.6 |
| Silty clay | |||||||||||
| CTL | 353 ± 28.4 | 22.7 ± 5.3 bc | 642 ± 109 | 23.6 ± 1.0 d | 4924 ± 954.5 | 403 ± 51.8 | 687 ± 107.1 | 88.2 ± 17.4 | 64.8 ± 8.0 | 17.3 ± 1.5 abc | 26.5 ± 3.4 |
| NPK | 354 ± 27.4 | 15.7 ± 1.8 c | 753 ± 89.9 | 31.4 ± 2.5 cd | 3656 ± 423.3 | 301 ± 39.3 | 534 ± 69.4 | 69.5 ± 15.4 | 49.9 ± 1.8 | 14.2 ± 0.6 c | 19.0 ± 1.9 |
| LSM | 357 ± 33.6 | 24.0 ± 1.9 abc | 821 ± 75.4 | 40.1 ± 2.7 ab | 4652 ± 187.6 | 404 ± 8.0 | 371 ± 15.8 | 78.6 ±10.9 | 58.3 ± 9.7 | 20.6 ± 1.1 ab | 26.4 ± 1.9 |
| LCM | 361 ± 38.9 | 33.0 ± 3.6 ab | 788 ± 124 | 43.8 ± 1.1 a | 5413 ± 942.2 | 464 ± 51.7 | 380 ± 51.1 | 54.2 ± 5.85 | 67.8 ± 5.7 | 22.0 ± 2.1 a | 27.4 ± 2.2 |
| SPL | 348 ± 34.3 | 35.6 ± 1.6 a | 680 ± 50.7 | 33.5 ± 2.2 bc | 4856 ± 661.1 | 423 ± 74.8 | 416 ± 17.0 | 72.3 ± 13.3 | 56.9 ± 5.0 | 15.2 ± 0.6 bc | 25.6 ± 2.7 |
- aCTL, Zero-N control; NPK, mineral NPK; LSM, liquid swine manure; LCM, liquid dairy cattle manure; SPL, solid poultry litter.
- bWEON and WEOC were extracted with 20°C deionized water.
- cHot-WEON and Hot-WEOC were extracted sequentially after removal of WEON and WEOC, in a water bath at 50°C for 16 h.
- dSNS = Soil N supply (mg N kg−1 dry soil) = Post-harvest mineral N + N accumulation in above-ground plant tissue.
- eValues are the mean and standard error.
- fData was subjected to one-way Analysis of Variance (ANOVA), if a significant difference was detected at a P < 0.05 confidence level in the ANOVA model, a LSD multiple comparison test with a Tukey adjustment was conducted to detect differences between fertility treatment means at a P < 0.05 confidence level. Within each column, values with the same letter or no letters are not significantly different (P > 0.05).
For the sandy loam, the SPL-amended soil had significantly more WEON than the LSM-amended and CTL soils but was not different from LCM-amended and NPK fertilized soils. In the silty clay, the LCM- and SPL-amended soils had significantly greater WEON concentrations than the NPK fertilized soil, and the SPL-amended soil had more WEON than the CTL soil, but there was no difference among manure amendments. Manure-amended and NPK fertilized soils provided a wide range of WEON values. There was 2.5-fold more WEON in the manure-amended (manured soils average) and NPK fertilized soils than in the unfertilized sandy loam soils. In the silty clay soil, the WEON concentration was 1.6-fold greater in the manure-amended (manured soils average) than the NPK-fertilized and unfertilized treatments.
In the silty clay soil, the SEON concentration was significantly greater with the LCM than the NPK and SPL treatments. The hot-WEON concentration was significantly greater in the manured treatments than the CTL, and the LSM- and LCM-amended soils had more hot-WEON than the NPK-treatment (Table 2).
Generally, there was no difference in C/N ratios of labile OM fractions due to fertilizer treatments, the only exception being that the WEOC:WEON ratio was significantly affected by fertilizer treatments in the sandy loam soil (P < 0.01; Table 3). The WEOC:WEON ratio was 4 times higher in the CTL than the NPK and SPL treatments, and this was statistically significant (Table 3).
| Treatmenta (n = 3) | WEOC:WEONb | Hot-WEOC:WEONc | POMC:POMNd | MBC:MBNe | SEOC:SEONf | SOC:SONg |
|---|---|---|---|---|---|---|
| Sandy loam | ||||||
| CTL | 26.5 ± 7.0h ai | 46.1 ± 9.4 | 29.1 ± 8.24 | 7.2 ± 2.1 | 5.6 ± 0.5 | 15.4 ± 3.7 |
| NPK | 6.7 ± 1.0 b | 42.1 ± 4.7 | 29.4 ± 4.42 | 18.2 ± 4.2 | 4.4 ± 0.8 | 15.5 ± 3.7 |
| LSM | 15.2 ± 4.1 ab | 58.5 ± 7.2 | 43.5 ± 12.1 | 25.0 ± 21.6 | 4.5 ± 0.9 | 12.7 ± 1.4 |
| LCM | 12.4 ± 2.3 ab | 63.3 ± 8.5 | 24.5 ± 5.40 | 3.1 ± 0.5 | 5.1 ± 0.6 | 9.8 ± 0.8 |
| SPL | 6.53 ± 0.9 b | 50.7 ± 5.0 | 45.4 ± 16.1 | 8.92 ± 1.6 | 4.7 ± 0.7 | 15.2 ± 2.7 |
| Silty clay | ||||||
| CTL | 18.0 ± 5.6 | 27.3 ± 4.7 | 12.0 ± 1.0 | 8.0 ± 0.4 | 3.8 ± 0.4 | 9.9 ± 1.8 |
| NPK | 23.4 ± 4.2 | 23.9 ± 1.7 | 12.2 ± 0.2 | 8.0 ± 0.8 | 3.5 ± 0.1 | 10.6 ± 0.9 |
| LSM | 15.3 ± 2.8 | 20.1 ± 0.7 | 11.5 ± 0.3 | 5.0 ± 0.9 | 2.8 ± 0.3 | 9.2 ± 0.5 |
| LCM | 11.5 ± 2.5 | 17.9 ± 2.4 | 11.5 ± 0.9 | 7.3 ± 1.6 | 3.1 ± 0.1 | 8.8 ± 0.8 |
| SPL | 9.88 ± 1.4 | 20.5 ± 2.2 | 11.7 ± 0.6 | 6.1 ± 0.8 | 3.8 ± 0.5 | 10.9 ± 1.8 |
- aCTL, Zero-N control; NPK, mineral NPK; LSM, liquid swine manure; LCM, liquid dairy cattle manure; SPL, solid poultry litter.
- bWEOC:WEON is the C/N ratio of water extractable organic matter (WEOM) extracted with 20°C deionized water.
- cHot-WEOC:WEON is the C/N ratio of hot-water extractable organic matter extracted sequentially after removal of WEOM, in a water bath at 50°C for 16 h.
- dPOMC:POMN is the C/N ratio of particulate organic matter.
- eMBC:MBN is the C/N ratio of the microbial biomass.
- fSEOC:SEON is the C/N ratio of the salt extractable organic matter extracted with 0.5 M K2SO4.
- gSOC:SON ratio is the C/N ratio of the whole soil.
- hValues are the mean and ± standard error.
- iData was subjected to one-way analysis of variance (ANOVA), if a significant difference was detected at a P < 0.05 confidence level in the ANOVA model, a LSD multiple comparison test with a Tukey adjustment was conducted to detect differences between fertility treatment means at a P < 0.05 confidence level. Within each column, values with the same letter or no letters are not significantly different (P > 0.05).
3.1 Soil N supply
Since the SNS was not affected by fertilizer treatments in soils with contrasting texture (Table 2), data were pooled among soil types to evaluate the relationships between labile OM fractions and the SNS. The strongest correlations were between the SNS and the POMN concentration (r = 0.87; P < 0.001; Table 4, Fig. 1), the POMC concentration (r = 0.83; P < 0.001), the SOC concentration (r = 0.82, P < 0.001), and the hot-WEON concentration (r = 0.81, P < 0.001). When the C/N ratios of labile OM fractions were correlated with SNS, the correlation coefficients were lower than those of the individual components (i.e., POMN > POMC:POMN), suggesting a stronger association between POMN and other measured parameters to the SNS than the C/N ratio of any labile OM fraction (Table 4).
| Parameter | Correlation Coefficient |
|---|---|
| SNSb | |
| POMN | 0.87*** |
| POMC | 0.83*** |
| SOC | 0.82*** |
| Hot WEON | 0.81*** |
| HotWEOC:WEONc | –0.74*** |
| SON | 0.73*** |
| MBC | 0.69*** |
| WEOC | 0.67*** |
| SEON | 0.65*** |
| MBN | 0.62*** |
| Hot-WEOC | 0.61*** |
| POMC:POMNd | –0.59** |
| SEOC:SEONe | –0.47** |
| SOC:SONf | –0.38* |
- a* Significant at a P < 0.05 confidence level; ** Significant at a P < 0.01 confidence level (n = 4).
- bSNS is soil N supply (mg N kg−1 dry soil) = Post-harvest mineral N + N accumulation in above-ground plant tissue
- cHot-WEOC:WEON is the C/N ratio of hot-water extractable organic matter extracted sequentially after removal of WEOM, in a water bath at 50°C for 16 h.
- dPOMC:POMN is the C/N ratio of particulate organic matter.
- eSEOC:SEON is the C/N ratio of the salt extractable organic matter extracted with 0.5 M K2SO4.
- fSOC:SON ratio is the C/N ratio of the whole soil.

Simple linear regression and Pearson correlation coefficient (r) between particulate organic matter N (left) and C (right) (POMN and POMC) and the soil N supply to wheat during a 42 d period for each replicate in sandy loam and silty clay soils (n = 30) as affected by a 3-y history of annually applied N-P-K fertilizer (NPK), liquid swine manure (LSM), liquid dairy cattle manure (LCM) ,or solid poultry litter (SPL) applied at 90 kg available N (ha · y)−1, and a zero-N control (CTL).
4 Discussion
4.1 Labile OM concentrations
Fertilized soils in this study exhibited a range of C and N concentrations in labile OM fractions. The WEON was the only fraction that supported the hypothesis of higher labile organic N concentration in SPL-amended soils than other fertilizer treatments, implying that SPL increased the WEON concentration. However, there was more hot-WEON and SEON in the LCM-amended silty clay soil than the SPL treatment, which suggests that 50°C water extraction and salt extraction procedures retrieve potentially soluble substances such as microbial by-products (Courtier-Murias et al., 2013) that cannot be extracted with 20°C water. Lack of difference among fertilizer treatments was not unexpected because soil samples were collected in the spring after the field plots—which were not planted with a cover crop after wheat harvest—were subjected to 7 months of high precipitation (rainfall and snow), freeze–thaw cycles during winter and spring snowmelt. In this humid temperate climate, such conditions are expected to result in N losses through runoff, leaching, and in gaseous emissions (Rasouli et al., 2014), which appears to deplete the C and N concentrations in labile OM fractions towards baseline levels, regardless of the fertilizer history.
4.2 Labile OM as an indicator of SNS
In a humid temperate climate, it is considered practical to perform soil test analyses for N at pre-planting (e.g., in the week before the field is seeded) to predict the SNS to field crops during vegetative growth stages when crop N demands are high. This was the rationale for measuring labile OM fractions prior to beginning the controlled environment study with wheat. Although the SNS after 42 d was not affected by fertilizer treatments, the variation in labile OM fractions across 5 fertilizer treatments in two soil textures permitted correlation analysis. The best indicators of the SNS were the POMN and POMC concentrations, which is consistent with our hypothesis, but the SOC content in the whole soil was also strongly correlated with the soil N supply and slightly better than the hot-WEON concentration. It seems logical that soils with higher SOC content also possess greater POMN, POMC, and other labile OM fractions. The question is whether we should be measuring the labile OM fractions to predict SNS or whether whole soil measurements provide insight into the SNS.
The fact that POMN was the strongest indicator of the SNS in this study is consistent with findings in soils amended with crop residues from W Canada (St. Luce et al., 2011), E Canada (St. Luce et al., 2014) and a loam soil with a 13-y history of solid beef cattle manure in E Canada (Sharifi et al., 2008). Since the top two indicators of SNS were based on the POM fraction, it suggests that the biological processes that are responsible for producing mineral N are controlled by POM. Two distinct possibilities emerge: (1) the POM fraction is a source of organic N that is transformed into mineral N; and (2) the POM fraction is an accessible organic C substrate for microbial biomass that controls N mineralization from other sources (e.g., WEON). The first possibility may be supported in the silty clay soil, which was a N-rich substrate with a low POMC:POMN ratio of 11 to 12, larger microbial biomass C and N and a greater SNS than the sandy loam soil. On the other hand, the POMC:POMN ratio ≥ 25 in the sandy loam soil implies that POM is primarily a C substrate and so N mineralization is likely occurring from other labile OM fractions. Given the rapid turnover of soluble OM fractions, the hot-WEON fraction warrants further consideration as an indicator of SNS.
5 Conclusion
Although correlation does not imply causation, it does identify relationships that merit further study, which seems to be the case for the pre-planting concentrations of POMN, POMC, and hot-WEON that could be indicators of SNS in fertilized soils from the Saint Lawrence River Lowlands in Quebec, Canada. We recommend further studies of these labile OM fractions in the context of developing a robust indicator of SNS in manure-amended soils, which will necessitate in-field testing in this and other regions with humid temperate climates. Still, we require research using 13C and 15N enriched OM (e.g., soil OM, crop residues, and manure) to describe the dynamics of the labile organic OM fractions in relation to the SNS for crops. We need to know which labile OM fractions contribute directly to the SNS (i.e., they are transformed into mineral N) and which ones exert an indirect effect on the soil N supply through coupled C-N cycling.
Acknowledgements
We appreciate the helpful comments from two anonymous peer reviewers, who evaluated this manuscript, and the Soil Ecology Research Group (SERG) at McGill University for critical review of earlier versions of this manuscript. Thanks to the staff of Les Moulins de Soulanges, Universite Laval, and Agriculture and Agri-Food Canada for maintaining the experimental sites and to David Burton for advice on the experimental design. Special thanks to Johanne Tremblay, Hicham Benslim, and Hélène Lalande for their technical assistance. This project was funded by a Natural Sciences and Engineering Research Council Discovery Grant to M. Sharifi.




