The use of boron isotopes to evaluate boron uptake by rape grown in acid soil treated with boron containing goethite
Abstract
Boron (B), taken up by plants, comes mainly from boron adsorbed by soil constituents, in particular by metal hydrous oxides, organic matter, and edges of clay minerals. The extent and availability of B adsorbed or occluded by soil minerals is unknown due to the lack of a methodology for probing activity of this type of boron. In this study, 10B labeled boron-containing goethites, i.e., goethite with adsorbed B (ad-B-goethite) and occluded B (oc-B-goethite), were added individually to an Ultisol for pot experiments to probe soil B bioavailability. The fraction of soil B extracted from B-containing goethite showed a sigmoidal extraction pattern similar to that of B adsorbed on soil minerals. The rape seedling uptake of B from ad-B-goethite treatment of soil was close to that from soil background (50%), while that from oc-B-goethite treatment of soil was about 66%. The B absorbed from both B-containing goethite and soil was mainly accumulated in the shoot; less than a tenth of the B was accumulated in the root. In summary, the behavior of B in B-containing goethite was generally similar to that of soil B, indicating that B containing goethite can be used to probe migration of B from soil to plant.
1 Introduction
Boron (B) is an essential micronutrient for plants, with its uptake being controlled by the B concentration in the soil solution, which is buffered by the adsorption and desorption reactions of B adsorbed on mineral surfaces (Majidi et al., 2010). Acidic soils in which B is deficient for plant growth are widespread in China. Thus, desorption of B from B containing goethite applied to B deficient acidic soils plays an important role in the plant availability of soil B. Boric acid is a very weak monobasic acid with a pKa of 9.2. In acidic environments, B prevails as non-ionized H3BO3 species and has little propensity for adsorption onto negatively charged soil colloids (Zhu et al., 2000). Under conditions of high rainfall, B leaches from the surface of light-textured soils, which results in B deficiency in these soils (De, 1997).
The adsorption capacity of B in soils is dependent on the composition and properties of soil charge. While not adsorbing strongly on negatively charged sites, B can adsorb onto metal hydrous oxides, and the oxides thus may play an important role in B adsorption behavior (Elrashidi and O'Connor, 1982; Gu et al., 1994; Chen et al., 2009). Organic matter (OM) also strongly and more extensively binds B than negatively charged clay minerals (Yermiyahu et al., 1995). Adsorption capacity of B decreases with the decrease of clay content and CEC of such soils (Abu-Sharar et al., 2014). Goethite (α-FeOOH) is one of the most abundant metal hydrous oxides in soils and has been used as a model system in numerous studies of the interaction of cations, anions or organic substances with mineral surfaces (Antelo et al., 2007).
Liao et al. (2007) evaluated the effect of B on Mn2+ adsorption on goethite using synthesized goethite and two types of B-containing goethite: adsorbed B goethite (ad-B-goethite) and occluded B goethite (oc-B-goethite). Boron adsorption behavior on reactive surface hydroxyl groups of oxides is commonly simulated using the constant capacitance model (Goldberg et al., 2000). Su and Suarez (1995) reported for the first time the coordination of adsorbed B on mineral surfaces and found that both B(OH)30 and B(OH)
 species are adsorbed via a ligand exchange mechanism. Both inner-sphere and outer-sphere surface complexes on amorphous Fe-oxide were observed by ATR-FTIR spectroscopy (Peak et al., 2003). FTIR showed that both trigonal and tetrahedral B were present when B reacted with goethite, and the ratio of tetrahedral B to trigonal B increased with increased reaction time (Cheng et al., 2002).
species are adsorbed via a ligand exchange mechanism. Both inner-sphere and outer-sphere surface complexes on amorphous Fe-oxide were observed by ATR-FTIR spectroscopy (Peak et al., 2003). FTIR showed that both trigonal and tetrahedral B were present when B reacted with goethite, and the ratio of tetrahedral B to trigonal B increased with increased reaction time (Cheng et al., 2002).
Relatively few researches have been conducted on the availability of B to plants and the B distribution in crops from applied fertilizers (Pfeffer et al., 2001; Konsaeng et al., 2010). Despite previous studies of B adsorption by oxides (Goldberg and Glaubig, 1985, 1988; Goldberg et al., 1993; Keren and Gast, 1983), little is known about the behavior of B bound to oxide in variable charge soils, which is vital to the understanding of availability and uptake of soil B. Recently, studies of the properties and reactivity of adsorbed (surface) and occluded (inner) B in goethite and manganite have been conducted. These synthetic minerals were used to simulate B-containing oxides in soil, had relatively high heterogeneous activity (Liao et al., 2007; Liu et al., 2010; Liao et al., 2011c), and influence the B fraction in soil (Ren et al., 2014). Boron-containing oxides can also mitigate toxicity of acid soil and have high B uptake efficiency by rape seedlings (Liao et al., 2011a, 2011b; Cui et al., 2010; Ren et al., 2009, 2010, 2014). However, the behavioral similarity of synthetic boron-containing goethite to soil B has not been carefully evaluated.
A combined study of B concentrations and isotopic ratios has been used to illustrate B behavior during water-rock and soil-plant interactions (Komor, 1997; Rose et al., 2001; Williams et al., 2001; Perica et al., 2001; Franco et al., 2009; Panagopoulos, 2009; Boaretto et al., 2011; Lemarchand et al., 2012; Louvat et al., 2014). The content of B is only 0.001% of the Earth's crust. Boron has two stable, naturally occurring isotopes: 11B and 10B, with mean relative abundance of 80.182% and 19.822%, varying from 81.073% to 79.663% and 18.927% to 20.337%, respectively (Rosman and Taylor, 1998). By using the significant shifting back and forth of natural B isotopic compositions (δ11B), a precise quantification of respective contributions of reacting sites to B being released during biotite weathering (Voinot et al., 2013) and of the recharge of sites was obtained (Cary et al., 2013).
In most cases, the plant availability of B is examined indirectly through crop yields or is based on the total B content of succeeding crops. The utilization rate of B fertilizer is often measured through the difference in amounts of B uptake by crops in the presence and absence of B fertilizer. However, this approach results in poor precision since crops receive B not only from B fertilizer but also from soil. Consequently, there is uncertainty whether the source of B was the B fertilizer, the soil or both. Techniques, such as isotope labeling, are often used to address such problems (Waltar, 2004). Franco et al. (2009) used 10B-labeled fertilizers to investigate the absorbed B distribution in sugar cane and the utilization of B from fertilizers.
Since it is difficult to isolate B-containing oxides from soil, we synthesized two types of 10B-labled boron-containing goethite and applied them to an acid soil in which the growth of rape was investigated. The goal was to follow the release of B from 10B-labeled goethite in soil, to monitor the B uptake in rape, to calculate the plant available B, and estimate the utilization of B in the B-containing goethite.
2 Material and methods
2.1 Properties of soil sample
The soil sample (0–20 cm depth) was collected from a rich citrus orchard in Hunan Province of China (E 113°6', N 28°12′). The soil is an Ultisol with kaolinite and hydromica as major clay minerals. The sample was hand-picked to remove discrete plant material, sieved moist (< 5 mm), air-dried, and mixed uniformly. The soil was chemically and physically analyzed prior to use according to standard methods (Bao, 2000). Organic matter and alkalized N were determined by the wet oxidation method and alkali-hydrolyzed reduction diffusion method, respectively. Available P was extracted by NH4F-HCl and was determined by using the molybdate blue spectrophotometric method. Exchangeable K was extracted by NH4OAc and measured by atomic absorption spectrometry (AAS). Total B was digested with HCl-HNO3 and hot water soluble B (HWSB) was extracted with boiling deionized water, and B was determined using the curcumin colorimetry method. The soil pH (1:1 soil-to-water) was analyzed by using a pH meter, the exchangeable cation capacity (CEC) was determined by extraction with 1 M NH4OAc and analysis using AAS, and the amorphous Fe was extracted with oxalic acid NH4-oxalate (pH 3.3) and determined by using AAS. The soil mechanical composition was determined with the pipette method.
The analytical data showed that the soil sample contained 20.93 g kg−1 OM, 84.48 mg kg−1 alkalized N, 19.02 mg kg−1 available P, 179.9 mg kg−1 exchangeable K, 21.70 mg kg−1 total B, 0.40 mg kg−1 hot water soluble B, and 355.1 mg kg−1 amorphous Fe, respectively. The soil also had a pH of 5.61, CEC of 12.73 cmolc kg−1, exchangeable Ca of 9.30 cmolc kg−1, exchangeable Mg of 0.87 cmolc kg−1, clay content of 22.6%, silt content of 39.7%, and sand content of 37.7%. Since to the citrus orchard Ca-superphosphate as phosphate fertilizer had been applied chronically, which carried a lot of S to soil, the S content of soil sample was not determined.
2.2 Preparation of 10B labeled B-containing goethite
The original goethite was prepared according to the method of Atkinson et al. (1967). It was prepared by titrating 5 L of 0.15 M Fe(NO3)3 with 2.5 M NaOH at a rate of 5 mL min−1 to pH 11.9. The suspension was then equilibrated in a closed polyethylene bottle at 60°C for 48 h.
Goethite with adsorbed boron (ad-B-goethite) was prepared by treatment of the original goethite with 0.01 M borax solution (solid:liquid mass of 1:5) at room temperature for 24 h with shaking (Wang et al., 2006). 10B labeled ad-B-goethite was obtained by the same process except K210B4O7 · H2O (10B abundant 93.6%) solution replacing borax solution. The goethite with occluded B (oc-B-goethite) was prepared by titrating 5 L of 0.15 M Fe(NO3)3 with a mixture of 0.75 M boric acid and 2.5 M NaOH (Wang et al., 2006). 10B labeled oc-B-goethite was synthesized using H310BO3 (10B abundant 93.6%) instead of unlabeled boric acid. Other steps were identical to the preparation of unlabeled oc-B-goethite.
The two labeled substances were filtered and washed several times with deionized water until the conductivity of the suspensions was < 1×102 μS cm−1, centrifuged, dried at 60°C, sieved moist (< 0.154 mm), and stored in a desiccator. Total B was determined after preparation of ad-B-goethite and oc-B-goethite. The analytical data showed that ad-B-goethite and oc-B-goethite contained 282.9 mg kg−1 and 394.4 mg kg−1 B, respectively.
2.3 Rape seedling pot experiments
Four trials were designed in the pot experiments. The trials included: (1) ad-B-goethite treated soil with rape growth; (2) ad-B-goethite treated soil without rape growth, as control treatment; (3) oc-B-goethite treated soil with rape growth; and (4) oc-B-goethite treated soil without rape, as control treatment. Portions of the conditioned soil, each containing 250 g soil, 1.10 g B-containing goethite (getting 311 µg B for soil treated with ad-B-goethite and 434 µg B for soil treated with oc-B-goethite), and fertilizers containing 0.15 g N and 0.17 g S kg−1 soil [0.714 g (NH4)2SO4 kg−1 soil], 0.065 g P kg−1 soil (0.285 g KH2PO4 kg−1 soil), and 0.124 g K kg−1 soil (0.285 g KH2PO4 and 0.081 g KCl kg−1 soil), were weighed into 250 mL paper cups after uniform mixing. Rape seeds were sown into the soil cups on November 13, 2009, while controls received no seeds. The rape seedlings were harvested alternately from February 9 to March 13, 2010. There were 14 paper cups in each trial treated with boron-containing goethite and 5 paper cups in each control trial. Following germination, three rape seedlings were maintained within each cup. During the rape seedling growth period, no extra fertilizers were applied, only deionized water was used to irrigate plants at intervals to sustain the soil sample at 70% filed capacity.
2.4 Soil sampling and plant harvest for B analysis
Soil samples of about 20 g were taken at random from each cup at intervals for analysis between December 2, 2009, and March 28, 2010. Two seedlings were randomly selected from each treatment after 86 d of growth, between February 2 and March 28, 2010. Harvested seedlings were washed with deionized water, dried at 60°C, and the dry weights of shoots and roots were determined. Air-dried soil samples were sieved through 20 mesh and homogenized for B analysis.
HWSB in the soil with B-containing goethite was extracted for 5 min into boiling deionized water at a water : soil ratio of 2:1 contained in an aluminum container. This was followed by addition of two drops of 1 M CaCl2 and deionized water to make up the weight to its initial value before the container was heated. The suspension was filtered into dried plastic containers for storage and analysis. Extraction of HWSB of B-containing goethite was similar to that described for HWSB in soil except that a water:solid ratio of 20 mL : 0.1 g was used. Total B in B-containing goethite was digested by 1 M HCl at an acid:goethite ratio of 100:1. The digested suspensions were brought to a final volume of 100 mL with deionized water. The suspensions were filtered and stored in plastic containers prior to determination.
The B concentration of the filtrates was then determined according to the standard method (Bao, 2000). The HWSB of ad-B-goethite and oc-B-goethite were 163.1 mg kg−1 and 182.0 mg kg−1, respectively, and the total B content of ad-B-goethite and oc-B-goethite were 216.5 mg kg−1 and 238.8 mg kg−1, respectively.
Boron in plant tissue was obtained by shaking 0.2 g plant tissue with 20 mL 1 M HCl at 25°C for 2 h. The suspensions were filtered into dried plastic containers for storage and determination.
Boron concentration of soil and plant samples was determined according to standard methods (Bao, 2000). Boron isotopes were determined by inductively coupled plasma-mass spectroscopy (ICP-MS, Mode: ELAN-DRC-e, Producer: Perkin Elmer).
2.5 Data treatment
The %10B abundance determined by ICP-MS was transformed into the atom %10B excess. The data set was analyzed using Excel 5.0. Statistical analyses and correlation analyses were performed using SAS 8.1.
10B atom percent excess = 10B abundance in samples – 10B abundance in nature,
()where 10B abundance in samples was detected by ICP-MS and 10B abundance in nature is 19.8%.
BDFG% = (Epl / Eg) × 100,
()BG = (Epl / Eg) × Bpl,
()where Epl is 10B atom percent excess in the plant, Eg is 10B atom percentage excess in B-containing goethite, and Bpl is the amount of B taken up by the plant.
BU (%) = (Bpl × Epl / Bg × Eg) × 100,
()where Bpl is the amount of B taken up by the plant, Bg is the total amount of B in B-containing goethite, Epl is 10B atom percentage excess in the plant, and Eg is 10B atom percent excess in B-containing goethite.
BR = (Bs × Es/ Eg) × 100,
()where Bs is the amount of B derived from the soil, Es is 10B atom percent excess in soil, and Eg is 10B atom percent excess in B-containing goethite.
3 Results
3.1 10B concentrations in soil and plant
Hot water soluble 10B (HWS10B) in soil changed with time as 10B was released from 10B labeled goethite and was taken up in rape seedlings. For the four treatments, HWS10B in soil increased relative to that in the original soil. Except for oc-B-goethite without rape growth, HWS10B in soil remained stable for up to 98 d and then increased (Fig. 1). Soil with added ad-B-goethite or oc-B-goethite and with rape growth showed higher HWS10B than soil without rape growth.
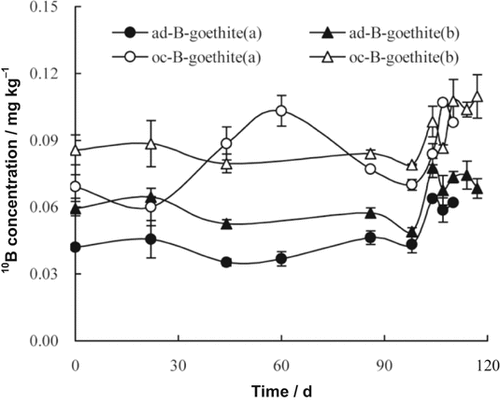
Changes of hot water soluble 10B (HWS10B) in soil treated with ad-B-goethite and oc-B-goethite without rape growth (a) and with rape growth (b).
For soils with rape growth, 10B concentration of shoot decreased and the concentration difference between ad-B-goethite soil treatment and oc-B-goethite soil treatment was consistently 3 mg kg−1. There were two peaks in 10B concentration of shoot in the test time range occurring at days 104 and 110, while 10B concentration of root decreased rapidly and remained largely stable after 107 d of growth (Fig. 2). Unlike the shoot, the 10B root concentration difference between ad-B-goethite soil treatment and oc-B-goethite soil treatment was not obvious.
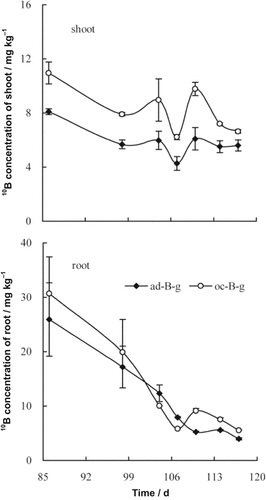
Changes of 10B concentration of shoot and root of rape seedlings grown in ad-B-goethite treated soil and oc-B-goethite treated soil
3.2 Boron release from B-containing goethite
Application of B-containing goethite to soil led to an increase of soil hot water soluble B (HWSB). In order to measure plant availability of B in B-containing goethite, the total HWSB amount, instead of concentration, was also considered in the analysis of B in soil and rape. The amount of HWSB from B-containing goethite that remained in soil (BR) increased and changed with time for the four treatments (Fig. 3). With rape growth, soil with ad-B-goethite addition yielded BR that varied from 11.34 to 18.11 μg, while BR for soil with oc-B-goethite addition varied from 18.52 to 29.09 μg. Without rape growth, BR ranged from 8.23 to 14.94 μg for ad-B-goethite soil treatment and from 9.27 to 16.32 μg for oc-B-goethite soil treatment. Soil with rape growth showed a higher content of HWSB compared with the control without rape growth. There was an abrupt rise in BR after 98 d of growth for all of the treatments.
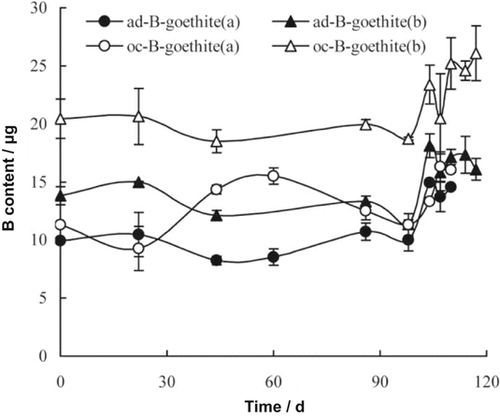
Hot water soluble B remaining (BR) in soil treated with ad-B-goethite and oc-B-goethite, without rape growth (a) and with rape growth (b).
3.3 Boron absorption by rape from B-containing goethite
Boron-containing goethite was not the sole source of B for the growth of rape, soil was also a source, both involving labeled (10B) and unlabeled (11B) B. In the calculation of B uptake in the pot experiments, the first sampling day was affirmed as the origin day (day 0) and the sampling was continued for 32 d in all (Fig. 4). As for ad-B-goethite treated soil with rape growth, boron uptake from the mineral and from soil were stable before day 12 but increased significantly thereafter along with total B in the rape seedlings. The oc-B-goethite treated soil with rape growth showed a gradual increase of B uptake after day 18. In the soils treated with ad-B-goethite or oc-B-goethite and with rape growth, the curves of total B uptake by rape, and of B uptake from B-containing goethite and uptake from untreated soil, all showed a sigmoidal model (Fig. 4).
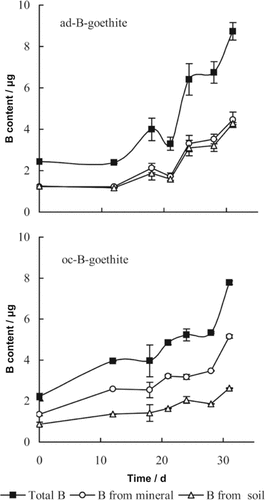
Uptake of B by rape from soil treated with boron-containing goethite in the form of ad-B-goethite and oc-B-goethite. The first sampling time was as origin (day 0). Note: Total B in rape (µg) = content of B in shoot (µg) + content of B in root (µg). Content of B in shoot / root (µg) = concentration of B in shoot / root (µg g−1) × dry weight of the shoot / root (g). B in rape derived from boron-containing goethite (µg) = Total B in rape (µg) × BDFG of plant.
3.4 Boron uptake in plants
Rape grown in ad-B-goethite treated soil absorbed an equal amount of B from the ad-B-goethite and from the soil. For rape grown in oc-B-goethite treated soil, 66% of the plant B was derived from oc-B-goethite, while only 34% was from soil (Fig. 4). In shoots, the proportions of B were 50% to 54% and 62% to 67% for soil treatments with ad-B-goethite and oc-B-goethite, respectively (Table 1). However, the corresponding proportions for the roots were 43% to 49% and 40% to 57%. The maximum B utilization of ad-B-goethite was nearly 2.06%, which was almost the same as that for oc-B-goethite (2.15%).
| Treatment | Date | Total B / μg | BDFG / % | BU / % | |||
|---|---|---|---|---|---|---|---|
| shoot | root | plant | shoot | root | |||
| ad-B- goethite | 26.02.2010 | 2.20 ± 0.06 | 0.20 ± 0.07 | 49.53 ± 0.09 | 50.41 ± 0.17 | 42.88 ± 0.73 | 0.57 ± 0.00 |
| 09.03.2010 | 2.07 ± 0.11 | 0.32 ± 0.07 | 51.36 ± 0.24 | 52.36 ± 0.16 | 44.77 ± 0.99 | 0.57 ± 0.01 | |
| 15.03.2010 | 3.30 ± 0.44 | 0.70 ± 0.10 | 53.35 ± 1.65 | 54.54 ± 1.63 | 47.72 ± 1.62 | 0.98 ± 0.10 | |
| 18.03.2010 | 2.71 ± 0.30 | 0.59 ± 0.02 | 51.91 ± 0.37 | 52.72 ± 0.66 | 48.16 ± 1.24 | 0.79 ± 0.08 | |
| 21.03.2010 | 5.62 ± 0.78 | 0.78 ± 0.02 | 51.69 ± 0.08 | 52.14 ± 0.14 | 49.49 ± 0.16 | 1.53 ± 0.18 | |
| 25.03.2010 | 6.03 ± 0.54 | 0.72 ± 0.02 | 52.32 ± 0.58 | 52.73 ± 0.79 | 49.03 ± 0.82 | 1.63 ± 0.11 | |
| 28.03.2010 | 8.14 ± 0.40 | 0.59 ± 0.03 | 51.01 ± 1.81 | 51.22 ± 1.92 | 48.11 ± 0.35 | 2.06 ± 0.17 | |
| oc-B- goethite | 26.02.2010 | 2.02 ± 0.20 | 0.20 ± 0.04 | 60.74 ± 1.40 | 62.86 ± 2.24 | 39.70 ± 0.34 | 0.66 ± 0.03 |
| 09.03.2010 | 3.65 ± 0.07 | 0.30 ± 0.08 | 65.48 ± 0.13 | 67.18 ± 0.19 | 44.28 ± 2.07 | 1.08 ± 0.00 | |
| 15.03.2010 | 3.61 ± 0.77 | 0.35 ± 0.00 | 64.47 ± 3.24 | 65.92 ± 3.74 | 49.97 ± 1.10 | 1.06 ± 0.16 | |
| 18.03.2010 | 4.20 ± 0.10 | 0.65 ± 0.00 | 66.37 ± 0.39 | 67.75 ± 0.64 | 57.38 ± 1.37 | 1.34 ± 0.03 | |
| 21.03.2010 | 4.85 ± 0.31 | 0.38 ± 0.02 | 60.75 ± 1.03 | 61.75 ± 1.19 | 48.12 ± 0.36 | 1.33 ± 0.05 | |
| 25.03.2010 | 4.84 ± 0.06 | 0.50 ± 0.02 | 65.13 ± 0.01 | 66.28 ± 0.01 | 53.88 ± 0.61 | 1.45 ± 0.01 | |
| 28.03.2010 | 7.15 ± 0.11 | 0.62 ± 0.02 | 66.23 ± 0.23 | 67.07 ± 0.17 | 56.62 ± 0.52 | 2.15 ± 0.03 | |
3.5 Relationships among B content in soils and plants
Correlation analyses were undertaken to determine the relationships between total soil B content, soil B content from B-containing goethite, rape B content, and rape B derived from B-containing goethite (Table 2). The results show that total soil B content was significantly correlated with soil B in ad-B-goethite treated soil with rape growth (r2 = 0.995, P < 0.01) and in oc-B-goethite treated soil with rape growth (r2 = 0.944, P < 0.01), respectively. And total rape N content showed high correlation with rape boron content in ad-B-goethite treated soil (r2 = 0.999, P < 0.01) and in oc-B-goethite treated soil (r2 = 0.987, P < 0.01), respectively. It was found that after rape growth, soil B content was not correlated with rape boron content in the soils treated with ad-B-goethite or oc-B-goethite.
| B-containing goethite | Factors | Correlation coefficients | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| ad-B-goethite | 1 Total B content in soil | – | |||
| 2 Soil B content deprived from ad-B-goethite | 0.9948** | – | |||
| 3 Total B content in the rape | 0.3086 | 0.3459 | – | ||
| 4 Rape B from ad-B-goethite | 0.3335 | 0.3710 | 0.9988** | – | |
| oc-B-goethite | 1 Total B content in soil | – | |||
| 2 Soil B content deprived from oc-B-goethite | 0.9441** | – | |||
| 3 Total B content in the rape | 0.4031 | 0.4760 | – | ||
| 4 Rape B from oc-B-goethite | 0.3131 | 0.3958 | 0.9870** | – | |
- aNote: * and **—significance of correlation at the 0.05 and 0.01 probability levels, respectively.
4 Discussion
Boron behavior in the soil and vegetation cycle is difficult to characterize (Louvat et al., 2014). Plants respond only to B in the soil solution (Keren et al., 1985), and it has been shown that B-containing goethite increased the available B content of soil and, thus, also of the soil solution (Ren et al., 2009, 2014; Cui et al., 2010). In this study, for soil treated with ad-B-goethite without rape growth, the B that remained in soil (BR) increased at a rate of 0.02–0.08 μg d−1 for the initial 98 d and at the rate of 0.2–0.8 μg d−1 thereafter. The soil with oc-B-goethite without rape growth demonstrated a higher rate, 0.07–0.2 μg d−1 in the initial 98 d period and 0.3–1.0 μg d−1 thereafter. However, for soils with rape growth, BR increased by the rate of 0.02–0.05 μg d−1 in the initial 98 d period and 0.05–1.2 μg d−1 thereafter for ad-B-goethite, while the corresponding ranges for oc-B-goethite were 0.01–0.03 μg d−1 and in 0.5–1.6 μg d−1, respectively. In the study by Xie et al. (2011), who synthesized a slow-release N and B fertilizer (SNBF), it was shown that the boron in SNBF released 10.7, 60.1, and 95.4% within 1, 3, and 10 d, respectively, which was consistent with a sigmoid release pattern. Our study of soils treated with synthesized B-containing goethite also followed the sigmoid release pattern. Apparently, after mixing the B-containing goethite with soil, equilibration reactions lead to B stabilization and subsequent release by an S-shaped extraction curve.
Ligand exchange with surface hydroxyl groups has been invoked as the mechanism of B adsorption on Al- and Fe-oxides (Goldberg and Glaubig, 1985). Goldberg (1999) defined two reactions based on spectroscopic observations and interpreted the adsorption of B(OH)30 and B(OH)
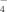 in terms of the constant capacitance model. In the formation of B-containing goethite, hydroxyl groups in the surface of the mineral would react with H3BO3 to form surface complexes of B(OH)30 and B(OH)
in terms of the constant capacitance model. In the formation of B-containing goethite, hydroxyl groups in the surface of the mineral would react with H3BO3 to form surface complexes of B(OH)30 and B(OH)
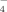 . Since the B released from B-containing goethite (BR) follows a sigmoidal curve, the release of B from the B-containing goethite (BR) might be rationalized as follows: (1) On contact with soil solution after addition of the minerals to the soil, the external surfaces of the B-containing goethite hydrate and swell slowly. Slow release of B adsorbed on the external surfaces occurs. (2) As hydration continues, more surfaces are exposed to the acidic soil solution, the weakly adsorbed surface complex B(OH)30, which dominates adsorbed B are rapidly released into free soil water (Goldberg and Glaubig, 1985). (3) Eventually, the release of B slows as available surface B is depleted and B is released from inner surfaces by slow diffusion processes.
. Since the B released from B-containing goethite (BR) follows a sigmoidal curve, the release of B from the B-containing goethite (BR) might be rationalized as follows: (1) On contact with soil solution after addition of the minerals to the soil, the external surfaces of the B-containing goethite hydrate and swell slowly. Slow release of B adsorbed on the external surfaces occurs. (2) As hydration continues, more surfaces are exposed to the acidic soil solution, the weakly adsorbed surface complex B(OH)30, which dominates adsorbed B are rapidly released into free soil water (Goldberg and Glaubig, 1985). (3) Eventually, the release of B slows as available surface B is depleted and B is released from inner surfaces by slow diffusion processes.
Comparison of soil treated with B-containing goethite, with and without rape growth, showed that soil planted with rape contained higher HWSB derived from B-containing goethite (Figs. 1 and 3). This is likely to be due to the solubilizing effect of the root exudates containing many organic acids that would have diffused into the soil. This process would lead to more unavailable B (including B from B-containing goethite and from soil) being transformed into available B. In our study, there was no correlation between total B content in soil and plant B content (Table 2) although B-containing goethites were applied to soil. Ren et al. (2009) have shown that HWSB was correlated with rape boron content (r2 = 0.898, P < 0.01). This means that HWSB is still the optimal method for determining availability of soil B. We have proposed that the surface of B-containing goethite minerals added to the soil would be slowly hydrated and swollen by soil solution during the growth of rape. As the goethites are in contact with water, adsorbed B may release into free water due to a concentration gradient with the release rate of B increasing over time, which was more obvious 98 d later (Fig. 1). The adsorbed B in the surface of B-containing goethites was released gradually and then the combined B in the crystal lattice dissolved, which tends to release more boron. The B content of oc-B-goethite was 12% greater than that of ad-B-goethite.
Goethite has often been used as a model oxide to trace the mechanism of ion adsorption (Goli et al., 2011), and biotite was chosen as a test-mineral because it is reactive with acids (Voinot et al., 2013). Boron reacts easily with the surface hydroxyl of minerals in variable charge soils (Goldberg et al., 2000). In our study, we used synthesized B-containing goethite to investigate the release of B in acid soil. The similarity of rape absorption of B from soil with absorption from soil treated with B-containing goethite (Fig. 4) indicated that the behavior of B in B-containing goethite was similar to that of B in soil. Although B gradually accumulated in rape, the proportion of rape B derived from soil treated with B-containing goethite (BDFF%) was constant, around 50% for ad-B-goethite soil treatment and 66% for oc-B-goethite soil treatment (Table 1). The total boron content in rape showed a high correlation with rape boron from B-containing goethite (Table 2), indicating that the boron released from the goethite had been taken up by the rape.
Using XRD analysis, Liao et al. (2007) concluded that B in ad-B-goethite was adsorbed simply on the surface of goethite, but in the case of oc-B-goethite, the B might enter the lattice of goethite. It might be expected that B would be more readily released from the goethite's surface than from its lattice. In our study, more HWSB was released from oc-B-goethite than from ad-B-goethite implying that either the uptake of B had destabilized the lattice in the freshly prepared material that was used or that the observed reduced crystallinity and corresponding increased surface area of the oc-B-goethite gave rise to more of the readily available adsorbed B (Liao et al., 2007).
As shown in Table 1, most of B assimilated by plants was distributed in the shoot. Less than a tenth of boron was distributed to the root. Given that 50% and 66% of the B taken up by rape were absorbed from ad-B-goethite and oc-B-goethite, respectively, B-containing goethite can provide a major source of boron for plant. In our experiments, the bioavailability of ad-B-goethite and oc-B-goethite in the growth season of rape were 2.06% and 2.15%, respectively.
In short, the B release mechanism of B-containing goethite can be explained by initial hydration and swelling, rapid release of adsorbed B once contacted by acid soil solution, followed by slow diffusion of B released from inner soil surfaces.
5 Conclusions
In general, the behavior of B in B-containing goethite (ad-B-goethite or oc-B-goethite) in soil is similar to that of soil B. Therefore, ad-B-goethite or oc-B-goethite can be used to imitate migration of B from soil to plant. Boron uptake from B-containing goethite and soil is mainly accumulated in shoot. Less than a tenth of boron is accumulated in root. The rape seedling uptake of B from ad-B-goethite treatment of soil was close to that from soil background (50%), while that from oc-B-goethite treatment of soil was about 66%. The highest boron utilization rates of ad-B-goethite and oc-B-goethite in this work were 2.06% and 2.15%, respectively.
Acknowledgements
This work was supported by the National Science Foundation of China (40973056 and 40371064). The authors are grateful to Dr. Bo Gao and Dr. Linghua Liu (Department of Water Environment, China Institute of Water Resources and Hydropower Research–IWHR), for their help on B-isotopes measurements. We also thank Professor Shoou-Yuh Chang (Department of Civil, Architectural and Environmental Engineering of North Carolina A&T State University, USA), Associate Professor Alan Langdon (Waikato Centre for Advanced Materials, University of Waikato, New Zealand) for their review and comments to this manuscript.




