Effect of local anesthetics on platelet physiology and function
Abstract
Local anesthetics are often used at the site of injury or mixed with platelet-rich plasma to reduce pain when treating orthopedic and sports-related injuries. Local anesthetics have been shown to have deleterious effects on stromal cells, but their impact on platelets has not been investigated. In this study, we aimed to assess the effects of lidocaine, bupivacaine, and ropivacaine on platelet health. Based on the deleterious effects of local anesthetics on nucleated cells, we hypothesized that these compounds would affect platelet viability, intracellular physiology, and function. Platelet preparations were derived from randomly selected donors and exposed to lidocaine 1%, bupivacaine 0.75%, ropivacaine 0.5%, and saline at 1:1 and 1:3 ratios. Platelet morphology, viability, intracellular calcium, production of radical oxygen species (ROS), apoptosis, and adhesion were assessed via fluorescent microscopy and flow cytometry. Bupivacaine resulted in increased ROS production, calcium dysregulation, apoptosis, and reduced platelet adhesion. By contrast, ropivacaine and lidocaine were similar to saline in most assays, except for a low degree of mitochondrial stress as evidenced by increased ROS production. Ultimately, bupivacaine 0.75% was harmful to platelets as evidenced by reduced platelet viability, adhesion, and increased apoptosis, whereas lidocaine 1% and ropivacaine 0.5% were relatively safe at the 1:1 and 1:3 dilutions.
Clinical significance: Lidocaine 1% and ropivacaine 0.5% can be used at up to a 1:1 ratio with platelet preparations to reduce the pain and discomfort of PRP procedures while maintaining platelet therapeutic potential.
1 INTRODUCTION
Platelets are cell fragments, derived from megakaryocytes in the bone marrow where they are pre-loaded with organelle fragments and secretory granules.1 Platelets lack traditional cell components, namely a nucleus, but do possess mitochondria, endoplasmic reticulum, lysosomes, and unique secretory granules.2 There are two granule types: dense granules and alpha-granules (α-granules). Dense granules are loaded with calcium, ADP, ATP, histamine, and serotonin, which upon release result in activation of adjacent platelets and promotion of hemostasis and thrombosis.3, 4 The α-granules contain a variety of proteins to assist in clot formation, including thrombin, fibrinogen, von Willebrand Factor, and growth factors which recruit hematopoietic and mesenchymal cells during healing and injury resolution.5, 6 Specific subpopulations of dense and α-granules are released in a temporal fashion, with the initial release of proinflammatory and angiogenic factors followed by anti-inflammatory factors in the later stages of wound healing.7
To date, medical interest in the use of autologous platelets in the form of platelet-rich plasma (PRP) has focused on the release of growth factors by the α-granules.8 These factors are thought to be involved in the resolution of inflammation and healing of chronic musculoskeletal injuries,9-13 including via the regulation of mesenchymal stem/stromal cell activity.6 In addition, platelets have to adhere to collagens and other substrates at the site of injury, using their filopodia and actomyosin to pull together torn collagen, reduce microscopic tissue defects and stabilize a fibrin scaffold.14
To improve comfort and reduce pain, PRP procedures are often performed in conjunction with low-dose steroids and local (amide-type) anesthetics (LA).15, 16 LAs have been researched with regard to their effect on chondrocytes,17, 18 tenocytes,18 and mesenchymal stem cells,19 though little research has been done on the effect of LAs on platelet physiology pertaining to their clinical use in orthobiologics. In vitro studies have shown that LAs impact platelets to some extent, affecting platelet-dependent hemostasis,20 aggregations,20, 21 microspike formation,22 and reduced efficiency of PRP-induced tenocyte proliferation.23 However, there have been no detailed investigations regarding the physiological response of the platelet to LAs at clinically relevant doses (on the order of milliliters [ml]).24, 25 While LAs are often used in platelet procedures for musculoskeletal (MSK) conditions,24, 25 the safe dose and concentration of LAs relative to platelet preparations have not yet been defined,16, 26 largely due to the lack of scientific data.
In this study, we aimed to investigate the effect of the LAs lidocaine, ropivacaine, and bupivacaine on platelet viability and function, specifically with regard to calcium regulation, radical oxygen species production, and platelet adhesion. LAs were mixed at 1:1 and 1:3 ratios relative to platelet preparations, similar to what is currently used in practice to reduce procedural discomfort.24, 25 Our overall goal is to improve the comfort, consistency, and efficacy of PRP procedures in orthopedic and sports medicine.
2 METHODS
2.1 Preparation of platelet suspensions
Platelet suspensions were produced from fresh blood collected from consenting male and female donors ranging in age from 34 to 90-years-old, randomized, and blinded to the investigators. Leukocyte-poor (and red blood cell-poor) platelet suspensions used in this study were created analogous to the production of leukocyte-poor platelet-rich plasma as previously reported27 with some modifications, where the final platelet pellet was diluted in a serial fashion to allow detection of fluorophore-conjugated antibodies and dyes via flow cytometry. A total of 36 mL of whole blood was drawn into 9 mL acid citrate dextrose solution A (ACD) (20% final volume/volume), centrifuged at ~1200 relative centrifugal force (RCF) for 5 min, followed by an additional cycle at ~270 RCF for 5 min. Then 2 ml of the upper plasma fraction was collected and recentrifuged at 2200 RCF for 5 min for the purposes of this study, while the remaining ~20 mL was processed further for LP-PRP preparation for patient use. The remaining platelet-poor plasma was discarded and the platelet pellet was resuspended in 2 mL calcium- and magnesium-free phosphate-buffered saline (PBS) containing 20% ACD to mitigate platelet aggregation, retain single-platelet suspension, and remove fibrinogen/fibrin which would interfere with flow cytometry analysis. The platelet preparation was diluted 1:3 in PBS for all staining protocols to allow accurate quantitation of fluorescent signal (from antibodies/dyes) via flow cytometry. An IRB Waiver was obtained for use of the platelet specimens. Each experimental series was repeated with a platelet suspension from a different donor; apoptosis assays were repeated three times.
2.2 Platelet labeling and staining
Platelets were labeled for flow cytometry using anti-human allophycocyanin (APC)-conjugated CD61 (CD61; Biolegend, Cat.# 336412) and 5 µl stock antibody per 100 µl of platelet solution, then incubated at room temperature for 45 min. For viability assessment, platelets were costained with calcein acetoxymethyl (calcein-AM) (Thermo Scientific Ref#. C3099) diluted to 2.5 µg/ml for 60 min at 37°C and fluorescent signal (calcein) was confirmed via fluorescent microscopy (AMG EVOS FL, 470/22BP nm excitation and 510/42BP nm emission light cube). For assessment of radical oxygen species (ROS), platelets were costained using MitoTracker Orange CMH2TMRos (MTO) as previously described28 (Thermo Scientific, Ref.# M7511) used at a final concentration of 1 µM. MTO is in a reduced form and localizes to the mitochondria based on the mitochondrial membrane potential.29 The molecule is then oxidized via mitochondrial ROS, resulting in fluorescence and immobilization in the mitochondria.30 For intracellular calcium assessment, platelets were costained with Fluo-4 (Thermo Scientific Ref.# F14201) at 1 µM at 37°C for 45 min. For apoptosis assessment, platelets were incubated with 5 µl of stock phycoerythrin (PE)-conjugated annexin-V (AV; Biolegend, Cat.# 640908) per 100 µl platelet suspension. Platelet activation was determined by positive-labeling with fluorescein (FITC)-conjugated anti-human CD62P (anti-CD62P; Biolegend, Cat.# 304904) in the cell suspension at 5 µl per 100 µl. An unlabeled or isotype control was used to establish gating criteria for all assays.
2.3 LA and compound preparation
LAs used throughout the study included preservative-free stock solutions of 0.75% bupivacaine (7.5 mg/ml Hospira Inc, NDC 0409-1165-18), 0.5% ropivacaine (5 mg/ml, Akorn Inc, NDC 17478-081-30), and 1% lidocaine (10 mg/ml, Hospira, Inc, NCD 0409-4713-75). These stock solutions were diluted into platelet preparations at either a 1-to-1 ratio (1:1) or a 1-to-3 ratio (1:3). The respective final concentrations used in the experiments were 3.75 mg/ml (0.375%) and 1.875 mg/ml (0.1875%) for bupivacaine, 2.5 mg/ml (0.25%) and 1.25 mg/ml (0.125%) for ropivacaine and 5.0 mg/ml (0.5%) and 2.5 mg/ml (0.25%) for lidocaine. The 1:1 dilution was used in all assays and 1:3 in the apoptotic assay to determine whether apoptosis was dose-dependent. Ionophore A23187 (A23187; Sigma Aldrich, Ref.# C7522) allows free transport of calcium across the cell membrane and was used as a control for calcium dysregulation in Fluo-4 assays at 10 µM31 and as the apoptotic control32 at final concentrations of 10 µM and 2.5 µM.
2.4 Flow cytometry
For all experimental series, platelets were analyzed using the Beckman Coulter Cytoflex S equipped with 488 nm and 638 nm lasers. Reporter channels for all experiments included 525 nm/40BP, 585 nm/42BP, and 660 nm/20BP. Color compensation for multicolor experiments was performed using unstained and individually stained platelets according to modern flow cytometry practices and in accordance with Beckman Coulter instructions. Acquisition setting and gating criteria were based on thresholds that revealed the CD61+ platelet population against isotype controls. For each experiment, 50 000 platelets (CD61+) were assayed.
2.5 Assessment of platelet adhesion
Platelets were prepared and labeled with calcein-AM as described above. Glass slides (Fisher Cat.# 12-544-2) and coverslips (Fisher Cat.# 12548B) were pre-cleaned with 70% denatured ethanol, rinsed with ultrapure water (Invitrogen Cat.# 10977015), and wiped dry. Platelets were incubated at room temperature for 10 min for all conditions including saline controls and each of the LAs at a 1:1 dilution. A 7.5 µL aliquot of each condition was seeded onto glass slides in duplicate. After 5 min, platelets were counted in four fields of view in two series, one unwashed (labeled as “prewash”) and the other washed in a PBS bath (labeled as “post-wash”) which displaced the coverslip and allowed us to quantify platelets adhering to the glass slide. Platelets were imaged and scored using an AMG EVOS FL inverted microscope under fluorescence with a 470/22BP nm excitation and 510/42BP nm emission light cube.
2.6 Data normalization
Using flow cytometry, we assessed calcein (viability dye) and Fluo-4 (calcium dye) signal to determine the percentage of viable platelets for each of the LA conditions relative to the saline control. Relative percentages were determined by dividing values from the treated sample by the saline control (relative % = sample/saline), wherein the saline control always equals 100%. Assessment of ROS production was based on MTO intensity via flow cytometry. LA-treated samples were normalized to the saline control using the median fluorescent intensity (MFI) and robust standard deviation (rSD) determined by CytExpert software. Normalization of the MFI was achieved by calculating the resolution metric (RD): (sampleMFI − controlMFI)/(samplerSD + controlrSD). The resulting output reports the fold-change over a background of the signal,33 wherein the saline value always equals “0%” (the sampleMFI and controlMFI subtracted are the same value, equaling “0”). No normalization was required for apoptotic assays. Platelet adhesion percentage was determined by dividing the average post-wash platelet count by the prewash platelet count of the respective condition.
2.7 Statistical analysis
Statistical significance of normalized data was determined by one-way ANOVA analysis with a 95% confidence interval using the Tukey multiple comparisons test in GraphPad Prism v8.1.2. Standard deviations are representative of repeated experiments from separate blood donors.
3 RESULTS
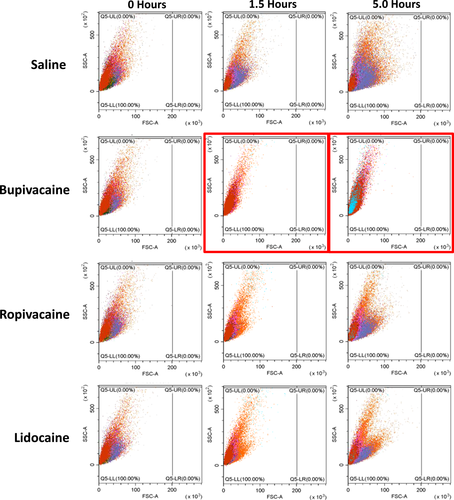
3.1 Platelets shrink when exposed to local bupivacaine
Initially, we observed a dramatic shift in platelet size (forward scatter channel [FSC] to the left) indicating platelet shrinkage within 5 h of exposure at a 1:1 dilution with bupivacaine 0.75%, ropivacaine 0.5%, and lidocaine 1% (thus at final concentrations of 0.375%, 0.25%, and 0.5% respectively). In bupivacaine samples, shrinkage occurred at an accelerated rate with platelets being the smallest compared with ropivacaine or lidocaine (Figure 1). By contrast, there was no change in platelet size or morphology in the saline control.
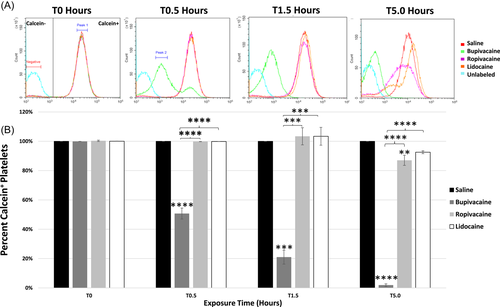
3.2 LAs diluted 1:1 affect platelet calcein signal
Bupivacaine induced a rapid loss of calcein signal at 0.5 h compared to all other conditions (p < .0001). This occurred in a biphasic fashion, indicating a temporal loss of calcein in two separate subcellular locations (Figure 2: peak 1, peak 2). After the initial separation of the two peaks, the overall intensity of the calcein signal reduced, with only a single peak observed (peak 2), indicating a substantial loss of cytoplasmic calcein and localization of signal to a specific organelle. Over the time course, calcein signal in platelets exposed to bupivacaine was reduced at each successive time point, differing from saline, ropivacaine, and lidocaine at 1.5 h (p < .001 for all) and 5.0 h (p < .0001 for all; Figure 2B). Depletion of calcein signal in ropivacaine-treated platelets differed from the saline control only at 5 h (p < .001). Calcein signal in platelets treated with lidocaine did not differ from ropivacaine or saline at any point.
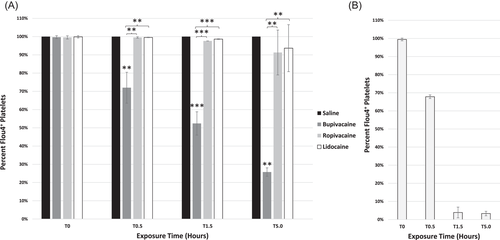
3.3 Dysregulation of intracellular calcium following platelet exposure to bupivacaine
Similar to calcein, the intracellular Flou-4 (calcium) signal was reduced in a biphasic fashion followed by depletion. At 30 min, bupivacaine-treated platelets differed from the saline control, ropivacaine, and lidocaine conditions (p < .01 for each comparison; Figure 3A). This negative trend with bupivacaine continued throughout the 5-h time course, differing from platelets treated with saline, ropivacaine, and lidocaine (1.5 h, p < .001 compared to all; 5 h, p < .01 for each comparison; Figure 3A). Ionophore A23187 was used as a control, inducing calcium dysregulation, resulting in the depletion of intracellular calcium (Figure 3B).
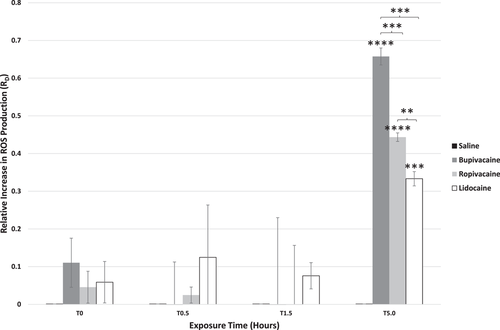
3.4 LAs increase ROS production in platelets
ROS production was assessed by labeling platelets with MTO to further confirm cellular stress and mitochondrial dysfunction noted by loss of calcein signal and dysregulation of calcium. Increases in ROS production were seen with all LA exposures and were inversely correlated with calcein signal (Figure 4). At 5 h, sharp increases in platelet ROS production were observed in all LAs. This was most prominent with bupivacaine, differing from saline (p < .0001), ropivacaine, and lidocaine (p < .001 for each). Ropivacaine and lidocaine induced increases in ROS over the saline control at 5 h (p < .0001 and p < .001, respectively) and platelets treated with ropivacaine produced significantly more ROS compared to those treated with lidocaine (p < .01).
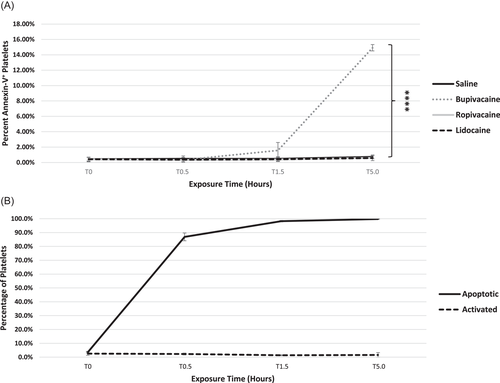
3.5 Bupivacaine induces platelet apoptosis
For each of the LAs, there was little change in the percentage of apoptotic platelets through 1.5 h. However, by 5 h, ~15% were apoptotic (AV+) in bupivacaine-treated samples differing from ropivacaine, lidocaine, and saline (p < .0001; Figure 5A). In platelets treated with the A23187 apoptotic control, there was a dose and time-dependent induction of platelet apoptosis (Figure 5B), with the percentage of apoptotic platelets exceeding 90% by 5 h. A23187-treated platelets which were incubated with CD62P did not show activation concurrent with apoptosis via AV+ labeling (Figure 5B). This was also the case in LA-treated platelets, where apoptosis occurred without significant activation (data not shown).
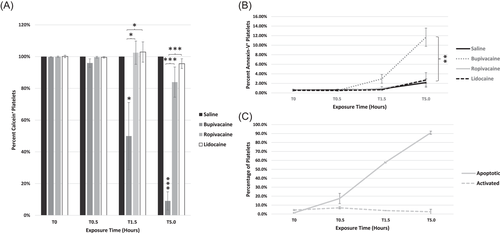
3.6 Bupivacaine diluted 1:3 to platelet preparation results in Calcein-Depletion and platelet apoptosis
To determine if further diluted LAs have a similar impact on platelet viability, the percentage of calcein-positive and apoptotic platelets was determined following exposure to LAs diluted 1:3. Bupivacaine resulted in a significant loss of calcein signal compared to all other conditions at 1.5 h (p < .05) and 5 h (p < .001; Figure 6A). The percentage of apoptotic (AV+) platelets exposed to any of the LAs diluted 1:3 was not significant compared to the saline control through 1.5 h of exposure (Figure 6B). However, by 5 h, the percentage of apoptotic platelets reached 12% with bupivacaine, differing from the saline control, ropivacaine, and lidocaine (p < .01 for all comparisons). By contrast, ropivacaine and lidocaine treated platelets did not show an increase in apoptotic cells compared to the saline control at any time point (Figure 6B). The apoptotic control A23187 at 2.5 µM induced apoptosis but not activation of platelets (Figure 6C).
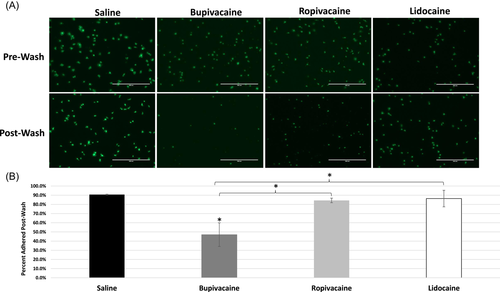
3.7 Local anesthetics impair platelet adhesion
To determine if LAs affect platelet adhesion, platelets were briefly exposed to saline or LAs, incubated on a glass slide, then counted pre- and postwash (Figure 7). In the saline control, adhesion occurred rapidly, with clear filopodia extending from the platelets (Figure 7A). Washing the slide resulted in minimal loss of platelets, indicating normal platelet adhesion (Figures 7A,B). In ropivacaine and lidocaine-treated platelets, filopodia formation appeared less robust and there were slightly fewer platelets, but this was not statistically different than saline. In sharp contrast, platelets briefly exposed to bupivacaine showed minimal filopodia formation and reduced adherence, differing from all other conditions (Figure 6B; p < .05 for all comparisons).
4 DISCUSSION
Over the course of our experiment, we observed significant changes in platelet physiology following exposure to lidocaine, ropivacaine, and bupivacaine. Bupivacaine showed the most deleterious effect on platelets, resulting in shrinkage, loss of calcein fluorescence, dysregulation of intracellular calcium, increase in mitochondrial ROS production, apoptosis, and impaired adhesion to glass. By contrast, lidocaine and ropivacaine at 1:1 dilutions were similar to saline and only differed with regard to ROS production at 5 h. Further, bupivacaine differed from lidocaine and ropivacaine in each platelet assay at each dilution tested over 5 h. Our findings demonstrating the toxicity of bupivacaine on platelets parallel those of others demonstrating its toxicity on chondrocytes and mesenchymal stromal cells (MSCs).17, 19
The duration of observation and measurement in our study was 5 h, given that the half-lives of lidocaine, bupivacaine and ropivacaine within joints are 1.5, 3, and 6 h respectively.17 In clinical practice, typically the final PRP preparations range from 2–4 mL in volume, and 1–2 mL of LA are often injected into the tissues beforehand or mixed with the platelet preparation before injection.24, 25 Thus, the ratio of LA to platelet preparation may range from 1:4 to 1:1. We, therefore, used ratios of LA to platelet preparations of 1:1 or 1:3. As early as 0.5 h, we observed that platelets exposed to bupivacaine (1:1) resulted in platelet shrinkage, a morphological change associated with platelet dysfunction, degranulation, apoptosis, or death.34, 35 To investigate the possibility of platelet death, platelets were stained with the viability dye calcein-AM. There was a rapid and robust reduction of intracellular calcein signal in bupivacaine-treated platelets compared to saline, ropivacaine, and lidocaine. Interestingly, the reduction of signal in platelets was biphasic, indicating the occurrence of an intracellular event beyond viability and subcellular retention of calcein before the complete loss. The same trend was observed for both ropivacaine and lidocaine but to a substantially lower degree. Normally mitochondria are impermeable to calcein, but in pathologic states, their permeability transition pores open. When this occurs, calcein localizes to the mitochondria from the cytosol,36 possibly producing the biphasic shift we observed. In support of this concept, Panel et al.37 showed that dysregulation of intracellular calcium resulted in the temporary localization of calcein to the mitochondria, followed by loss of mitochondrial integrity and calcein signal resulting in cell death.37
While calcein, which also binds magnesium, is a nonspecific indicator of calcium, it has historically been used for this purpose.38, 39 To determine if the shift in calcein signal was indicative of intracellular calcium movement, we labeled platelets with Fluo-4 dye which binds calcium with high affinity and becomes intensely fluorescent. Typically in events of calcium dysregulation, cytosolic calcium increases as the result of both calcium release from intracellular stores and calcium entering from the extracellular environment because of increased plasma membrane permeability.40 We suspended platelets in calcium-free saline, thereby reversing the normal extracellular–intracellular calcium gradient. In the event that intracellular calcium stores are released and the plasma membrane becomes permeable to calcium, intracellular calcium would be depleted. Platelets labeled with Fluo-4 showed a rapid loss of intracellular calcium following exposure to bupivacaine. The bupivacaine-treated platelets mirrored those treated with ionophore A23187, designed specifically to dysregulate calcium, thus providing evidence that bupivacaine exerts its effect at least in-part via calcium dysregulation.
Calcium dysregulation is often associated with mitochondrial dysfunction. To assess changes in mitochondrial activity, platelets were labeled with MTO, where an increase in signal within reflects the production of mitochondrial ROS.28, 40 The intensity of the dye will accumulate in control conditions indicative of mitochondrial activity; however, sharp increases indicate mitochondrial stress. Elevated ROS is seen with mitochondrial dysfunction and often followed by apoptosis.42 We found that 5 h of platelet exposure to all LAs tested resulted in significant increases in ROS production, particularly with bupivacaine. Others have found that bupivacaine can uncouple mitochondrial complex I,43 lead to calcium loss44 and increased ROS45 in mitochondria isolation experiments, while ropivacaine and lidocaine can affect mitochondrial O2 consumption and ATP production, but not cell toxicity to the same degree as bupivacaine.43
Our findings of reduced intracellular calcein and calcium signal and elevated ROS production with bupivacaine suggest improper mitochondrial function and probable apoptosis. Using Annexin-V (AV), we found that bupivacaine exposure resulted in dose-dependent platelet apoptosis affecting ~12% and ~15% of platelets at respective 1:3 and 1:1 dilutions at 5 h. We used the ionophore A23187 as a control to successfully induce apoptosis in platelets at 10 µM and 2.5 µM as expected. Neither A23187 nor any of the LAs resulted in platelet activation, verifying that AV+ labeling was an accurate measure of apoptosis without concurrent activation as noted by others.46, 47
While it is clear that bupivacaine induces platelet apoptosis, it is unclear whether loss of calcein indicated other modes of platelet death such as necrosis. Because platelets do not have cell nuclei, we were unable to assess necrosis via the usual methods. Conservatively, the estimate of nonviable platelets should include the calcein-depleted populations as well, indicating the potential negative impact of lidocaine and ropivacaine.
The disruption of intracellular calcium after exposure to LAs, especially bupivacaine, raised the possibility of defective calcium-dependent processes in platelets, including adhesion, and spreading/filopodia formation.14, 48 In our study, platelet adhesion was significantly impaired with bupivacaine. Nachmias et al.22 observed reduced platelet microspike (sub-filopodia) formation following exposure to lidocaine and suggested this was possibly attributable to LA-dependent changes in intracellular calcium levels.22 These findings have significant clinical implications, as platelet adhesion, activation, degranulation, and contraction appear to be important in treating orthopedic injuries.27, 14, 49
A limitation of our study is that we do not precisely know the pharmacokinetics of the LAs used in vitro and in vivo. The 5-h duration of our experiments was at the upper limit of the duration of effect we see clinically when using these LAs with PRP (1–4 h) and similar to the published duration of effect for these LAs at the concentrations used for peripheral nerve and soft tissue blocks.50 An additional limitation was the inability to measure absolute platelet death. While calcein fluorescence serves as a conservative and reasonable marker of viability, it is unclear if its decline was an indicator of cell death or simply movement of divalent cations, including calcium, escaping through compromised cell membranes, paralleling Flou-4 signal. Finally, the platelet preparations used were diluted below the threshold for therapeutic-graded PRP to allow real-time assessment via flow cytometry at each time interval with the respective LA concentrations. While it is theoretically possible that platelets concentrated to >1,000,000/µl as in the clinical setting may respond to these LAs differently to some extent, each individual platelet is exposed to the same final concentration of the LAs and the presence of other platelets farther or closer is unlikely to have an influence on LA toxicity.
In this study, we aimed to investigate the impact of LAs on platelet physiology, viability, and function. The results clearly demonstrate that bupivacaine should be avoided, while ropivacaine 0.5% and lidocaine 1% were safe at the 1:1 dilution used with PRP. Further research on ropivacaine and lidocaine would be indicated to assess the lower threshold for mitochondrial stress and a more sensitive assessment of platelet contraction force. Finally, it should be noted that any dilution of PRP with LAs will reduce platelet concentrations accordingly.
5 CONCLUSION
Bupivacaine was detrimental to platelets, causing shrinkage, calcium dysregulation, reduction of adhesion, and apoptosis. Lidocaine and ropivacaine at the concentrations used had minimal effect on platelet metabolism, viability, and adhesion. Our results stress the importance of carefully selecting the type of LA, concentration, and volume when performing treatments involving platelets and PRP. Our findings should be considered when interpreting the results of clinical research studies in which LAs were used.
AUTHOR CONTRIBUTIONS
R.C.D. analyzed the data, conceived, designed, drafted, revised the manuscript, and finally approved the submitted draft. Y.U. acquired and analyzed the data and drafted and revised the manuscript and finally approved the submitted draft. M.B. conceived and revised the manuscript and finally approved the submitted draft.




