Activation of canonical Wnt signaling accelerates intramembranous bone regeneration in male mice
Abstract
Canonical Wnt signaling plays an important role in skeletal development, homeostasis, and both endochondral and intramembranous repair. While studies have demonstrated that the inhibition of Wnt signaling impairs intramembranous bone regeneration, how its activation affects intramembranous bone regeneration has been underexplored. Therefore, we sought to determine the effects of activation of canonical Wnt signaling on intramembranous bone regeneration by using the well-established marrow ablation model. We hypothesized that mice with a mutation in the Wnt ligand coreceptor gene Lrp5 would have accelerated intramembranous bone regeneration. Male and female wild-type and Lrp5-mutant mice underwent unilateral femoral bone marrow ablation surgery in the right femur at 4 weeks of age. Both the left intact and right operated femurs were assessed at Days 3, 5, 7, 10, and 14. The intact femur of Lrp5 mutant mice of both sexes had higher bone mass than wild-type littermates, although to a greater degree in males than females. Overall, the regenerated bone volume in Lrp5 mutant male mice was 1.8-fold higher than that of littermate controls, whereas no changes were observed between female Lrp5 mutant and littermate control mice. In addition, the rate of intramembranous bone regeneration (from Day 3 to Day 7) was higher in Lrp5 mutant male mice compared to their same-sex littermate controls with no difference in the females. Thus, activation of canonical Wnt signaling increases bone mass in intact bones of both sexes, but accelerates intramembranous bone regeneration following an injury challenge only in male mice.
1 INTRODUCTION
Wnt signaling plays an important role during the development of the skeleton, and its homeostasis, disease, and repair.1 Prior studies demonstrated that activating canonical Wnt (cWnt) signaling pharmacologically or genetically leads to accelerated long bone fracture healing, a process which includes both endochondral and intramembranous bone repair.2 Also, inhibition of cWnt signaling impairs fracture repair in mice by reducing the callus area and strength.3 However, how cWnt signaling activation affects intramembranous bone regeneration in the absence of endochondral healing has been underexplored.
Intramembranous bone regeneration occurs where interfragmentary strain is minimized and lacks the histologically evident cartilaginous template emblematic of endochondral repair. This form of bone repair resembles the embryonic development of flat bones.4 Many surgical treatments, such as the placement of dental and joint replacement implants, rely exclusively on intramembranous bone regeneration and lack histological evidence of an endochondral component. Administration of the cWnt activator sclerostin antibody following implant placement in rodent models leads to enhanced bone repair and mechanical fixation of the implant to the skeleton.5-8 Given that in the United States alone ∼900,000 joint replacement surgeries and ∼2 million dental implant procedures are performed every year,9-11 further elucidating the underlying mechanisms driving intramembranous bone regeneration may ultimately lead to enhanced care for patients undergoing these procedures.
The bone marrow ablation model induces intramembranous bone regeneration in the absence of an endochondral component12 and mimics joint replacement surgery.13, 14 Our previous transcriptomic profiling experiment in this model demonstrated an increase in β-catenin gene expression before increases in osteogenic genes, suggesting a key role for cWnt signaling in the intramembranous bone regeneration pathway.15 While the molecular basis for endochondral bone regeneration, including the role of cWnt signaling during long bone fracture healing, has received widespread attention,16-19 its role during intramembranous bone regeneration has been relatively unexplored. Studies that sought to understand the role of cWnt signaling on intramembranous bone regeneration also relied largely on endochondral healing models. Therefore, we sought to examine the role of cWnt signaling in intramembranous bone regeneration by performing bone marrow ablation surgery in C57Bl/6 and Lrp5 mutant (HBM) mice. cWnt signaling is active in HBM mice and these mice have previously been shown to have higher bone mass compared to littermate controls.20, 21 We hypothesized that the activation of cWnt signaling accelerates intramembranous bone regeneration.
2 MATERIALS AND METHODS
2.1 Mouse husbandry
Animal studies were approved by the Rush University Medical Center Institutional Animal Care and Use Committee. In the first experiment, C57Bl/6 mice (Jax#664) were bred in-house and generated to assess intramembranous bone regeneration and β-catenin expression (n = 3–4/time point). In the second experiment, Lrp5 mutant (HBM) mice, generously provided from the laboratory of Mark Johnson PhD,20, 21 were bred with C57Bl/6 mice to generate HBM and wild-type (WT) littermate control mice. These offspring were used to determine the effects of activation of cWnt signaling on intramembranous bone regeneration (n = 4–5/genotype/time point/sex). The generated HBM offspring are heterozygotes for Lrp5 transgene linked downstream of the 3.6-kb rat collagen type 1 promoter, which is a dominant trait and has been shown to exhibit high bone mass phenotype compared to their WT littermate controls with C57Bl/6 background.20 At 3 weeks of age, mice were weaned and group-housed two to five mice per cage. Mice were maintained in a pathogen-free facility, subjected to a 12/12 h light/dark cycle, had ad libitum access to standard laboratory rodent chow and water.
2.2 Bone marrow ablation surgery
Unilateral bone marrow ablation surgery was performed in the right femur of male and female 4-week-old mice using a previously published surgical procedure.12 In brief, the distal femur was breached through the intercondylar groove with a 23-gauge needle, followed by marrow reaming with a 25-gauge needle. After further mechanical ablation of the marrow with a 30-gauge needle, the marrow contents were flushed with 0.5 ml of sterile saline. Bone wax was applied to seal the entrance hole at the intercondylar groove. Mice were administered 0.05 mg/kg of buprenorphine for analgesia up to a day postsurgery and 5.0 mg/kg of cefazolin as an antibiotic for the 2 days postsurgery. Mice were sacrificed using CO2 inhalation and secondary cervical dislocation. Femurs were collected, fixed in 4% paraformaldehyde overnight, and transferred to 70% EtOH for microcomputed tomography scanning and further processing for histology and immunohistochemistry.
2.3 Microcomputed tomography
Microcomputed tomographic (µCT) scanning was performed on the bone regenerating area of the right (marrow ablated) femur and the distal metaphysis and mid-diaphysis of the left (intact) femur using a high-resolution laboratory imaging system (µCT50; Scanco Medical AG) in accordance with the American Society of Bone and Mineral Research guidelines for the use of µCT in rodents.22 Scans were acquired using a 7.4 µm3 isotropic voxel, 70 kVp and 114 µA peak X-ray tube potential and intensity, 300 ms integration time, and were subjected to Gaussian filtration. The region of interest (ROI) for microCT analysis of the regenerating bone was the medullary space from 40% to 70% of the total bone length proximal to the distal condyles. This compartment is normally devoid of bone. The ROIs for the intact left femur included the distal metaphysis for analysis of trabecular bone and the midshaft for cortical bone. In the intact metaphysis, the most distal slice was ∼200 µm (27 slices) proximal to the distal growth plate with the ROI extending proximally to 30% of the femur length. The trabecular bone was segmented from the cortical bone by manual contouring. Cortical bone morphology was evaluated in an ROI centered on the femoral mid-diaphysis that included 10% of the bone length. Thresholds of 289, 350, and 460 mg HA/cm3 were used for evaluation of the regenerating bone, intact trabecular bone, and cortical bone, respectively. Regenerating bone-dependent variables included bone volume fraction (BV/TV, mm3/mm3). Trabecular bone outcomes included trabecular bone volume fraction (BV/TV, mm3/mm3), thickness (Tb.Th, mm), separation (Tb.Sp, mm), and number (Tb.N, 1/mm). Cortical bone outcomes included cortical tissue mineral density (Ct.TMD, mg HA/cm3), cortical thickness (Ct.Th, mm), total cross-sectional (Tt.Ar, mm2), cortical bone (Ct.Ar, mm2), and medullary (Ma.Ar, mm2) areas, and the maximum and minimum moments of inertia (Imax and Imin, mm4).
2.4 Histology and immunohistochemistry
Following μCT scanning, femurs were decalcified in 20% EDTA for 2 weeks, dehydrated, and embedded in paraffin. Hematoxylin and Eosin staining was performed on 6 μm paraffin sections to evaluate the regenerated bone. For immunohistochemical staining, paraffin sections were rehydrated and, following antigen retrieval by trypsin digestion (for β-catenin and osteocalcin) or no antigen retrieval (for PCNA) for 15 min at 37°C, quenched using endogenous peroxidase and slides were blocked with TNB (Tris, NaCl, Blocking reagent, Perkin Elmer) before overnight incubation with unphosphorylated β-catenin (1:500; #8814, Cell Signaling), osteocalcin (1:200; ab93876, Abcam), or PCNA (1:200; #13110, Cell Signaling) antibodies to examine activation of cWnt signaling, osteoblasts, or proliferating cells, respectively.23 After detection with biotinylated secondary antibodies and tyramide amplification (TSA Plus Biotin Kit; Perkin Elmer), signals were visualized using chromogen substrates and counterstained with fast green or hematoxylin. Paraffin sections were also used to evaluate osteoclasts in the regenerated bone by enzymatic TRAP staining.24
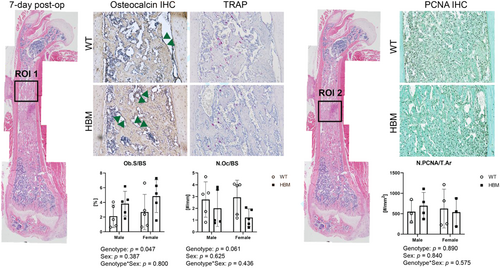
To quantitate osteoblasts, osteoclasts, and proliferating cells, we divided the regenerating marrow into two ROIs: ROI 1 was defined as a region where the de novo bone formation has occurred; ROI 2 was defined as a region where the stromal cells were highly populated (Figure 4). In ROI 1, osteocalcin positive osteoblast surface (Ob.S) or the number of TRAP-positive osteoclasts (N.Oc) were normalized by bone surface (BS). In ROI 2, the number of PCNA positive proliferating cells (N.PCNA) was normalized by the total area (T.Ar). All measurements were performed using Osteomeasure software (OsteoMetrics).
2.5 Statistical analysis
Data were analyzed with Prism 9 (GraphPad Software) and RStudio version 1.3.1093. Three-way analysis of variance was used to test the main effects of genotype, time point, sex, and their interactions on outcome parameters. Tukey's HSD posthoc comparisons of means test was used to identify significant differences between groups. To determine if HBM mice exhibit accelerated intramembranous bone regeneration, we performed linear regressions using the Day 3–7 data and compared the slopes from these models. Using coefficients from linear regression, slopes from experimental groups were compared pair-wise using Tukey's HSD posthoc comparisons of means. Differences were considered significant at p < 0.05. Data are reported as mean ± SD.
3 RESULTS
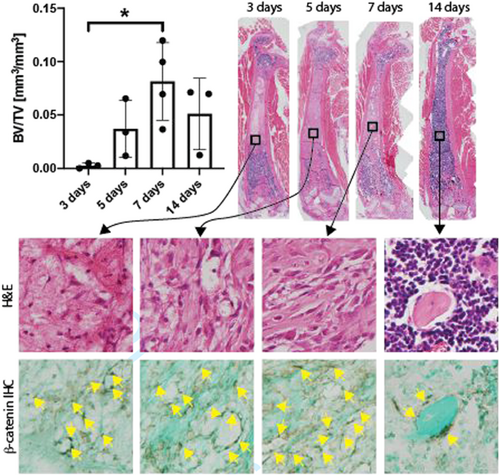
In the first experiment, regenerated bone within the ablated medullary compartment peaked at Day 7 after surgery, where the mean BV/TV was 8.1 ± 1.8% (Figure 1). Increased immunoreactivity to unphosphorylated β-catenin within the ablated marrow compartment preceded the peak in BV/TV, where β-catenin positive marrow stromal and endothelial cells (DAB positive (brown) cells) appeared at Day 3. This repopulation of the empty medullary space by β-catenin positive cells was accompanied by extracellular matrix (fast green positive) deposition beginning at Day 3 that mineralized beginning around Day 5 and was partially resorbed by Day 14. On Day 14, β-catenin positive cells (yellow triangles) were limited to bone lining cells.
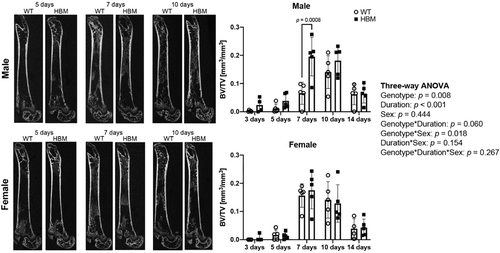
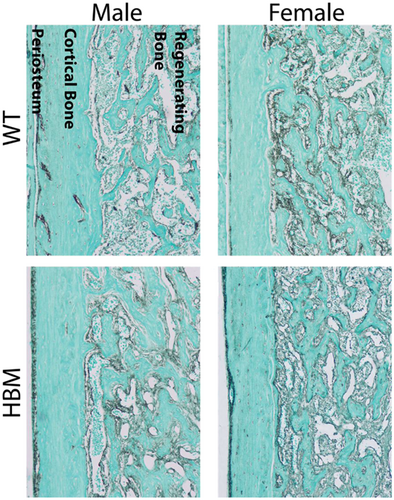
In the second experiment, intramembranous bone regeneration BV/TV was dependent on time point (p < 0.001) and genotype (p = 0.008) (Figure 2). While sex did not affect intramembranous bone regeneration as a main effect (p = 0.444), the amount of regenerated BV/TV was dependent on the sex-by-genotype interaction (p = 0.018). Specifically, when the time points were pooled, regenerated BV/TV was 1.8-fold higher (p = 0.028) in HBM male mice compared to their same-sex WT littermate controls, whereas in females, no change was observed (p = 0.964).
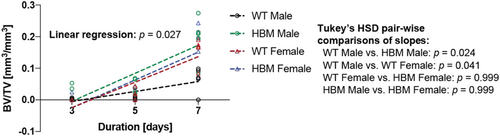
HBM male mice had a larger increase in regenerated BV/TV from Days 3 to 7 than their same-sex WT littermate controls (Figure 3, p = 0.024), suggesting an accelerated rate of intramembranous bone regeneration. This trend was absent in female HBM mice (p = 0.999). The intramembranous bone regeneration rate was also different between WT males and females (p = 0.041).
Osteoblast surface was significantly different by genotype (p = 0.047) but was not dependent on sex or sex-by-genotype interaction (Figure 4). When both males and females were pooled, osteoblast surface was 1.8-fold higher in HBM mice compared to WT littermate controls. The number of osteoclasts or proliferating cells were not by differed genotype, sex, or their interactions.
β-catenin immunohistochemistry demonstrated that both male and female HBM mice exhibit higher immunoreactivity on the intact periosteal surface compared to WT mice (Figure 5). However, when compared within the regenerating bone region, female WT mice were shown to have higher β-catenin immunoreactivity along the bone surface compared to male WT mice.
In intact bone, activation of canonical Wnt signaling led to increased trabecular and cortical bone and 5 of the 11 genotype effects were sex-dependent (Table 1, genotype-by-sex interaction term p < 0.050). For instance, the greater amount of distal femoral metaphyseal trabecular bone mass in HBM mice was dependent on sex (genotype × sex; p < 0.001). Specifically, BV/TV was 70% greater in male HBM mice compared to WT mice, but only 30% greater in female HBM mice compared to WT mice. The greater trabecular BV/TV in HBM mice was due to 8% thicker trabeculae (p = 0.003), 25% greater trabecular number (p < 0.001), and 18% less trabecular separation (p < 0.001), regardless of sex or time point. Trabecular thickness was dependent upon both genotype and sex (genotype × sex; p = 0.030), being 14% thicker in male HBM mice compared to WT mice, but only 3.4% thicker in female HBM mice compared to WT mice.
| 3 | 5 | 7 | 10 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| WT | HBM | WT | HBM | WT | HBM | WT | HBM | ||
| BV/TV (mm3/mm3) | Male | 0.038 ± 0.02 | 0.084 ± 0.02 | 0.073 ± 0.03 | 0.13 ± 0.04 | 0.057 ± 0.03 | 0.095 ± 0.04 | 0.082 ± 0.02 | 0.13 ± 0.04 |
| Female | 0.035 ± 0.01 | 0.039 ± 0.01 | 0.044 ± 0.01 | 0.048 ± 0.02 | 0.036 ± 0.01 | 0.066 ± 0.01 | 0.042 ± 0.01 | 0.059 ± 0.02 | |
| Tb.Th (mm) | Male | 0.029 ± 0.004 | 0.036 ± 0.007 | 0.036 ± 0.007 | 0.040 ± 0.004 | 0.033 ± 0.005 | 0.037 ± 0.004 | 0.037 ± 0.005 | 0.043 ± 0.007 |
| Female | 0.029 ± 0.001 | 0.029 ± 0.002 | 0.031 ± 0.002 | 0.030 ± 0.004 | 0.031 ± 0.002 | 0.034 ± 0.002 | 0.031 ± 0.002 | 0.035 ± 0.001 | |
| Tb.Sp (mm) | Male | 0.38 ± 0.04 | 0.27 ± 0.03 | 0.34 ± 0.05 | 0.23 ± 0.03 | 0.34 ± 0.07 | 0.27 ± 0.07 | 0.27 ± 0.04 | 0.25 ± 0.03 |
| Female | 0.45 ± 0.07 | 0.35 ± 0.05 | 0.39 ± 0.1 | 0.31 ± 0.04 | 0.39 ± 0.1 | 0.31 ± 0.03 | 0.37 ± 0.05 | 0.34 ± 0.06 | |
| Tb.N (1/mm) | Male | 2.7 ± 0.3 | 3.8 ± 0.5 | 3.1 ± 0.5 | 4.5 ± 0.5 | 3.1 ± 0.6 | 3.9 ± 0.9 | 3.9 ± 0.5 | 4.2 ± 0.4 |
| Female | 2.3 ± 0.4 | 3.0 ± 0.4 | 2.8 ± 0.7 | 3.3 ± 0.4 | 2.6 ± 0.7 | 3.6 ± 0.3 | 2.7 ± 0.4 | 3.1 ± 0.5 | |
| Ct.Th (mm) | Male | 0.10 ± 0.007 | 0.11 ± 0.02 | 0.11 ± 0.01 | 0.13 ± 0.01 | 0.11 ± 0.02 | 0.13 ± 0.01 | 0.11 ± 0.004 | 0.13 ± 0.02 |
| Female | 0.10 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.003 | 0.11 ± 0.005 | 0.10 ± 0.01 | 0.11 ± 0.003 | 0.12 ± 0.02 | 0.12 ± 0.006 | |
| TA (mm2) | Male | 1.2 ± 0.07 | 1.3 ± 0.9 | 1.3 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.3 | 1.5 ± 0.1 | 1.4 ± 0.1 |
| Female | 1.2 ± 0.07 | 1.2 ± 0.07 | 1.3 ± 0.2 | 1.3 ± 0.08 | 1.1 ± 0.2 | 1.2 ± 0.05 | 1.4 ± 0.1 | 1.4 ± 0.1 | |
| MA (mm2) | Male | 0.86 ± 0.05 | 0.87 ± 0.03 | 0.87 ± 0.05 | 0.90 ± 0.04 | 0.88 ± 0.06 | 0.85 ± 0.18 | 0.99 ± 0.08 | 0.93 ± 0.05 |
| Female | 0.80 ± 0.05 | 0.83 ± 0.04 | 0.91 ± 0.1 | 0.89 ± 0.06 | 0.78 ± 0.1 | 0.83 ± 0.04 | 0.94 ± 0.04 | 0.89 ± 0.06 | |
| BA (mm2) | Male | 0.37 ± 0.03 | 0.44 ± 0.08 | 0.42 ± 0.04 | 0.54 ± 0.03 | 0.44 ± 0.07 | 0.48 ± 0.1 | 0.46 ± 0.03 | 0.52 ± 0.07 |
| Female | 0.37 ± 0.04 | 0.41 ± 0.04 | 0.42 ± 0.03 | 0.42 ± 0.03 | 0.36 ± 0.06 | 0.41 ± 0.02 | 0.47 ± 0.1 | 0.47 ± 0.04 | |
| Ct.TMD (mg HA/cm3) | Male | 845 ± 56 | 921 ± 20 | 927 ± 40 | 927 ± 50 | 853 ± 28 | 929 ± 67 | 913 ± 30 | 924 ± 42 |
| Female | 872 ± 43 | 892 ± 51 | 916 ± 47 | 909 ± 96 | 967 ± 59 | 958 ± 28 | 911 ± 30 | 904 ± 29 | |
| Imax (mm4) | Male | 0.075 ± 0.01 | 0.094 ± 0.2 | 0.092 ± 0.02 | 0.13 ± 0.01 | 0.10 ± 0.02 | 0.11 ± 0.04 | 0.12 ± 0.01 | 0.13 ± 0.02 |
| Female | 0.072 ± 0.01 | 0.084 ± 0.01 | 0.094 ± 0.02 | 0.092 ± 0.01 | 0.070 ± 0.02 | 0.088 ± 0.01 | 0.11 ± 0.04 | 0.11 ± 0.02 | |
| Imin (mm4) | Male | 0.046 ± 0.01 | 0.059 ± 0.01 | 0.056 ± 0.01 | 0.074 ± 0.01 | 0.058 ± 0.01 | 0.066 ± 0.02 | 0.068 ± 0.01 | 0.071 ± 0.01 |
| Female | 0.044 ± 0.005 | 0.050 ± 0.006 | 0.054 ± 0.01 | 0.055 ± 0.008 | 0.044 ± 0.01 | 0.05 ± 0.004 | 0.066 ± 0.02 | 0.061 ± 0.009 | |
| 14 | p value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WT | HBM | genotype | time point | sex | genotype*time point | genotype*sex | time point*sex | genotype*time point*sex | ||
| BV/TV (mm3/mm3) | Male | 0.11 ± 0.04 | 0.15 ± 0.03 | <0.001 | <0.001 | <0.001 | 0.848 | <0.001 | 0.014 | 0.634 |
| Female | 0.061 ± 0.01 | 0.063 ± 0.02 | ||||||||
| Tb.Th (mm) | Male | 0.040 ± 0.001 | 0.044 ± 0.005 | 0.003 | <0.001 | <0.001 | 0.632 | 0.030 | 0.139 | 0.764 |
| Female | 0.035 ± 0.002 | 0.033 ± 0.003 | ||||||||
| Tb.Sp (mm) | Male | 0.24 ± 0.03 | 0.21 ± 0.04 | <0.001 | <0.001 | <0.001 | 0.040 | 0.757 | 0.694 | 0.929 |
| Female | 0.31 ± 0.01 | 0.31 ± 0.06 | ||||||||
| Tb.N (1/mm) | Male | 4.5 ± 0.4 | 4.8 ± 0.8 | <0.001 | <0.001 | <0.001 | 0.109 | 0.169 | 0.151 | 0.540 |
| Female | 3.2 ± 0.2 | 3.6 ± 0.5 | ||||||||
| Ct.Th (mm) | Male | 0.13 ± 0.01 | 0.15 ± 0.01 | <0.001 | <0.001 | <0.001 | 0.990 | 0.032 | 0.047 | 0.735 |
| Female | 0.12 ± 0.006 | 0.13 ± 0.008 | ||||||||
| TA (mm2) | Male | 1.5 ± 0.2 | 1.6 ± 0.2 | 0.042 | <0.001 | <0.001 | 0.711 | 0.370 | 0.510 | 0.637 |
| Female | 1.4 ± 0.1 | 1.4 ± 0.1 | ||||||||
| MA (mm2) | Male | 0.98 ± 0.1 | 1.0 ± 0.1 | 0.945 | <0.001 | 0.018 | 0.702 | 0.945 | 0.475 | 0.738 |
| Female | 0.93 ± 0.06 | 0.92 ± 0.1 | ||||||||
| BA (mm2) | Male | 0.54 ± 0.06 | 0.62 ± 0.07 | <0.001 | <0.001 | <0.001 | 0.807 | 0.034 | 0.117 | 0.594 |
| Female | 0.46 ± 0.04 | 0.50 ± 0.04 | ||||||||
| Ct.TMD (mg HA/cm3) | Male | 933 ± 50 | 993 ± 53 | 0.043 | <0.001 | 0.270 | 0.366 | 0.007 | 0.045 | 0.400 |
| Female | 1006 ± 17 | 952 ± 62 | ||||||||
| Imax (mm4) | Male | 0.14 ± 0.04 | 0.18 ± 0.04 | 0.001 | <0.001 | <0.001 | 0.871 | 0.075 | 0.119 | 0.573 |
| Female | 0.11 ± 0.02 | 0.12 ± 0.02 | ||||||||
| Imin (mm4) | Male | 0.078 ± 0.01 | 0.095 ± 0.02 | <0.001 | <0.001 | <0.001 | 0.515 | 0.052 | 0.311 | 0.849 |
| Female | 0.065 ± 0.01 | 0.070 ± 0.01 | ||||||||
- Note: Data presented as mean ± SD. n = 5/time point/genotype except Day 3-HBM-male, Day 3-HBM-female, and Day 14-WT-female (n = 4).
At the midshaft, HBM mice had greater total area (4.0%, p = 0.042), cortical bone area (13%, p < 0.001), cortical thickness (12%, p < 0.001), and cortical tissue mineral density (2.2%, p = 0.043) than WT mice, regardless of sex or time point. Larger total area and cortical thickness led to increased moments of inertia in HBM mice, where Imax was higher by 16% (p = 0.001) and Imin higher by 14% (p < 0.001).
4 DISCUSSION
Our study demonstrates that activation of Wnt signaling accelerates intramembranous bone regeneration in male mice. The delayed regenerating BV/TV in male WT mice at postsurgery was rescued by activation of cWnt signaling. Intramembranous bone regeneration was not accelerated in female HBM mice compared to their same-sex WT littermate controls. In both male and female HBM mice, the trabecular and cortical bone properties in the intact femur were higher than in WT littermate controls, confirming prior studies in HBM mice.20
While the role of cWnt signaling has not been explored extensively in intramembranous bone regeneration, studies using animal models of diaphyseal fracture healing demonstrate the importance of cWnt signaling in endochondral bone regeneration. In newly formed fracture calluses, cWnt signaling is more active compared to intact bones and the impairment of cWnt signaling delays fracture healing in mice.25-28 When cWnt signaling was activated by pharmacological therapies in mice, fracture healing was accelerated29, 30 and we have previously shown that the use of a neutralizing antibody to sclerostin increases intramembranous bone regeneration in animal models of implant placement.5-7 Our prior study that examined transcriptomic profiles of regenerating intramembranous bone tissue showed significant increases in cWnt signaling-related genes, such as Wnt5a and β-catenin, before increases in several osteogenic genes.15 Together, these and the present study demonstrate the importance of cWnt signaling in both intramembranous and endochondral bone regeneration.
The molecular mechanisms of how activation of cWnt signaling accelerates intramembranous bone regeneration needs to be further explored, but embryonic development of membranous bone provides insights to how this may occur.4 During membranous bone development as occurs in the calvaria and scapula, activation of Wnt signaling by Axin2 deletion increases proliferation and reduces apoptosis in osteoprogenitor cells whereas inactivation of Wnt signaling leads to craniofacial defects.31, 32 Also, Wnt signaling is required for calvarial vasculature development,33, 34 suggesting that in HBM mice, upregulation of these pathways may explain the observed accelerated intramembranous bone regeneration.
The sexually dimorphic outcome in our study was surprising. While this may be attributed to sex-dependent differences in mesenchymal cell proliferation and differentiation,35, 36 our data that show no changes in proliferation suggest that cWnt activation and estrogen signaling interplay may be predominantly affecting mesenchymal cell differentiation in females. Estrogen receptor and Wnt signaling pathways have been shown to interact in the skeletal and nervous systems.37, 38 In bone, activation of estrogen receptor led to synergistically enhanced bone formation in Wnt3a-transduced cells and fetal limb explant.39 Also, estrogen receptors have been shown to regulate the Wnt antagonist Sost expression in osteoblasts.40 Estradiol itself has also been shown to suppress Sost level by interacting with BMP2 signaling.41 In light of these prior studies as well as increased β-catenin immunoreactivity in the female regenerating bone region, our studies suggest a possible interplay between estrogen receptor and Wnt signaling during intramembranous bone regeneration. These studies along with our results highlight the need to examine both males and females to reveal potential sexual dimorphic outcomes in bone regeneration.
We found that some of the measurements in intact bone from our WT and HBM mice showed blunted effects of cWnt signaling activation, such as similar BV/TV in female mice at day 3 (3.5% vs. 3.9%), compared to the previous study which showed 2- to 3-fold BV/TV differences between the two groups.20 This discrepancy may be due to variation in the microCT measurements such as scanning parameters, region of interest, or thresholds. Another possibility is that bone marrow surgery may lead to unexpected consequences to non-operated intact limb. For example in long bone fracture healing studies, intact limbs from fractured animals exhibited higher osteoclast number compared to non-fractured mice.42 Given these observations, our future experimental design will include non-operated animals to determine potential surgery effects on the intact skeleton.
Future studies need to explore whether intramembranous bone regeneration is also accelerated in older HBM mice. In aging C57Bl/6 mice, Wnt gene expression was decreased during osteoblast differentiation of primary bone marrow stromal cells.43 Also, activation of Wnt signaling following mechanical loading was decreased in aged C57Bl/6 mice.44 These studies suggest that decreased basal level of Wnt signaling may lead to delayed intramembranous bone regeneration in aged mice compared to young mice.
In conclusion, our study demonstrates that activation of cWnt signaling accelerates intramembranous bone regeneration in male mice. Further elucidating the role of cWnt signaling in bone regeneration may improve current treatments and provide novel therapeutic opportunities for multiple dental and orthopedic procedures that are dependent upon successful intramembranous bone regeneration.
ACKNOWLEDGMENTS
This study was supported by Cohn Research Fellowship and K01AR077679 (Frank C. Ko). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Rush University Medical Center MicroCT/Histology Core provided experimental support. The authors would like to thank Dr Mark Johnson for sharing HBM mice.
AUTHOR CONTRIBUTIONS
Study design: Frank C. Ko, D. Rick Sumner. Study conduct and data collection: Frank C. Ko and Meghan M. Moran. All authors contributed to data analysis, drafted and revised manuscript content, and approved the final and submitted version of the manuscript. D. Rick Sumner takes responsibility for the integrity of the data analysis.




