The effect of estrogen-like compound on rotator cuff tendon healing in a murine model
Level of Evidence: Basic Science
Abstract
Estrogen deficiency has been shown to negatively influence rotator cuff tendon healing. Therefore, the addition of an estrogen-like-compound (ELC) in a nonestrogen-deficient animal may improve the quality of a rotator cuff repair. The purpose of this study was to evaluate the effects of an ELC, diethylstilbestrol (DES), on tendon healing in a murine rotator cuff repair model. Thirty-three male wild-type mice (C57BL/6NJ) were randomly divided into three study groups. Group 1—unoperated mice with normal rotator cuff tendons. Groups 2 and 3 consisted of surgically repaired rotator cuff tendons; Group 2 (repair-only) was the standard repair group (no DES injected), whereas Group 3 (repair + DES) was the experimental repair group (injected with DES). Comparing the maximal thickness of calcified fibrocartilage to uncalcified fibrocartilage, the ratios for the control (intact tendon), repair-only, and repair + DES groups were 2:1, 0.9:1, and 1.7:1. RNA expression data demonstrated upregulation of chondrogenic, angiogenic, and tendon modulation genes in the repair- only group compared to the control (intact tendon) group (p < 0.04 for all), and that addition of DES further increased the osteogenic, angiogenic, and tendon modulation gene expression compared to the repair-only group (p < 0.02). Immunohistochemical analysis indicated that the addition of DES further increased osteogenic, angiogenic, and tendon maturation protein expression at the enthesis compared to standard repairs.
1 INTRODUCTION
Tendon healing after rotator cuff repair can be challenging with failure of healing in up to 60% of patients utilizing current, biomechanically optimal surgical constructs.1, 2 Rotator cuff tendons will typically heal after repair in a disorganized, scar-mediated fashion not replicating the original native tendon–bone enthesis transitioning from tendon to uncalcified fibrocartilage (UFC), to calcified fibrocartilage (CFC), to bone.3 Recreation of the native tendon insertion with reduction of the amount of immature fibrovascular tissue at the repair site is a primary goal in improving tendon healing. Hormone-related modulation of tendon repair has been previously demonstrated to be important in appropriate tendon repair maturation.4 Estrogen-like compounds (ELC) likely have an important positive effect on tendon healing through cell proliferation and matrix synthesis.5 Limited data exist on the influence of hormones or ELCson rotator cuff repair healing.6
Various biologic augmentations have been examined in animal rotator cuff repair models. A majority of the research on biologic augmentation of rotator cuff repairs have focused on the addition of growth factors to accelerate or strengthen rotator cuff repairs. Vascular endothelial growth factor-A (VEGFA), Fibroblast growth factor 2 (FGF2), Platelet-derived growth factor-BB (PDGF-BB), and Transforming growth factor-β1 (TGF-β1) have all been shown to positively influence histologic or biomechanical characteristics after rotator cuff repair in mouse and rat models.7-11 Hormonal regulation has been evaluated as well. Local administration of parathyroid hormone in conjunction with an absorbable scaffold demonstrated positive effects on biomechanical and histologic outcomes of repairs in a rat model.12 Tanaka et al.,6 recently evaluated the effects of the estrogen-deficient state on rotator cuff tendon healing in a rat ovariectomized model. The authors reported inferiorbiomechanical strength and histologic properties (greater immature granulation tissue, absent chondroid tissue) in the ovariectomy group, suggesting the importance of estrogen with respect to tendon repair healing.6 However, a literature review indicates that no studies have evaluated the effects of an ELC following a rotator cuff repair in a nondeficient state.
This study evaluated the effects of the ELC diethylstilbestrol (DES) on tendon repair in a nonestrogen-deficient state in a mouse rotator cuff repair model. Both histologic and gene expression evaluations were performed comparing repairs with and without the addition of DES. We hypothesized that the addition of DES would improve the histology and gene expression profiles of rotator cuff repairs comparable to the intact rotator cuff tendon enthesis.
2 METHODS
2.1 Diethylstilbestrol
United States Pharmacopeia grade DES (Sigma-Aldrich) was dissolved in sesame oil (Sigma-Aldrich) at a final concentration of 0.15 mg/ml. Mice in Group 3 (treatment group, see groupings below) received a daily intraperitoneal (ip) injection with 0.2 ml of 0.15 mg/ml DES (30 μg/day). Mice in Group 2 (vehicle control group, see groupings below) received a daily ip injection of sesame oil. DES was chosen as the ELC due to its high affinity for estrogen receptors with more than fivefold potency compared to estradiol and that it has been utilized in a male murine model with success.13
2.2 Animal model and groups
Animal work was performed in accordance with laws for the care and use of laboratory animals with approval by the University of Utah's Institutional Animal Care and Use Committee (IACUC Protocol number 18-09005). Thirty-three male wild-type mice (C57BL/6NJ) were utilized for all experiments.
For histological and scanning electron microscopy (SEM) evaluation, a total of 23 mice were utilized. Mice were randomly divided into three groups (Table 1). Group 1 consisted of unoperated mice, which served as intact “control” shoulders. The mice were sacrificed without any surgical procedure to observe an intact, normal rotator cuff tendon–bone interface. Groups 2 and 3 consisted of surgically repaired rotator cuff tendons (surgical detachment and reattachment); Group 2 (repair-only group) was the “standard” repair group (sesame oil injection; no DES), whereas Group 3 (repair + DES group) was the experimental repair group (injected with DES).
| Study groups | Histological analysis (4 weeks) | Gene expression (2 weeks) |
|---|---|---|
| Control | n = 5 | n = 6 |
| Repair-only | n = 10 | n = 3 |
| Repair + DES | n = 8 | n = 3 |
| Total | n = 23 | n = 6 |
- Note: The contralateral shoulders were used for the “control” specimens for gene expression analysis.
- Abbreviation: DES, diethylstilbestrol.
For gene expression experiments a total of six mice were utilized; three mice were in the repair group injected with vehicle (repair-only group) and three in the repair group injected with DES (repair + DES group). The contralateral shoulder was used as a control intact rotator cuff tendon tissue for each repair group.
For immunohistochemical analysis, four mice had surgically repaired rotator cuff tendons. Two mice were injected with vehicle and two were injected with DES.
2.3 Surgical procedure, DES injections, and postprocedural monitoring
The surgical technique was modified from Lebaschi et al.14 Animals were placed under anesthesia using isoflurane and positioned in a holder for the duration of the surgical procedure. The surgery was performed under ×3.5-loupe magnification to ensure proper repositioning of the tendon to the bone interface. An incision was made starting just above the clavicle extending distally over the acromion directed to the elbow for ~2 cm. Subcutaneous flaps were mobilized with blunt dissection until the deltoid was identified with the overlying vasculature delineating the borders of the muscle. The deltoid was sharply elevated off the acromion to expose the underlying supraspinatus tendon. The arm was adducted and externally rotated to deliver the rotator cuff out from under the coracoacromial arch (Figure 1A). A suture hook was placed under the tendon and a 6-0 Prolene double-armed suture (Ethicon) secured in a modified Kessler suture pattern starting midsubstance of the supraspinatus tendon with one needle and completing the configuration with the second needle (Figure 1B). With traction on the stitch, the tendon was released from the humerus with a No.-15 blade (Figure 1C). Full mobility of the tendon was confirmed and debridement of the insertion site performed (Figure 1D). Two crossing bone tunnels were drilled using a 0.0138-in. jewelers drill bit by hand (Figure 1E). The first was drilled from the anterior footprint in a posteroinferior direction and the second drilled from the posterior footprint in an anteroinferior direction. The tunnels exited at the surgical neck.
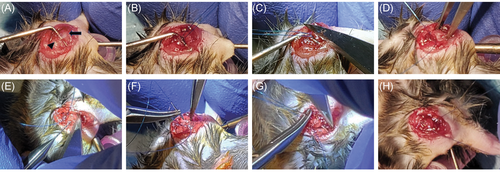
The needles were passed through the tunnels and tied (Figures 1F-H). The deltoid was reflected back over the humerus and the skin incision closed with running 6–0 Prolene sutures. Local anesthesia was then infiltrated into the skin incision. Following the surgical procedure, the mice were recovered and monitored daily for any signs of distress.
The mice were housed in cages and maintained on a normal chow diet with water ad libitum. Group 2 mice (repair-only) were injected IP daily with 0.2 ml of sesame oil as a control vehicle substance. Group 3 (repair + DES) mice were injected ip daily with 0.2 ml of a 0.15-mg/ml DES solution (30 μg/day). At 2 and 4 weeks postoperation, the mice were humanely euthanized for gene expression (n = 6), histological/SEM (n = 23), and immunohistochemical (n = 4) analyses, respectively (Table 1).
2.4 Histological processing
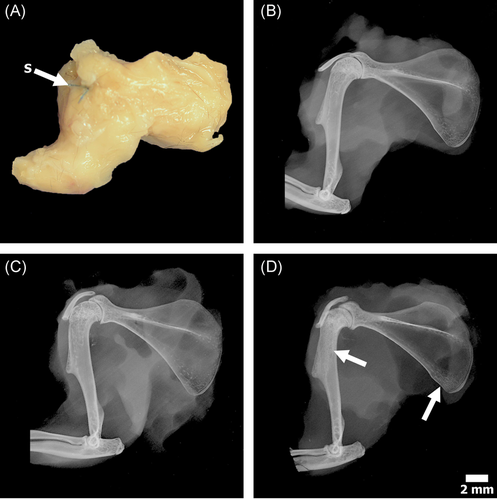
Shoulders were dissected before histological processing; skin and unnecessary tissue were removed leaving a construct consisting of the humerus, scapula, the four attached rotator cuff musculotendinous units, and coracoacromial arch (Figure 2A). The specimens were radiographed in a cabinet X-ray system (43855A; Faxitron) set at 90 kV for 1 min and then fixed in 10% neutral buffered formalin for a minimum of 72 h (Figure 2B-D). Following fixation, the specimens were dehydrated in ascending grades of ethanol, infiltrated, and embedded in poly-methyl-methacrylate (PMMA).15 The PMMA blocks were trimmed of excess PMMA and faced using an IsoMet 1000 Precision Cutter, then ground by hand using a variable-speed grinding wheel (Buehler Incorporated). The coarsely ground specimens were surface stained with Acid Fuchsin (~10 s) throughout the grinding process to enhance the visualization of the supraspinatus, ensuring the tendon–bone interface was captured. When the plane of interest was located, the specimen was polished to an optical finish for SEM imagining.
2.5 Scanning electron microscopy
The polished specimens were coated with a conductive layer of carbon for ~25 s with a High Vacuum Turbo Carbon Coater 208C (Ted Pella) for backscattered electron (BSE) imaging using a JEOL JSM-6610 (JEOL) SEM. The BSE detector allows for high-resolution images of bone morphology, as well as the ability to observe areas of CFC due to the structure and higher mineral content.16, 17 Images were captured at ×40 magnification (3.2 × 2.4 mm field of view) with a resolution of 2560 × 1920 pixels predominantly focused on the proximal humeral head (Figure 3). SEM images were used to evaluate the overall bone density of the proximal humerus measured as bone volume (BV) (% bone within the humeral head), as well as the area (CFC area; mm2) and maximal thickness (CFC thickness; μm) of the CFC at the tendon insertion.
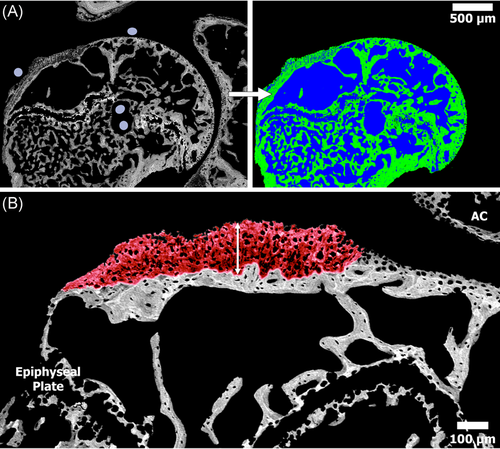
More specifically, to analyze the %BV, digital BSE images were opened in Adobe Photoshop to remove the glenoid and acromion leaving only the proximal humeral head. The images were imported into a custom MATLAB program which uses gray-level thresholding to determine the percentage of bone (gray) versus pore (black) space (Figure 4A).18 A similar process was used to determine the CFC area (mm2) from the digital BSE images; CFC from the tendon attachment site was able to be distinguished from the CFC of the articular cartilage due to the structural and mineralization difference as described by Karns et al.17 (Figure 4B). The CFC thickness measurements were obtained using the calibrated BSE images and analysis software, FIJI (ImageJ), which allowed for the maximal thickness of the CFC at the tendon–bone interface to be measured.
2.6 Light microscopy
Following SEM imaging, PMMA blocks were further polished to remove the thin layer of carbon and adhered to plastic slides using ExaktTechnovit 7210 with a precision adhesive press (EXAKT Technologies). The specimens were then ground to a final thickness of ~75 µm. The slides were stained with Sanderson's rapid bone stain (tissue/cellular components)and acid fuchsin (bone/CFC mineralization) to visualize the tendon–bone interface.19, 20 The stained sections were viewed under a light microscope (Nikon E600; Nikon Inc.) equipped with associated image capturing software (Camera Control Pro; Nikon) and digital images were captured at the tendon–bone interface (Figure 5). On the basis of prior mouse rotator cuff repair data of Lebaschi et al.,14 all repairs will typically heal; consequently, analysis of the repairs were focused on the quality of the repair instead of if healing or lack of healing (gap formation) occurred. Due to the slight variations within the mice and sectioning plane, a uniform 0.28-mm2 (700 × 400 μm) area was extracted at the tendon–bone interface for all data analyses. The extracted region of the tendon–bone interface was analyzed for the total number of chondrocytes within the UFC (chondrocyte count), the area of UFC (UFC area; mm2), and the maximum thickness of the UFC (UFC thickness; μm). All light microscopy measurements were obtained on the calibrated extracted region of the tendon–bone interface using the analysis software, FIJI (ImageJ).
3 GENE EXPRESSION ANALYSIS
To determine if we could detect changes in RNA gene expression in our tendon repair model, we performed differential gene expression on RNA using a custom Nanostring panel. We isolated RNA from the whole mouse repaired shoulders and the unoperated contralateral control shoulder as control 14 days postsurgery. Six mice were subjected to the tendon repair surgery; three were injected daily with sesame oil (repair-only), and three with DES (repair + DES) for 14 days. Mice were euthanized and the skin over both shoulders was incised to reveal the acromioclavicular joint. The surrounding muscle was removed and the scapula and humerus were cut approximately 2-4-mm distal from the acromioclavicular joint. The entire joint was placed in RNAlater (Sigma-Aldrich) and stored at −80°C. Total RNA was extracted from tissue samples using the Direct-zol RNA Miniprep kit (Zymo Research). Both shoulders were utilized for analysis with the contralateral shoulder being used as the control intact rotator cuff for each group.
RNA gene expression was analyzed using the Nanostring nCounter platform by the Molecular Diagnostics Core at the University of Utah. A custom nCounter® assay (NanoString Technologies) was designed for quantitative assessment of expression of 28 gene elements. In addition to the 28 genes, there were three housekeeping genes, six positive control genes, and eight negative control genes included in the same panel totaling 45 genes analyzed. The 28 genes we evaluated various biologic aspects of rotator cuff repair healing and hormonal control are listed in Table 2.
| Gene | Type |
|---|---|
| Aggrecan (Acan) | Chondrogenic |
| Sex-determining region Y-box 9 (Sox9) | Chondrogenic |
| Collagen, type II, alpha 1 (Col2a1) | Chondrogenic |
| Hypoxia-inducible factor 1 alpha (Hif1α) | Angiogenic |
| Platelet/endothelial cell adhesion molecule 1 (Pecam1) | Angiogenic |
| Vascular endothelial growth factor a (Vegfa) | Angiogenic |
| Alkaline phosphatase liver/bone/kidney (Alpl) | Osteogenic |
| Osterix (Osx/Sp7) | Osteogenic |
| Beta-catenin (β-catenin) | Osteogenic |
| Wnt | Osteogenic |
| Indian hedgehog (Ihh) | Osteogenic |
| Bone sialoprotein (Ibsp) | Osteogenic |
| Collagen, type 1, alpha1 (Col1a1) | Tendon |
| Matrix metalloproteinases 3, 9, 13 and 14 (Mmp3, 9, 13, 14) | Tendon |
| Tenomodulin (Tnmd) | Tendon |
| Glioma-associated oncogene 1 (Gli1) | Tendon |
| Transforming growth factor-beta 1 (Tgf-β1) | Tendon |
| Platelet-derived growth factor-a (Pdgfa) | Tendon |
| ScleraxisA/B (ScxA/B) | Tendon |
| Estrogen receptor 1 (Esr1) | Hormonal |
| Progesterone receptor (Pgr) | Hormonal |
| Growth regulation by estrogen in breast cancer 1 (Greb1) | Hormonal |
| Estrogen-related receptor beta (Esrrβ) | Hormonal |
| Cyclin F (Ccnf) | Hormonal |
| Cyclin-dependent kinase inhibitor p21 (Cdkn1a) | Hormonal |
| Bone morphogenic protein 4 (Bmp4) | Hormonal |
| Estrogen-related receptor alpha (Esrrα) | Hormonal |
| Glutaredoxin 2 (Glrx2) | ROS scavenger |
Gene counts were normalized in the nSolver™ Software package using background thresholding (threshold count value: 10) and positive control and housekeeping genes (using the geometric mean). Normalized data was exported and the Linear Models for Microarray Data (limma) R package (https://bioconductor.org/packages/release/bioc/html/limma.html) was used to compute differential gene expression.21 We analyzed gene expression using two models, a combined and additive model. For the combined model, we compared differential gene expression between vehicle-treated repaired shoulders (repair-only) to contralateral controls and vehicle-treated repaired shoulders (repair-only) to DES-treated repaired shoulders (repair + DES). For the additive model, we compared the effect of DES on repair by using the average of drug-repaired (repair + DES) and drug-contralateral shoulder RNA gene counts versus the average of vehicle-repaired (repair-only) and vehicle-contralateral shoulder RNA gene counts. For each condition we analyzed three biological replicates.
4 IMMUNOHISTOCHEMISTRY
Shoulders were dissected as described above and fixed in 10% neutral buffered formalin at 4°C for 48 h, processed through an ethanol series, equilibrated in xylenes, and embedded in paraffin. Five to six micrometers of coronal sections tissue sections were cut and mounted onto slides. Tissue sections were deparaffinized and rehydrated as follows: Xylene 3 × 5 min, 95% EtOH 2 × 3 min, 70% EtOH 2 × 3 min, ddH2O 2 min, and 1× PBS for 5 min. Antigen retrieval was performed by incubating all slides in citrate buffer (10 mM, pH 6.0) at 90°C for 10 min followed by peroxidase blocking for 10 min at 25°C using BLOXALL (SP-6000-100; Vector Laboratories). Slides were rinsed 2 × 5 min in phosphate-buffered saline [PBS], incubated for 1 h at 25°C in blocking solution (5% fetal bovine serum and 1% BSA in PBS), and then rinsed 2× 5 min in PBS at 25°C.
All antibodies were obtained through Abcam: Sp7/Osterix (22552), Greb1 (72999), Sox9 (185966), p21 (188224), Tnmd (203676), and Pdgf-AA (216619). All antibodies were diluted 1:200 dilution in blocking solution. Tissue sections were incubated with primary antibody at 4°C, for 18–24 h in a humidified chamber, rinsed 3× with PBS, and then blocked with 2.5% horse serum (MP-7401; Vector Laboratories) for 20 min at 25°C. Block solution was replaced with ImmPress-HRP horse anti-rabbit secondary antibody (MP-7401; Vector) and incubated for 30 min at 25°C followed by 2 × 5 min rinses with PBS. Lastly, slides were stained with 3,3′-diaminobenzidine (SK-4100; Vector Laboratories) for 10–20 min, rinsed for 5 min with PBS, dried, and mounted.
5 QUANTITATIVE POLYMERASE CHAIN REACTION (QPCR)
The same RNA used for Nanostring analysis was used for qPCR analysis. Briefly, 1 µg of total RNA was reverse-transcribed into complementary DNA, and Tnc and β-actin were amplified as previously described.22 The copy number of Tnc was normalized to 1,000 copies of β-actin as previously described.23 Primers used are as follows: β-actin F 5’-GTAACAATGCCATGTTCAAT-3’, β-actin R 5’- CTCCATCGTGGGCCGCTCTAG, Tnc F 5’-GAGACCTGACACGGAGTATGAG-3’, and Tnc R 5’-CTCCAAGGTGATGCTGTTGTCTG-3’.
5.1 Statistical analysis
Student's t tests were utilized to determine significance between histological (SEM and light microscopy) data sets. p Values with α = 0.05 were considered statistically significant. For the determination of gene expression differences in limma, we used the Benjamini and Hochberg false discovery rate (FDR) for multiple testing corrections. An FDR of 5% was used and p < 0.05 were considered statistically significant. To determine the statistical significance of Tnc expression between control, repair-only, and repair + DES samples by qPCR, we used a two-way analysis of variance with Tukey's test to adjust for multiple comparisons. p Values < 0.05 were considered statistically significant.
6 RESULTS
6.1 Gross and SEM analyses
Qualitatively, no gross differences were observed between the dissected shoulders, that is, no infection, hematoma and/or tissue necrosis were apparent across all specimens. The contact radiography of the repair + DES (Group 3) specimens demonstrated increased trabecular density at the surgical neck of the humerus and inferior angle of the scapula. However, the diaphysis of the long bone appeared unaltered (Figure 2B-D). No radiographic differences were observed between the control (Group 1) and repair-only (Group 2) shoulders with the exception of the small defect created by the suture.
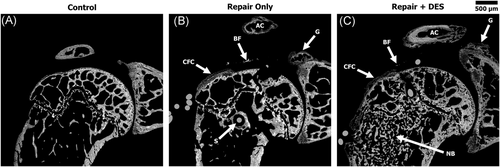
BSE images provided a more detailed analysis of the bone/CFC response compared to the radiography. Qualitatively, both the repair-only (Group 2) and repair + DES (Group 3) bone appeared to tolerate the surgery well. Thetrabeculaeconsolidated/remodeled around the suture tunnel leaving a small ~50-μm fibrous tissue gap with no significant reaction. Loose bone fragments from the drilling processes could be seen in the adjacent tissue, confirming the bone surface was prepped before reattachment of the tendon. Both repair groups demonstrated a bone response stemming from the neck of the glenoid and within the acromion (Figure 3).
Quantitatively, BV analysis indicated that the percentage of bone within the proximal humeral head was significantly greater (p < 0.001) in the repair + DES group (50.5% ± 7.4%) when compared to both the Control (29.3% ± 3.4%) and the repair-only (32% ± 4.5%) groups. No statistical difference in BV was observed between the control and repair-only groups (Table 3). In contrast to the BV data, the repair-only group contained the largest CFC area (0.086 ± 0.019 mm2) followed by the repair + DES (0.062 ± 0.026 mm2) and control (0.042 ± 0.02 mm2) groups. No statistical significance (p = 0.1739) was observed when comparing the CFC area of the repair + DES to control shoulders (Table 3 and Figure 4). The maximal thickness of the CFC correlated with the CFC area analysis demonstrating a greater CFC thickness in the repair-only group (178 ± 59 μm) followed by the repair + DES (139 ± 48 μm) and Control (41 ± 8 μm) groups (Table 3 and Figure 4).
| Data | p Value | |||||
|---|---|---|---|---|---|---|
| SEM analysis | Control | Repair-only | Repair + DES | Control versus repair-only | Control versus repair + DES | Repair versus repair + DES |
| Bone volume (%) | 29.3 ± 3.4 | 32 ± 4.5 | 50.5 ± 7.4 | 0.2608 | 0.0001 | 0.0001 |
| CFC area (mm2) | 0.042 ± 0.02 | 0.086 ± 0.019 | 0.062 ± 0.026 | 0.0012 | 0.1729 | 0.0346 |
| Max CFC thickness (μm) | 83 ± 35 | 178 ± 59 | 139 ± 48 | 0.0144 | 0.0477 | 0.2171 |
- Note: Boldface indicates statistical significance (p < 0.05).
- Abbreviations: CFC, calcified fibrocartilage; DES, diethylstilbestrol; SEM, scanning electron microscopy.
6.2 Light microscopy
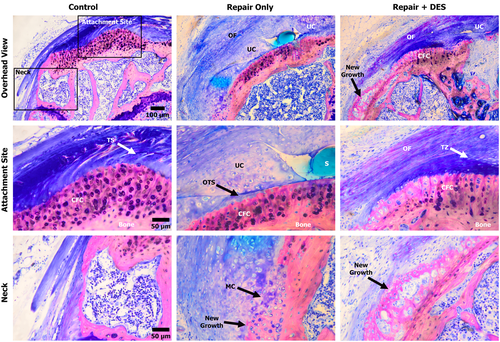
Qualitatively, the repair-only and repair + DES groups both showed organizing fibers above the tendon-healing site via polarized light and light microscopy. It was too early in the healing response to be comparable to the Control native enthesis with regard to fiber organization, however, the analysis did reveal a higher degree of fiber organization in the repair + DES shoulders compared to the repair-only shoulders (Figure 5). The alignment of fibers in the repair + DES shoulders could be seen stemming across the original tendon site down the neck of the humerus where the sutures were anchored to the bone (Figure 5). The chondrocytes within the UFC adjacent to the CFC at the tendon–bone interface appeared large and unorganized with limited columnar organization in both the repair-only and repair + DES groups.
Quantitively, the number of chondrocytes in the zone of UFC were significantly greater (p < 0.05) in the repair-only group (137 ± 75) compared to the repair + DES (67 ± 40) and Control (26 ± 6) groups (Table 4). UFC area analysis correlated with the chondrocyte count, showing the repair-only group contained the largest area (0.106 ± 0.053 mm2) followed by the repair + DES (0.051 ± 0.018 mm2) and Control (0.019 ± 0.006 mm2) groups. No statistical differences were observed when comparing the UFC area per chondrocyte, suggesting the number of chondrocytes predicts the area of the UFC (Table 4).
| Data | p Value | |||||
|---|---|---|---|---|---|---|
| Light microscopy analysis | Control | Repair-only | Repair + DES | Control versus repair-only | Control versus repair + DES | Repair versus repair + DES |
| Chondrocyte Count (#) | 26 ± 6 | 137 ± 75 | 67 ± 40 | 0.011 | 0.0463 | 0.0497 |
| UFC area (mm2) | 0.019 ± 0.006 | 0.106 ± 0.053 | 0.051 ± 0.018 | 0.0064 | 0.003 | 0.0173 |
| UFC area (μm2) per chondrocyte | 752 ± 173 | 798 ± 147 | 853 ± 243 | 0.6694 | 0.4382 | 0.6544 |
| Max UFC thickness (μm) | 41 ± 8 | 188 ± 63 | 84 ± 23 | 0.0009 | 0.0028 | 0.0012 |
| CFC versus UFC thickness (ratio) | 2:1 | 0.9:1 | 1.7:1 | - | - | - |
- Note: Boldface indicates statistical significance (p < 0.05).
- Abbreviations: CFC, calcified fibrocartilage; DES, diethylstilbestrol; UFC, uncalcified fibrocartilage.
The maximal UFC thickness of the repair-only group (188 ± 63 μm) was also determined significant (p < 0.0012) when compared to the repair + DES (84 ± 23 μm) and control (41 ± 8 μm) groups. When comparing the maximal thickness of the CFC from the SEM analysis to the maximal thickness of UFC from the light microscopy, the ratios for the control intact tendons, repair-only, and repair + DES groups were 2:1, 0.9:1, and 1.7:1, respectively. The data demonstrated that the addition of DES maintained a closer CFC/UFC ratio as the Control intact tendons (Table 4).
6.3 Gene expression
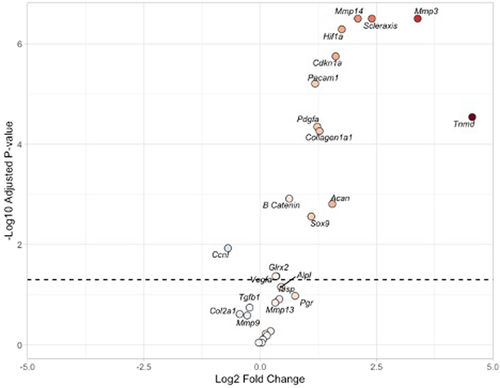
To determine if DES treatment altered transcription of genes following rotator cuff repair, we examined the expression of genes involved in joint tissue remodeling. In the comparison between repairs treated with vehicle (repair-only) and the control tissue, we found significant upregulation of tendon remodeling and maturation genes including Scx, Mmp 3 and 14, Tnmd, Pdgfa, and Col1a1. We also found upregulation of angiogenic genes including Hif1α, Pecam1, and Vegfa. Some chondrogenic genes were upregulated including Sox9 and Acan. The osteogenic gene β-catenin was upregulated. Reactive oxygen species scavenger Glrx2 was upregulated. Finally, hormonal downstream products of Esrrβ Cdkn1a (also called p21) were upregulated whereas Ccnf was downregulated (Table 5 and Figure 6).
| Gene | logFC | p Value | Adj. p value |
|---|---|---|---|
| Mmp3 | 3.620365 | 3.26E−10 | 4.8033E−09 |
| Scx | 2.473298 | 3.43E−10 | 4.8033E−09 |
| Mmp14 | 1.950306 | 1.33E−09 | 1.2405E−08 |
| Hif1α | 1.711377 | 2.06E−09 | 1.4391E−08 |
| Pecam1 | 1.141483 | 4.74E−08 | 2.6538E−07 |
| Cdkn1a | 1.336324 | 6.07E−08 | 2.8349E−07 |
| Tnmd | 4.650974 | 1.61E−07 | 6.4398E−07 |
| Pdgfa | 1.002044 | 3.03E−06 | 1.0594E−05 |
| Ccnf | −1.06277 | 9.99E−06 | 2.978E−05 |
| Acan | 1.727127 | 1.06E−05 | 2.978E−05 |
| Sox9 | 1.282258 | 1.56E−05 | 3.9731E−05 |
| Col1a1 | 0.878308 | 2.23E−05 | 5.2E−05 |
| β-catenin | 0.53523 | 8.32E−05 | 0.00017911 |
| Vegfa | 0.324049 | 0.003808 | 0.00761633 |
| Mmp13 | 0.42393 | 0.006019 | 0.01123485 |
| Glrx2 | 0.244233 | 0.02316 | 0.04053024 |
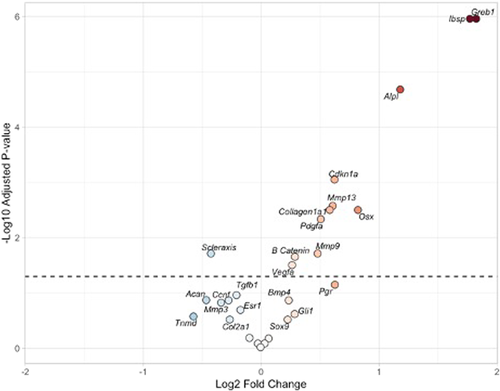
In the comparison between the repair + DES group and the repair-only group, we determined the addition of DES significantly increased the expression of a variety of gene products. First, Greb1 was upregulated which is a downstream product of estrogen receptor supporting DES activity through the estrogen receptor. Cdnk1a is a downstream product of Esrrβ and again showed an increased activity compared to repairs without DES (repair-only group). A significant increase in osteogenic genes including Ibsp, Alpl, β-catenin, and Osx (also called sp7) was identified in repair + DES versus repair-only. DES addition further increased angiogenic genes including Vegfa. Tendon-remodeling genes also increased including Mmp9, Mmp13, and Pdgfa supporting further maturation of the tendon insertion (Tables 6 and S1 and Figures 7 and S1). Together these data indicate that we can detect quantitative changes in gene expression in our tendon-repair model and that gene expression changes induced by DES treatment support our histological data demonstrating improved repair.
| Gene | logFC | p Value | Adj. p value |
|---|---|---|---|
| Ibsp | 1.769774 | 4.8E−08 | 1.27E−06 |
| Greb1 | 1.823336 | 9.11E−08 | 1.27E−06 |
| Alpl | 1.178954 | 1.98E−06 | 1.84E−05 |
| Mmp13 | 0.606352 | 0.000358 | 0.002503 |
| Cdkn1a | 0.621865 | 0.000494 | 0.002574 |
| Osx | 0.82048 | 0.000551 | 0.002574 |
| Pdgfa | 0.505288 | 0.002235 | 0.00894 |
| Col1a1 | 0.581333 | 0.004303 | 0.015062 |
| Scx | −0.42647 | 0.00516 | 0.015829 |
| Mmp9 | 0.478553 | 0.005653 | 0.015829 |
| β-catenin | 0.286243 | 0.008075 | 0.020554 |
| Vegfa | 0.261948 | 0.010181 | 0.023757 |
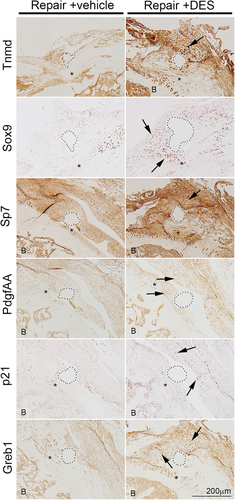
6.4 Immunohistochemistry
Gene expression analysis indicated that DES treatment alters gene expression in the shoulder joint following tendon repair but fails to provide spatial information on tissue-specific expression. To determine which tissues are responding to DES treatment following tendon repair, we examined immunohistochemical localization of Tnmd, Sox9, Sp7, PdgfAA, p21, and Greb1 in repair-only and repair + DES tissue sections. Consistent with our gene expression analysis, we found increased expression of Sox9, Sp7, PdgfAA, p21, and Greb1 in the repair + DES tissue compared to the repair-only tissue. Further, these proteins are upregulated in and adjacent to the repaired tendon (Figure 8). These data show that DES treatment resulted in a localized upregulation of several key genes involved in tendon, cartilage, and bone remodeling at and around the repaired tendon enthesis.
6.5 Tenascin C expression and localization in tendon repair
Genetic variants linked to Tenascn C (TNC) are associated with rotator cuff tears as well as the failure of healing after rotator cuff repair.24, 25 We wanted to determine if Tnc is upregulated following rotator cuff repair in the mouse and if its expression is regulated by DES. We performed quantitative reverse-transcription polymerase chain reaction (qRT-PCR) to measure changes in Tnc expression following rotator cuff repair in control, repair-only, and repair + DES mice. In unoperated animals (Control), there is a low level of Tnc messenger RNA (mRNA) and following surgery (repair-only) there is a 2.6-fold induction of Tnc mRNA expression in the shoulder joint. A 2-week treatment of DES (repair + DES) induced Tnc mRNA expression sevenfold compared to the controls and 2.7 fold compared to vehicle-treated animals (repair-only) (Figure 9A).
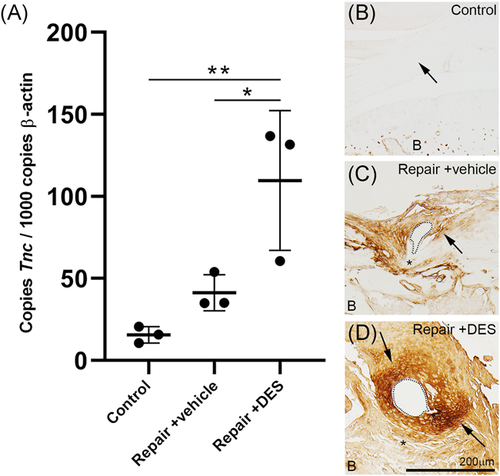
We next wanted to determine what tissues in the shoulder joint are expressing Tnc following rotator cuff repair and DES treatment. To determine Tnc protein localization in the shoulder, we performed immunohistochemistry for Tnc on control, repair-only, and repair + DES tissue sections. Consistent with our qRT-PCR analysis, Tnc protein expression is absent from the naïve supraspinatus tendon (Figure 9B) and induced in and around the supraspinatus tendon following repair-only treatment (Figure (9C). Tnc protein expression is further induced with the addition of DES in the supraspinatus tendon following repair (Figure 9D). Together these data indicate Tnc expression is induced by DES following rotator cuff repair and its expression is localized to the supraspinatus tendon and adjacent tissue.
7 DISCUSSION
In this study, we have demonstrated that the addition of the ELC DES significantly improves the histologic and biologic characteristics of rotator cuff repairs in a murine model. First, our repair model showed similar histologic findings of other animal models of repairs with high levels of disorganized UFC at the tendon insertion site. In terms of expression data, our model is consistent with other models showing an increase in chondrogenic, tendon modulating, and angiogenic gene expression with some osteogenic characteristics. New findings in the analysis not previously reported include upregulation of the reactive oxygen species scavenger Glrx2 as well as downstream products of Esrrβ notably Cdkn1a supporting their roles in tendon healing. The expression data support the addition of DES significantly increases osteogenic gene expression as well as further angiogenic and tendon-remodeling/maturation gene expression which appears to be a direct result of estrogen receptor (ER) activity shown by upregulation of the ER activity marker Greb1.
Within our study, the addition of the ELC DES altered gene expression in a manner that encourages enthesis maturation. Scx is a transcription factor that has been implicated in tendon development and regeneration. Specifically, Scx is believed to direct the tendinous attachments to bone through a signaling mechanism that directs the transition of tendon to bone through a cartilaginous transition zone.26 In the comparison of repairs without DES to control tissue, Scx expression is significantly upregulated in repairs, which has been previously reported in repairs postoperatively from 7 days out to 4 weeks.14, 19 In the comparison between the repairs with and without addition of DES, DES addition downregulated the Scx expression. Maman et al.27 evaluated the effect of estradiol administration to supraspinatus tenocytes in culture and found a similar decline in Scx expression as we found with the addition of DES. We also found an increasing expression of tendon extracellular matrix marker Col1a1 in both repairs versus controls, as has been previously shown by Lebaschi et al.14 and Killian et al.,19 and expression was further increased with the addition of DES. Lebaschi et al.14 also reported upregulation of extracellular matrix modulators Mmp 3 and 14 after repair similar to our data. In addition, Sox9 is recognized to play an important role in cartilage formation and is regulated by Scx expression.26 Similarly, Tnmd is a transmembrane protein expressed in tendons, is a useful marker for identifying mature tenocytes, and is also regulated by Scx expression.9 Acan is the predominant proteoglycan found in cartilage. We found upregulation of Sox9, Tnmd, and Acan in standard repairs as has been reported by Lebaschi et al.14 and Tokunaga et al.9 but we did not find any further upregulation of these genes with the addition of DES. Overall, the data would support the addition of DES transitions the repairs away from early fibrocartilage formation (Scx, Sox9, Tnmd, and Acan) and towards tendon maturation (Col1a1) which aligns with the histologic data.
Within our study, the addition of the ELC DES altered gene expression in a proangiogenesis manner. Angiogenesis is a fundamental factor required for healing at a tendon–bone interface.28 Various cytokines and transcription factors have been identified as critical for the angiogenic process including VEGF, HIF1α, and PECAM1. HIF1α is a transcription factor regulating the cellular response to hypoxia through accumulation in cytosol resulting from low oxygen allowing translocation to the nucleus influencing the direct expression of target genes including VEGF.29 VEGF has multiple roles including binding VEGF receptors on endothelial cells triggering a pathway leading to angiogenesis. PECAM1, otherwise known as CD31, is a marker of endothelial cells and is related to endothelial cell migration.30 VEGF and PECAM1 have been previously shown to be upregulated after rotator cuff repair and our data supports these findings.28, 30 We have also identified the upregulation of Hif1α after rotator cuff repair. After addition of DES, we see further increases in Vegfa. Estrogen receptors have been previously shown to promote the proteasomal degradation of HIF1α.20 Consequently, increased expression levels of VEGF would be the presumed result with activation of ER receptor activity without further expression of HIF1α which is consistent with our data. Consequently, DES further stimulates angiogenesis critical for tendon healing.
Hormonal changes likely have a significant impact on tendon–bone healing. Tanaka et al.6 has previously shown that the estrogen-deficient state by ovariectomy, compared with control rats, led to decreased biomechanical properties and poor development of chondroid tissue that influenced the repair of the tendon insertion after surgery. The current data would support beneficial effects on tendon healing even when estrogen deficiency is not present and these effects may be potentiated in the estrogen-deficient state. The addition of the ELC DES significantly increases the activity of estrogen receptor shown by increased expression of downstream product Greb1. Essrβ activity is also increased with increased expression of Cdkn1a. Clearly, the addition of DES impacts RNA expression driving primarily osteogenic and tendon modulation genes instead of primarily chondrogenic genes seen with standard repairs. Upregulated Mmp9 and Mmp13 would support collagen turnover consistent tendon maturation, similar to Col1a1, although because these are not specific to tendon caution needs to be taken with this interpretation. Mmp13 upregulation has been previously shown in tenocyte cell culture with the addition of a selective estrogen receptor modulator and confirmed in the current data in vivo.31 Finally, increased BV of the proximal humerus also may positively impact tendon healing through improved fixation strength of anchors or sutures within the proximal humerus as osteoporosis has been negatively correlated with tendon healing in clinical cases of rotator cuff repair.32 This data would support investigating the addition of estrogen in the early postoperative period as it may have a significant positive impact on the structural integrity of repairs.
Previous work indicated that genetic variants linked to TNC associate with rotator cuff repairs, and TNC mRNA is expressed in torn rotator cuff tendon tissue. Here we show that Tnc mRNA is upregulated in following tendon repair and its expression is augmented by DES treatment. Further, Tnc protein localizes to the repaired tendon. These data suggest that TNC may have an important role in tendon repair and healing.
There are several limitations of this study. First, we have only analyzed one time point postoperatively for histologic, SEM, immunohistochemical, and RNA expression analysis. Consequently, it is not possible to describe the timing of the changes occurring. It is also difficult to fully conclude that the DES definitely improves the maturation of the healing repair equivalent to a normal enthesis as it is only one time point. Nevertheless, we can still conclude that there is a different biologic response with the addition of DES even out to 1-month postoperative. Second, we are not able to determine what occurs if the DES is stopped or if a different dosage would provide an even stronger effect. Third, we are unable to determine if the DES simply speeds up the biologic healing process or rather changes it such that a healed repair in a wild-type animal would never be able to attain similar anatomy. Finally, the ultimate superiority of the repairs as determined by mechanical testing was not performed.
8 CONCLUSIONS
The addition of DES increased osteogenic, angiogenic and tendon maturation gene and protein expression at the repaired enthesis compared to standard repairs. Further research is reasonable to evaluate ELCs in the early postoperative phase after rotator cuff repair.
ACKNOWLEDGMENTS
The authors thank Brooke Kawaguchi for technical support. We thank the reviewers for their critical reading of our manuscript. This study was supported in part by the University of Utah. Resources and facilities were provided by the George E. Wahlen, Department of Veterans Affairs, Bone & Joint Research Laboratory, Salt Lake City, UT. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
Animal work was performed in accordance with laws for the care and use of laboratory animals with approval by the University of Utah's Institutional Animal Care and Use Committee (IACUC Protocol number 18-09005).




