Segmental defect healing in the presence or absence of recombinant human BMP2: Novel insights from a rat model
Abstract
This study defined and compared the course of native, impaired and growth factor-stimulated bone regeneration in a rat femoral defect model. A mid-diaphyseal defect with rigid internal fixation was surgically created in the right femur of male Fischer rats and serially analyzed over 36 weeks. Native bone regeneration was modeled using a sub-critical, 1 mm size defect, which healed uneventfully. Critical size defects of 5 mm were used to analyze impaired bone regeneration. In a third group, the 5 mm defects were filled with 11 µg of recombinant human bone morphogenetic protein 2 (rhBMP2) impregnated onto an absorbable collagen sponge, modeling its clinical use. Native bone regeneration was characterized by endochondral ossification with progressive remodeling to ultimately resemble intact femora. An endochondral response was also observed under conditions of impaired bone regeneration, but by week 8 medullary capping occurred with fibrofatty consolidation of the tissue within the defect, resembling an atrophic non-union. rhBMP2 treatment was associated with prolonged inflammatory cytokine expression and rapid intramembranous bone formation occurring with reduced expression of cartilage-associated collagens. Between weeks 4 and 36, rhBMP2-treated bones demonstrated decreased trabecular number and increased trabecular separation, which resulted in inferior mechanical properties compared with bones that healed naturally. Clinical Significance: Recombinant human bone morphogenetic protein 2 (rhBMP2) is used clinically to promote healing of long bones. Our data suggest that it drives intramembraneous ossification producing an inferior regenerate that deteriorates with time. Clinical outcomes would be improved by technologies favoring endochondral regenerative ossification.
1 INTRODUCTION
Long bone regeneration consists of multiple sequential and overlapping phases, ultimately resulting in reconstitution of the original structure without a scar. Following osseous disruption, bleeding from the bone marrow and surrounding tissue triggers the coagulation cascade and initiates the formation of a hematoma about the bone ends, serving to both contain and attract immune responders to the site of injury.1 These cells release growth and chemotactic factors to transform the provisional fibrin-rich hematoma into a vascular fibrous callus and recruit progenitor cells to the site of skeletal disruption. Centrally within the callus, where oxygen tension is low and local tissue strains are high, mesenchymal progenitors undergo chondrogenic differentiation to initiate endochondral repair. The bridging of the disrupted bone ends by a soft, cartilaginous callus confers a moderate degree of stability to permit revascularization and transformation of the soft callus, via chondrocyte hypertrophy and apoptosis2 or through direct chondrocyte–osteoblast transdifferentiation,3 to bone through endochondral ossification. Within lacunae of newly formed bone, terminally differentiated osteocytes coordinate bone formation by osteoblasts and resorption by osteoclasts via mechanosensitive and secreted mechanisms to remodel the hard callus into a form resembling the native structure.
Despite bone's innate regenerative capacity, large segmental defects in long bones fail to heal. These circumstances result in osseous non-union and represent a substantial clinical burden. Multiple factors, occurring as inappropriate or untimely components of the overall healing response, have been associated with non-union and broadly include inadequate mechanical stability about the injury site, an elevated or prolonged immune response, impaired vascularization, and insufficient cell and growth factor signaling.4 While certain nonmodifiable factors, including age, sex, and the severity of trauma,5-7 have also been associated with delayed or impaired bone healing, it is not immediately clear why certain skeletal lesions will regenerate while others will not.
Clinical management of these scenarios involves addressing the factors underlying failed bone regeneration and has been crystallized into the “diamond concept.”8, 9 In addition to optimizing the mechanical and vascular environment of the injury site, this paradigm emphasizes multiple biological prerequisites of bone regeneration, including local signalling by growth factors. Much research has been dedicated to the discovery and clinical translation of such biological adjuvants to augment bone regeneration; recombinant, human bone morphogenetic protein 2 (rhBMP2) is the most widely used, clinically-approved osteoinductive agent authorized for this purpose. Early preclinical and clinical studies document the efficacy of rhBMP2 in rodent femoral segmental defects10 and human open tibial fractures,11 respectively. However, contemporary reports note unintended effects of osteoclast activation and osteolysis,12-15 the formation of poorly-quality and cystic bone,16 and the frequency of side-effects,17 some serious, with rhBMP2 treatment.
Considering the outstanding and unexplained differences between natural, impaired, and rhBMP2-stimulated bone regeneration, we sought to characterize and compare their natural course of healing using a rat femoral segmental defect model. We demonstrate that native long bone healing of a sub-critical size segmental defect occurs via endochondral ossification and involves progressive remodeling, resulting in superior mechanical properties compared to rhBMP2-treated bones at long-term time points.
2 MATERIALS AND METHODS
2.1 Surgical procedure
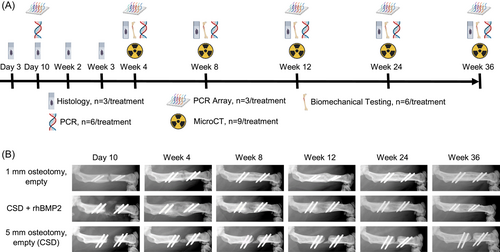
Animal care protocols were approved by the Institutional Animal Care and Use Committee at Mayo Clinic (Protocol A00005057-20). The surgical procedures were performed in skeletally mature male Fischer 344 rats aged 16 weeks, weighing 250–350 g (Charles River Laboratories) as described previously.18-20 Under sterile conditions and maintenance anesthesia with inhaled isoflurane, a 4 cm incision was made on the lateral right thigh to expose the femur. A custom-made, rigid polyether ether ketone plate was affixed to the anterolateral surface of the femur and used to guide four holes drilled with a 0.79 mm bit (RISystems AG). The plate was secured to the femur using four hand-driven threaded K-wires (MicroAire Surgical Instruments). Animals were randomly assigned to receive 1 mm (naturally healing defect) or 5 mm (non-healing, critical size defect (CSD)) femoral osteotomies using a 0.44 mm Gigli wire saw. Upon completion of the osteotomies, all of the 1 mm defects were left empty, whereas the 5 mm defects were left empty or filled with an absorbable collagen sponge (ACS) containing 11 µg of rhBMP2 (Infuse™ Bone Graft; Medtronic). The muscular layer surrounding the defect was closed with 4-0 Vicryl suture; the cutaneous skin incision was closed with 9 mm wound clips. Animals received buprenorphine slow release at 1 mg/kg for analgesia before surgery and 3 days after surgery. Bone regeneration was monitored with radiography using a MX-20 x-ray cabinet system (Faxitron Bioptics).
2.2 Microcomputed tomography (microCT) evaluation
Femora were imaged with a microCT imaging system equipped with a 10 mm focal spot microfocus x-ray tube (vivaCT 40, Scanco Medical AG). All bones were scanned with a 10.5 micron isotropic voxel size at 55 kV, with a 200 ms integration time and analyzed using Scanco proprietary software. The structure of the newly formed bone was segmented using automated threshold contouring. Bone structural and morphometric variables (bone volume fraction (BV/TV), trabecular number (Tb.N), thickness (Tb.Th), and separation (Tb.Sp)) were calculated using the integrated bone analysis scripts in the software.
2.3 Biomechanical testing
Femoral samples designated for biomechanical testing were explanted, cleaned of soft tissue, wrapped in saline-soaked gauze and frozen at −80°C until use. Before testing, frozen femora were thawed at 4°C overnight and tested under torsional loading until failure using a custom-made material testing system (Biomechanics Core Facility, Mayo Clinic) running a LabVIEW script (National Instruments) with a low-capacity torque sensor (Transducer Techniques LLC) generating time series curves of torque versus rotational displacement. A custom MATLAB script was used to define the following variables: torque to failure, rotation at failure, stiffness, and energy to failure; the output variables were normalized to the cross-sectional area of the bone callus. Following biomechanical testing, callus tissue was isolated, snap frozen, and stored in liquid nitrogen for RNA isolation.
2.4 Gene expression analysis
Total RNA was isolated from samples, including regenerated bone for rhBMP2-treated and 1 mm sized defects or capped bone ends for nonhealing 5 mm defects, using a RNeasy Plus Mini Kit (ThermoFisher). Assessment of RNA quantity and quality was undertaken using a NanoDrop spectrophotometer (ThermoFisher) with a 260/280 ratio ≥2.0 used as a threshold for RNA purity; 1 µg of cDNA was synthesized using a high-capacity reverse transcription Kit (AppliedBiosystems). To screen for gene expression changes between treatments and time points, polymerase chain reaction (PCR) arrays were performed for inflammatory, osteogenic, and bone turnover genes (Online Supporting Information). Based on the array data, inflammatory (interleukin-1β [IL-1β]; IL-6; interferon alpha 1, [Ifna1]; IL-2), osteogenic (phosphate regulating endopeptidase homolog X-linked (Phex); bone morphogenetic protein 7, (BMP7); collagen 10a1, (Col10a1); collagen 2a1, (Col2a1), and bone turnover (Dickkopf-related protein 1 (Dkk-1); sclerostin, (Sost), IL-6 receptor, (IL-6R); matrix metalloproteinase 8, (Mmp8) genes were selected for individual confirmation by PCR. Pre-amplification was performed using TaqMan PreAmp Master Mix with 150 µg of cDNA per sampling, according to the manufacturer's instructions (ThermoFisher). Subsequent PCR reactions were performed with 5 µL of the pre-amplified reaction solution using TaqMan Fast Advanced Master Mix (ThermoFisher). Gene expression values were normalized using to the GAPDH housekeeping gene and are depicted as 2−ΔCt.
2.5 Histology
Following euthanasia, femoral samples designated for histology were explanted, cleaned of soft tissue, fixed in 10% neutral buffered formalin for 96 h, decalcified in 10% EDTA for 28 days, processed in a tissue processor, and embedded in paraffin. Five-micron sections were mounted on poly l-lysine-coated slides, dried overnight, and stained with safranin orange and fast green and visualized using an Olympus BX43 microscope with an Olympus SC50 camera (Olympus).
2.6 Statistical analyses
GraphPad Prism 9.2.0 software was used for data analysis. Testing between multiple treatment groups over multiple time points, was performed using a two-way analysis of variance with post hoc testing and correction for multiple comparisons was performed by controlling the false discovery rate using the two-stage step-up method of Benjamini, Krieger, and Yekutieli. An a priori α value of 0.05 was used for all statistical tests.
3 RESULTS
3.1 Defect healing
Figure 1A outlines the study design. As shown by the representative X-ray images in Figures 1B, 5 mm defects failed to bridge but did so when 11 µg rhBMP2 were implanted. The 1 mm defects also bridged spontaneously, but more slowly than the defects treated with rhBMP2 and without a large, early callus.
3.2 Gene expression
A cytokine-predominant inflammatory response was evident with rhBMP2, characterized by elevated expression of IL-1β (Figure 2A). Similarly, after an initial reversal in expression patterns, IL-6 was also elevated with rhBMP2-treated versus naturally healing or non-healing osteotomies at Week 4. After this time point, expression progressively declined in naturally healing osteotomies, while rhBMP2-treatement and nonhealing osteotomies were associated with persistent elevation out to Week 12 and 24, respectively (Figure 2B). Ifna1 expression was initially decreased, but recovered to the level of uninjured bone by Week 12 (Figure 2C). IL-2 transcripts were increased with rhBMP2 treatment at Week 4 following surgery, after this time point expression rapidly declined to undetectable levels in rhBMP2-treated and naturally healing osteotomies; nonhealing defects displayed persistent expression out to Week 8 (Figure 2D).
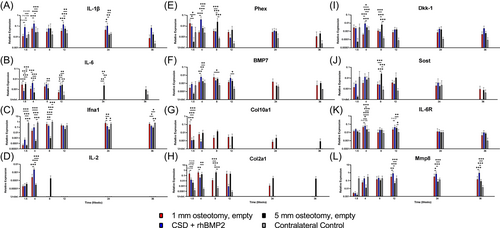
Analysis of osteogenic gene expression demonstrated that rhBMP2-treatment was associated with a peak in Phex and BMP7 expression at Week 4 following surgery (Figure 2E,F). In naturally healing bones, Phex expression gradually decreased after Day 10 while BMP7 expression peaked later, at Week 8 (Figure 2E,F). While expression of BMP7 in nonhealing defects largely mirrored that of naturally healing bones, a distinct peak in Phex expression among non-healing defects was observed at Week 8 following surgery (Figure 2E). Notable differences in the expression patterns of cartilage-associated collagens II and X were observed with and without rhBMP2 treatment. At Day 10 expression of Col10a1 and Col2a1 was up to 100-fold higher in the 1 mm defects compared with the 5 mm defects. Moreover, expression of these collagens rapidly decreased with rhBMP2 treatment after Week 4, while naturally healing bones demonstrated a more progressive decline throughout the 36-week time period; expression in nonhealing bones peaked at Week 8, as Col2a1 was significantly increased above 1 mm and rhBMP2-treated osteotomies (Figure 2G,H).
Expression of bone remodeling genes varied between treatments. While rhBMP2-treated and naturally healing osteotomies demonstrated early increases in Dkk-1 expression, differences in Dkk-1 expression were evident by Week 4, whereby rhBMP2-treated bones upregulated Dkk-1 before returning to the level of naturally healing bones by Week 8 (Figure 2I). Non-healing defects demonstrated a delayed pattern of Dkk-1 expression, peaking above naturally healing, intact contralateral, and rhBMP2-treated bones at Week 8 (Figure 2I). A similar trajectory of expression in non-healing defects was observed with respect to Sost, which was significantly increased above naturally healing, intact contralateral, and rhBMP2-treated osteotomies at Week 8 and remained elevated at Week 12 (Figure 2J). Conversely, expression of IL-6R only variably differed between time points or treatments (Figure 2K). Expression of Mmp8 was below that of the contralateral, intact bone at Day 10, but rapidly increased by Week 4 for all treatments, to the level of unoperated bones. At later time points, beyond Week 12, rhBMP2-treatment was associated with increased expression of Mmp8 above naturally and nonhealing defects (Figure 2L).
3.3 Histology of bone formation
In 1 mm osteotomies, a loose scaffold of connective tissue containing both nucleated cells and anucleate red blood cells (RBCs) was evident between Days 3 and 10 following surgery (Figure 3A,B). By Week 2, the native endochondral regenerative response from peri- and endosteal surfaces was detected, progressing to a bridging cartilage callus spanning the osteotomy ends by Week 3 (Figure 3C,D). Gradual conversion of this structure to a bony callus, with evidence of a transition zone of calcified cartilage, occurred between Weeks 4 and 8, giving way to a well-formed bony callus by Week 12 (Figure 3E–G). A continuous, outer neocortex was detectable by Week 24 ultimately resulting in concentric recortication, with segregated and well-developed inner and outer marrow cavities, by Week 36 following surgery (Figure 3H,I).
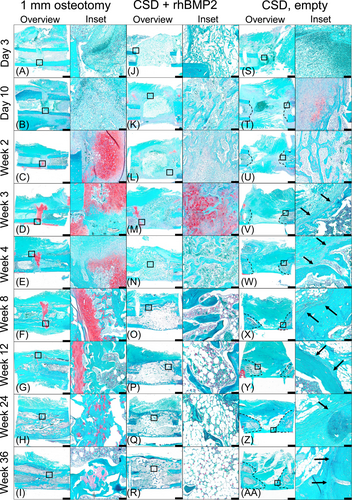
In rhBMP2-treated bones, the formation of a hematoma and cellular infiltration into the defect was largely prevented by the ACS, which occupied the entirety of the osteotomy at Day 3 (Figure 3J). By Day 10, a peripheral layer of calcified tissue formed on the outer edges of the ACS, the center of the sponge remained largely acellular, with small foci of safranin O-positive tissue visible outside of the defect. Between Dday 10 and Week 2, centripetal development of the peripheral calcified tissue was evident; surprisingly, large central portions of the ACS were still sparsely filled with cells (Figure 3K,L). By Week 3, the central portion of the defect was occupied by calcified tissue interspersed with RBCs and remnants of the ACS (Figure 3M). Dense spicules of calcified tissue, lined by presumptive osteoblasts, were observed at Week 4; this tissue was separated by a cellular compartment composed mainly of RBCs (Figure 3N, inset). A notable decline in the density of calcified tissue within the defect occurred between Weeks 4 and 8. By Week 8, a thin rim of calcified tissue outlined the top and bottom boundaries of the defect, with peripherally located marrow cavities (Figure 3O, inset). The central portion of the defect was composed predominantly of fatty marrow. Histology at Weeks 12, 24, and 36 was characterized by a peripheral shell of calcified tissue which gradually transitioned to a central cavity with few trabeculae interspersed with fatty, sparsely cellular marrow (Figure 3P,R).
In non-healing, empty 5 mm osteotomies, large areas of RBCs and nucleated cells integrated into a connective tissue scaffold were present within the defect at day 3 (Figure 3S). Surrounding muscle and fibrous tissue were evident at the top and bottom of the osteotomy gap at day 10, restricting the remaining RBCs and nucleated cells to an area within the defect between the top and bottom cortices. At this time, an endochondral osteogenic response was evident adjacent to the cut ends of the bone (Figure 3T). By Week 2, focal areas of hemorrhage had resolved and were replaced by muscle and fibrous tissue within the defect; the osteogenic response had progressed and areas of woven bone extended within the periphery of the osteotomy gap (Figure 3U). At Weeks 3 and 4, capping of a bone-end, with the presence of a well-developed marrow cavity, was evident (Figure 3V,W, inset). By Week 8, the loose fibrous tissue within the defect had condensed into a vascularized, fibrofatty strip connecting the capped bone ends (Figure 3X). This tissue connecting the capped bone ends progressively thickened between Weeks 12, 24, and 36 (Figure 3Y,AA).
3.4 Microarchitectural bone remodeling
Naturally healing, 1 mm osteotomies demonstrated progressive bridging and re-cortication, while rhBMP2 treatment resulted in a bridging callus by Week 4; bone was formed in empty 5 mm defects but served only to cap the cut ends of the bone without bridging the defect (Figure 4A).
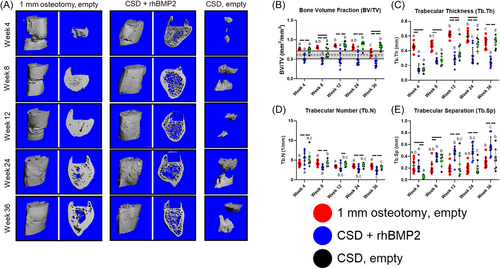
BV/TV of the developing callus peaked at Week 12 and ultimately decreased by Week 36 to a value resembling that of native femora. Conversely, BV/TV in animals treated with rhBMP2 significantly decreased from Week 4 to Week 36, below the range of uninjured femora. In non-healing defects, BV/TV plateaued at Week 12 where it was maintained out to Week 36 (Figure 4B).
In 1 mm osteotomies, Tb.Th increased between Weeks 8 and 12 and remained elevated at Week 24 until Week 36 where it decreased to the level of Week 8. rhBMP2 treatment resulted in a significant increase in Tb.Th between Weeks 4 and 8, that was maintained out to Week 36. Finally, in nonhealing defects, Tb.Th progressively increased between Weeks 4, 8, 12, and 24, where it remained until Week 36 (Figure 4C).
While Tb.N did not differ significantly at any time point in 1 mm osteotomies, rhBMP2 treated defects were characterized by a sequential decrease in Tb.N between Weeks 4 and 36. In nonhealing defects, Tb.N in the capped ends of the defect gradually decreased throughout the course of regeneration, although it did not significantly differ between Weeks 4 and 36 (Figure 4D).
Trabecular separation (Tb.Sp) remained relatively constant throughout healing of 1 mm osteotomies, differing only between Weeks 12 and 36; animals treated with rhBMP2 demonstrated a progressive increase in Tb.Sp throughout the course of healing. Finally, Tb.Sp in the capped ends of the non-healing defects increased between Weeks 4 and 8 where it remained relatively constant until Week 36 (Figure 4E).
3.5 Long-term mechanical properties
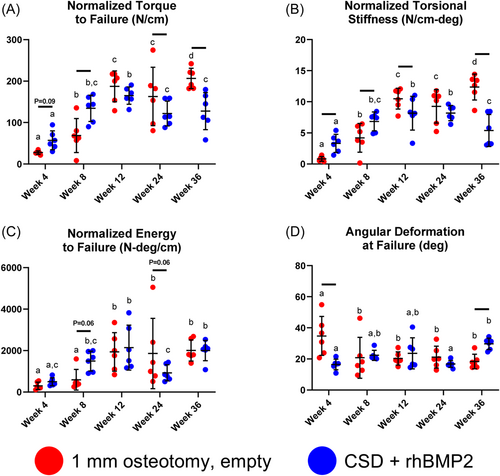
The mechanical properties of bone formed under bridging conditions (1 mm osteotomy and CSD + rhBMP2) were measured under torsional testing at intervals between 4 and 36 weeks following surgery. rhBMP2 treatment was associated with a plateau in normalized torque to failure, normalized torsional stiffness, and normalized energy by 8 weeks; 1 mm osteotomies reached an initial plateau for these values at 12 weeks (Figure 5A–C). By 36 weeks 1 mm osteotomies demonstrated significantly greater normalized failure torque and normalized torsional rigidity compared with rhBMP2 treatment (Figure 5A,B). Angular deformation at failure was significantly greater in 1 mm osteotomies at 4 weeks (Figure 5D).
4 DISCUSSION
Regeneration of the appendicular skeleton usually occurs without interruption; instances where it is impaired represent a substantial clinical burden and have attracted significant research directed towards the identification and clinical translation of osteoinductive growth factors such as rhBMP2. Despite its potent osteogenicity, rhBMP2 is used clinically at high doses that provide incremental benefit and produce poor quality bone. By comparing native, impaired, and rhBMP2-stimulated bone regeneration in a rat model, the objective of this study was to understand the biological basis for these circumstances; a summary of the salient differences between these conditions is provided in Table 1.
| Empty 5 mm defect (CSD) | CSD + rhBMP2 | 1 mm defect | |
|---|---|---|---|
| Early Inflammation | Low | High | Low |
| Gene expression | |||
| Inflammation | |||
| IL-1β | Minor changes | Early, sustained ↑ | Early ↑ , Gradual ↓ |
| IL-6 | Early ↑ , Transient ↓ | Delayed ↑ , Gradual ↓ | Early ↑ , Gradual ↓ |
| Ifn-α1 | Delayed ↑ to levels in native femora | Early ↑ to levels in native femora | Early ↑ to levels in native femora |
| IL-2 | Delayed ↑ , Subsequent ↓ | Large, early ↑ , Subsequent ↓ | Early ↑ , Subsequent ↓ |
| Osteogenesis | |||
| Col10a1 | Small, sustained ↑ | Small, transient ↑ | Large, sustained ↑ |
| Col2a1 | Modest, sustained ↑ | Modest, temporary ↑ | Large, sustained ↑ |
| Remodeling | |||
| Dkk-1 | Delayed ↑ , Gradual ↓ | Large, early ↑ | Minor changes |
| Sost | Delayed ↑ , Gradual ↓ | Minor changes | Minor changes |
| Osteoclast activity | Minimal | Transient ↑ (Weeks 2–4)21 | Minimal |
| Biology of healing | None (capping is endochondral) | Intramembranous | Endochondral |
| Mechanical strength | None | Early increase (plateau at Week 4); subsequent decline | Slow increase (plateau at Week 12); sustained |
| Bone quality | None | Low | High |
- Abbreviations: CSD, critical size defect; IL, interleukin; rhBMP2, human bone morphogenetic protein.
In naturally healing femoral osteotomies, we observed endochondral healing beginning at Week 3 and lasting until Week 12, when naturally healing bones reached their maximal mechanical properties and peak BV/TV. Beyond Week 12, naturally healing bones underwent progressive remodeling, evidenced by changes to BV/TV and Tb.Th, with evidence of re-cortication. rhBMP2-mediated bone regeneration, in contrast, was characterized by early histologic evidence of trabeculated bone spanning the defect with only small, focal areas of cartilage. Mechanical integrity was reached at Week 8, earlier than naturally healing bones, and was accompanied by decreased Tb.N and increased Tb.Sp at this time point. Beyond Week 8, few quantitative changes in mechanical strength and microarchitecture, aside from progressive increases to Tb.Sp, were observed. Ultimately, by Week 36 the failure torque and torsional rigidity of rhBMP2-treated CSD were significantly less than those of naturally healing bones. In nonhealing CSDs, osseous capping of the defect was observed by Week 8, with fibrous, vascular tissue occupying the defect, consolidating into a dense fibrofatty band spanning the osteotomy ends by Week 36. Trabecular features of new bone formed in the capped ends of nonhealing defects were similar to those of rhBMP2-treated bones at early time points, but evolved to more closely resemble naturally healing osteotomies at long-term follow-up.
Bone regeneration in stabilized, mid-diaphyseal segmental defects occurs as a balance of intramembranous and endochondral ossification. Our data confirm endochondral ossification as the predominant manner of native healing in long bones and support the potential for endochondral regeneration in CSD that will not heal. In contrast, genetic and histologic evidence of cartilage in CSD treated with clinically-relevant doses of rhBMP2 was minimal, suggesting regeneration predominantly via intramembranous bone formation. rhBMP2 treatment was also associated with a cytokine-predominant inflammatory response, characterized by elevated expression of IL-1β, IL-6, and IL-2. A similar inflammatory response in naturally healing osteotomies, that regenerate via endochondral ossification, was not detected, suggesting that the local inflammatory environment can influence osteogenic versus chondrogenic differentiation in rhBMP2-stimulated or native conditions of regeneration, respectively. While IL-1β is known to inhibit the chondrogenic differentiation of mesenchymal stromal cells in a dose-dependent fashion,22 IL-6 has been reported to inhibit chondrogenesis alone23 or promote chondrogenesis in the presence of the soluble IL-6 receptor.24 IL-1β induces IL-6 expression and combined treatment with IL-1β and the soluble IL-6 receptor promotes the expression of matrix metalloproteinases,25 suggesting that superimposed IL-1β activity can modulate the pro-chondrogenic effects of trans-signaling by IL-6 in the context of endochondral bone regeneration.
The role of IL-2 in determining the balance between intramembranous and endochondral bone formation is less studied. In mice lacking the common γ chain of the IL-2 receptor, bone regeneration is characterized by a larger callus, decreased bone volume fraction, and increased cartilage content,26 further suggesting that tuning of the immune response can modulate endochondral regeneration in segmental osseous defects. IL-2 is also an important T-cell activating cytokine; however, there are conflicting reports of enhanced27 or impaired28 fracture healing in mice lacking functional lymphocytes. Other studies suggest the ratio of cytotoxic to helper T-lymphocytes is prognostic for fracture healing; in a mouse model of fracture, selective depletion of cytotoxic T-lymphocytes was associated with increased bone mineral density and bone volume fraction versus adoptive transfer of cytotoxic T-lymphocytes.29 Additional T-cell subsets also have a complex role in bone regeneration, as some groups have demonstrated enhanced fracture healing in mice lacking γδ T-cells,30 while others have found that γδ T-cell-derived IL-17A promotes the regeneration of femoral cortical drill-hole injuries,31 an intramembranous process.
By Week 36, the mechanical properties of rhBMP2-treated bones were inferior to native healing, demonstrating functional consequences between intramembranous and endochondral bone regeneration. Preceding this time, progressive increases in Tb.Sp and decreases in Tb.Th were observed with rhBMP2 treatment, bearing striking resemblance to the trabecular changes that occur in osteoporosis. Osteoclasts are detectable as early as Day 10 with rhBMP2 treatment and remained increased above nonhealing conditions at Week 2 until Week 4,21 which is concordant with the peak expression of remodeling genes Dkk-1 and Sost. The knowledge that rhBMP2 stimulates the differentiation and survival of osteoclasts32 has led various groups to test whether anti-catabolic agents can improve bone formation with rhBMP2. The bisphosphonate zolendronate in combination with rhBMP2 treatment of a rat femoral CSD was associated with increased bone formation and failure loads versus rhBMP2 alone, although circulating markers of bone remodeling did not differ between groups.33 Similar findings were demonstrated using zolendronate and BMP7 in a rat femoral osteotomy model, where serial in vivo microCT demonstrated a delayed peak in callus volume for zolendronate-treated animals, suggesting differential remodeling kinetics versus BMP7 alone.34 Twice weekly administration of an antisclerostin antibody in a rat femoral CSD treated with rhBMP2 resulted in increased bone volume, bone area, and mechanical properties versus rhBMP2 alone.35 Interestingly, in empty CSDs, twice weekly anti-sclerostin antibody treatment for 12 weeks resulted in bridging in over one-third of cases, with significantly increased bone formation and increased circulating osteocalcin and type-1 procollagen.36 This is consistent with our finding that Sost transcripts peak at Week 8 in empty CSDs, offering an explanation for the modest efficacy of anti-sclerostin antibody treatment. Similar anabolic strategies have been pursued in the study of fracture healing. Dual inhibition of Sost and Dkk-1 increased bone volume fraction and peak failure loads compared to sole inhibition of Sost in a model of rat fracture repair, although these effects were not distinct from singular inhibition of Dkk-1.37 In natively healing 1 mm defects, low-level osteoclast activity is detectable, but does not differ throughout the first 12 weeks of regeneration (Supporting Information).
Although this study did not include a control group of animals whose defects received collagen sponge alone, data from a recent paper using this sponge to deliver mRNA encoding BMP-2 confirm that the sponge does not interfere with endochondral ossification or promote intramembranous bone formation.19 This supports our conclusion that the high dose of rhBMP2 is responsible for the latter.
Identifying prognostic factors that, at early time points, can distinguish osseous lesions that will heal versus those that will not, could provide a significant tool for clinicians in the treatment or early intervention in cases of impaired bone regeneration. Considering the expression of osteogenic agents at early time points in nonhealing defects, we observed differences in Phex and Bmp7 transcripts versus rhBMP2-treated and naturally healing osteotomies at Day 10 and Week 4 following surgery. Expression of both Phex and Bmp7 peaked at 8 weeks in nonhealing defects to a level that equaled or exceeded naturally healing and rhBMP2-treated bones, respectively. Histologically, nonhealing defects demonstrated evidence of an endochondral response with osseous capping of the medullary ends by Week 8, characteristic of an established atrophic non-union. This observation, in combination with the expression kinetics of Phex and Bmp7, is interesting in light of reports which document the decreased efficacy of growth factors in promoting regeneration of CSDs when administered in a delayed fashion.38, 39 In this manner, peak expression of Phex and Bmp7 concurrent with osseous capping at 8 weeks and arrest of bone repair, may invalidate the pro-regenerative effects of these growth factors, further suggesting that improperly tuned temporal profiles of osteogenic growth factor signaling may play a role in impaired healing. The finding that differences in Tb.Th and Tb.Sp of natural versus non-healing defects were apparent at early, but not later time points, further suggest that features exist during the initial regenerative process to distinguish bones that will heal from those that will not; the identification of such factors is a significant area for future work.
Bone regeneration is a complex biological process that, when impaired, can be stimulated to progress imperfectly using the osteoinductive cytokine rhBMP2. With rhBMP2 treatment we observed a significant early inflammatory response in conjunction with rapid direct bone formation, resulting in inferior mechanical characteristics and the deterioration of trabecular morphology versus natural healing osteotomies at later time points. Furthermore, the observation that endochondral ossification occurred over the course of nearly 12 weeks in naturally healing bones provides significant basis for the exploration of therapeutic strategies to expedite this process.
AUTHOR CONTRIBUTIONS
Conceptualization: Christopher H. Evans. Methodology: Joseph A. Panos, Michael J. Coenen, Christopher V. Nagelli, Erin B. McGlinch, Aysegul Atasoy-Zeybek, and Consuelo Lopez De Padilla. Investigation: Joseph A. Panos, Michael J. Coenen, Christopher V. Nagelli, Erin B. McGlinch, Aysegul Atasoy-Zeybek, RFC, and Consuelo Lopez De Padilla. Funding acquisition: Christopher H. Evans. Supervision: Rodolfo E. De la Vega and Christopher H. Evans. Writing–original draft: Joseph A. Panos and Christopher H. Evans. Writing–review & editing: Joseph A. Panos, Michael J. Coenen, Christopher V. Nagelli, Erin B. McGlinch, Aysegul Atasoy-Zeybek, Consuelo Lopez De Padilla, Rodolfo E. De la Vega, and Christopher H. Evans.
ACKNOWLEDGMENTS
This work was supported in part by the John and Posy Krehbiel Professorship in Orthopedics (C.H.E). Funding in support of this work was provided by grants from the National Institutes of Health (F31 AR079842-01A1 (J.A.P.), R01 AR074395 (C.H.E.), T32 AR56950 (J.A.P.)) and the Mayo Clinic Department of Molecular Medicine (J.A.P.).