Simulating the effect of glenohumeral capsulorrhaphy on kinematics and muscle function
Abstract
This study aimed to use a predictive simulation framework to examine shoulder kinematics, muscular effort, and task performance during functional upper limb movements under simulated selective glenohumeral capsulorrhaphy. A musculoskeletal model of the torso and upper limb was adapted to include passive restraints that simulated the changes in shoulder range of motion stemming from selective glenohumeral capsulorrhaphy procedures (anteroinferior, anterosuperior, posteroinferior, posterosuperior, and total anterior, inferior, posterior, and superior). Predictive muscle-driven simulations of three functional movements (upward reach, forward reach, and head touch) were generated with each model. Shoulder kinematics (elevation, elevation plane, and axial rotation), muscle cost (i.e., muscular effort), and task performance time were compared to a baseline model to assess the impact of the capsulorrhaphy procedures. Minimal differences in shoulder kinematics and task performance times were observed, suggesting that task performance could be maintained across the capsulorrhaphy conditions. Increased muscle cost was observed under the selective capsulorrhaphy conditions, however this was dependent on the task and capsulorrhaphy condition. Larger increases in muscle cost were observed under the capsulorrhaphy conditions that incurred the greatest reductions in shoulder range of motion (i.e., total inferior, total anterior, anteroinferior, and total posterior conditions) and during tasks that required shoulder kinematics closer to end range of motion (i.e., upward reach and head touch). The elevated muscle loading observed could present a risk to joint capsule repair. Appropriate rehabilitation following glenohumeral capsulorrhaphy is required to account for the elevated demands placed on muscles, particularly when a significant range of motion loss presents.
1 INTRODUCTION
Surgical glenohumeral capsulorrhaphy is used to treat glenohumeral instability caused by elongated, lax, or damaged capsuloligamentous structures.1-6 Glenohumeral capsulorrhaphy includes various techniques involving selective plication to different joint capsule sections.1, 2, 4-7 While primarily correcting instability, “tightening” the joint capsule will invariably impact glenohumeral joint motion and forces by altering passive resistance.1, 8, 9 Variable restrictions to range of motion have been observed under differing localized plications.1 Restricted range of motion following glenohumeral capsulorrhaphy may make achieving shoulder postures during daily activities difficult for patients. Furthermore, the muscular effort may increase during such tasks to counter the added passive resistance from the joint capsule. Understanding the impact of variations in glenohumeral capsulorrhaphy on movement and muscle function is relevant to the design of rehabilitation strategies. Knowledge of how movement may be altered and muscle function is affected will assist in providing specific rehabilitation targets.
Musculoskeletal simulations built from experimental movement data are used to examine how changes in the musculoskeletal system impact muscle and/or joint loading.10-14 Parameters in a musculoskeletal model are altered to represent relevant clinical pathologies, with the altered model/s used in simulations which estimate the control strategies (i.e., muscle activation and forces) that reproduce experimentally measured movement. This approach has been used to examine the effects of lower limb10 and deep core11 muscle weakness, as well as hip muscle strengthening12 on neuromuscular control and joint loading during both walking and running gait. Simulations of cerebral palsy gait have also been conducted to understand how common neuromuscular deficits associated with the condition impact gait function.13, 14
Studies using experimental kinematic data to generate muscle-driven simulations typically keep the movement strategy consistent to match the experimental data. This reveals information about how neuromuscular strategies may adapt to specific changes in the musculoskeletal system—but is limited in its ability to identify how movement may change. Keeping movement consistent may not reflect what naturally occurs with musculoskeletal changes. For example, kinematic differences during activities of daily living have been observed between elderly individuals with symptomatic rotator cuff impingements versus asymptomatic individuals;15 as well as patients with glenohumeral osteoarthritis and those who have undergone total shoulder arthroplasty compared to healthy controls.16 Predictive simulations that are generated de novo (i.e., without experimental data) can assist in identifying cause and effect relationships between changes to the musculoskeletal system, and movement and neuromuscular strategies. Predictive simulations have been more readily applied in a gait context.17, 18 Song and Geyer18 induced neural and musculoskeletal deficits associated with ageing, generating predictive simulations that identified muscle strength and mass as the predominant factors responsible for typical gait changes seen in elderly populations.18 Ong et al.17 simulated adaptations in the musculoskeletal system observed with cerebral palsy. Muscle weakness elucidated a slower “heel-walking” gait, while contracture resulted in a crouched “toe-walking” gait—aligning with the common gait adaptations seen in experimental evaluations.17 These studies reveal the power of predictive simulation for investigating how movement and control strategies change with adaptations in the musculoskeletal system.
Musculoskeletal modeling and predictive simulation provide a framework to alter parameters in a musculoskeletal model that simulate the changes in passive glenohumeral joint resistance observed with glenohumeral capsulorrhaphy, and examine how these changes impact movement and muscle function during upper limb tasks. This could provide surgeons with an understanding of the impact their procedures may have on daily function, and, subsequently, promote a more targeted approach to rehabilitation. This study aimed to use a predictive simulation framework to examine shoulder kinematics, muscular effort, and task performance time during functional upper limb movement tasks under various simulated selective glenohumeral capsulorrhaphy conditions. We manipulated passive restraints at the glenohumeral joint within a musculoskeletal model to simulate the impact of different selective capsulorrhaphy conditions. We hypothesized that the selective capsulorrhaphy conditions would: (i) alter movement strategies; (ii) increase muscular effort; and (iii) reduce performance (i.e., increased time to complete the task).
2 METHODS
2.1 Musculoskeletal model
A seven-segment musculoskeletal model of the torso and right upper limb was developed in OpenSim (version 4.0).19 The segment properties and inertial parameters reflected those of Wu et al.20 The kinematic foundation for the model21, 22 followed the recommendations of the International Society of Biomechanics.23 Muscle model parameters were set to those in De Groote et al.24 and tendon dynamics were ignored. Muscle-tendon paths and wrapping points provided by Wu et al.20 were used. A full description of the musculoskeletal model can be found in Document S1.
2.2 Models of selective capsulorrhaphy
A baseline model of no joint capsulorrhaphy was created (i.e., “None” model), followed by models representing anteroinferior, anterosuperior, posteroinferior, posterosuperior, total anterior, total inferior, total posterior, and total superior glenohumeral capsulorrhaphy procedures.1 The models were based on Gerber et al.1—whereby the passive restraints in the model were optimized so that similar passive joint angles were reached with identically applied torques as observed in their experiments. Glenohumeral capsulorrhaphy typically reduces the glenohumeral joint range of motion.1 To represent this in our musculoskeletal model, forces representing the passive restraints of the glenohumeral joint that responded to the joint position were included. An optimization routine identified the mathematical expressions required to accurately model the passive restraints under different capsulorrhaphy conditions (see Document S1).
2.3 Predictive simulations
Each capsulorrhaphy model was used in predictive muscle-driven simulations of functional movements (see Table 1 and Videos S1–S3) generated using OpenSim Moco (version 0.3.0)25 in MATLAB (version 2019b, The Mathworks Inc.). OpenSim Moco provides a framework for solving optimal control problems for musculoskeletal systems using direct collocation. The predictive simulations generate the control signals (i.e., muscle activations) required to produce the desired movement.
| Task | Description | Loada |
|---|---|---|
| Upward reach | Start with hand by side and reach to a point 15° above shoulder height at a horizontal distance two times that of forearm length | 1 kg |
| Forward reach | Start with hand by side and reach to a point at stomach height at a horizontal distance two times that of forearm length | 1 kg |
| Head touch | Start with hand by side and reach to touch a point 25 cm above the C7 vertebrae | No load |
- a Load simulated by adding specified mass to the hand body in the model.
The functional movements replicated experimental tasks measured in the literature.26, 27 The predictive simulations were generated by prescribing a series of task constraints and goals that contributed to an objective function value, with the optimal control problem aiming to minimize this value. First, all tasks were constrained to begin with the arm placed by the side (i.e., 0° of shoulder elevation and rotation, elbow flexion, and pronation/supination). Second, bounds were placed on the shoulder joint angles by extracting the maximum and minimum values recorded in similar movement tasks.26-28 Third, joint angular velocities were constrained to be zero at the end of the movement ensuring the limb finished in a static position. Fourth, a landmark or set of landmarks on the model (e.g., styloid processes of wrist, tip of middle finger) were required to reach an end-point to ensure the hand reached an appropriate position relevant to the task. Fifth, a goal to minimize the sum of squared control signals (i.e., muscle activations and torque actuators) was included. Sixth, a goal to minimize the time taken to reach the desired movement end-point was included. Overall, this generated predictive simulations of the tasks that achieved the desired movement (i.e., reaching the end task goals while remaining within the kinematic bounds), while minimizing effort (i.e., neuromuscular strategy used) and maximizing performance (i.e., time taken to perform the task). Predictive simulations of each movement task were repeated across the capsulorrhaphy models, resulting in a total of 27 predictive simulations (i.e., 3 tasks × 9 models).
Solving optimal control problems requires identifying an appropriate mesh interval (i.e., time step) and, subsequently, the number of nodes (i.e., “node” or “grid density,” or sample points) to use. We used a “grid refinement” approach as outlined in Lee and Umberger29 to determine an appropriate node density for each of the movement tasks (see Document S2).
2.4 Data analysis
The effect of capsulorrhaphy on shoulder function was assessed by comparing the shoulder kinematics and a metric of muscle cost, along with the performance indicator of time taken to complete the task. A single model was simulated for each capsulorrhaphy condition, therefore descriptive rather than inferential comparisons were made. Shoulder elevation, axial rotation, and elevation plane angles were extracted from the optimal control solutions. Joint angles were time normalized (i.e., 0%–100%) to the start and end points of the movement. Mean absolute differences across the shoulder joint angles were calculated for the selective capsulorrhaphy conditions relative to the “None” model to quantify differences in joint kinematics (i.e., average of the absolute difference in the joint angle at each time node relative to the “None” condition).
 ) (i.e., the ratio of force produced by the muscle relative to its potential maximum) was quantified for each muscle at each simulation time step
) (i.e., the ratio of force produced by the muscle relative to its potential maximum) was quantified for each muscle at each simulation time step
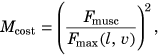
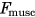 is the muscles current level of force output, and
is the muscles current level of force output, and 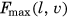 is the instantaneous maximum muscle force achievable considering the current length and velocity of the muscles fibers.10 Total muscle cost was calculated as the sum of all individual muscle costs integrated over time.10 The percentage change in total muscle cost was calculated for the selective capsulorrhaphy conditions relative to the “None” model. The relative contribution of individual muscles to the relative change in total muscle cost across the simulated capsulorrhaphy conditions was calculated to determine the muscles primarily responsible for the observed changes.
is the instantaneous maximum muscle force achievable considering the current length and velocity of the muscles fibers.10 Total muscle cost was calculated as the sum of all individual muscle costs integrated over time.10 The percentage change in total muscle cost was calculated for the selective capsulorrhaphy conditions relative to the “None” model. The relative contribution of individual muscles to the relative change in total muscle cost across the simulated capsulorrhaphy conditions was calculated to determine the muscles primarily responsible for the observed changes.All codes used to generate the models and simulations, along with the resulting data are available at the projects SimTK page.
3 RESULTS
3.1 Capsulorrhaphy models
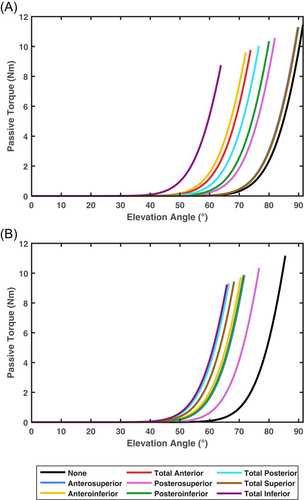
Under the simulated passive range of motion test conditions, all models fell within 0.1° of the data presented by Gerber et al.1 (see Table 2). Reductions in the range of motion were achieved via resistive force being applied earlier during movement (see Figures 1 and 2).
| Capsulorrhaphy model | Abduction | Flexion | Ext. Rot. (0° Abd.) | Int. Rot. (0° Abd.) | Ext. Rot. (45° Abd.) | Int. Rot. (45° Abd.) | Ext. Rot. (90° Abd.) | Int. Rot. (90° Abd.) |
|---|---|---|---|---|---|---|---|---|
| None | 91.50 | 85.52 | 53.37 | 44.59 | 104.33 | 38.99 | 132.96 | 30.74 |
| (91.5) | (85.6) | (53.4) | (44.6) | (10.4) | (39.0) | (133.0) | (30.8) | |
| Anteroinferior | 72.16 | 70.54 | 32.74 | 44.07 | 69.28 | 36.03 | 87.23 | 23.06 |
| (72.1) | (70.5) | (32.8) | (44.1) | (69.3) | (36.1) | (87.3) | (23.1) | |
| Anterosuperior | 89.74 | 71.75 | 23.28 | 44.07 | 85.74 | 36.34 | 123.86 | 26.88 |
| (89.8) | (71.8) | (23.3) | (44.1) | (85.8) | (36.4) | (123.9) | (26.9) | |
| Posteroinferior | 80.05 | 71.44 | 49.54 | 35.54 | 103.92 | 21.39 | 131.04 | 10.29 |
| (80.0) | (71.5) | (49.6) | (35.6) | (104.0) | (21.4) | (131.1) | (10.3) | |
| Posterosuperior | 82.02 | 76.71 | 48.83 | 28.43 | 103.33 | 28.24 | 130.74 | 27.49 |
| (82.1) | (76.8) | (48.9) | (28.5) | (103.4) | (28.3) | (130.8) | (27.5) | |
| Total anterior | 73.85 | 66.32 | 21.27 | 42.72 | 67.24 | 35.23 | 86.06 | 21.38 |
| (73.9) | (66.3) | (21.3) | (42.8) | (67.3) | (35.3) | (86.1) | (21.4) | |
| Total inferior | 63.82 | 65.82 | 32.74 | 37.49 | 74.54 | 19.18 | 87.23 | 3.99 |
| (63.8) | (65.8) | (32.8) | (37.5) | (74.6) | (19.2) | (87.3) | (4.0) | |
| Total posterior | 76.64 | 66.50 | 49.07 | 23.04 | 107.75 | 11.77 | 129.45 | 9.80 |
| (76.6) | (66.5) | (49.1) | (23.1) | (107.8) | (11.8) | (129.5) | (9.80) | |
| Total superior | 89.95 | 68.26 | 17.80 | 28.98 | 82.28 | 27.78 | 115.44 | 24.58 |
| (90.0) | (68.3) | (17.8) | (29.0) | (82.3) | (27.8) | (115.5) | (24.6) |
- Abbreviations: Abd., abduction; Ext., external; Int., internal; Rot., rotation.
3.2 Grid refinement
Node densities of 201, 201, and 151 were selected for the upward reach, forward reach, and head touch tasks, respectively. A complete summary of the grid refinement results is presented in Document S2.
3.3 Shoulder kinematics
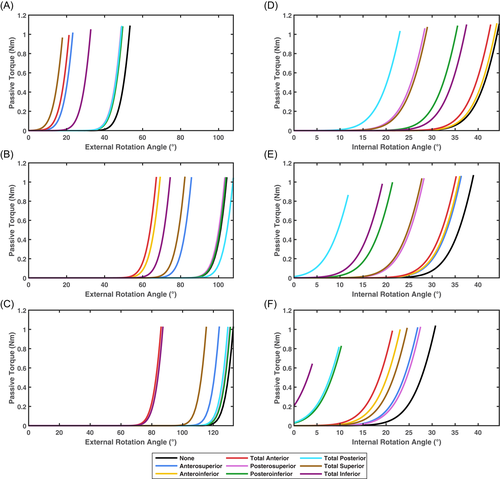
Joint angles for the selective capsulorrhaphy models across the three simulated tasks are presented in Figures 3-5. There were minimal differences in shoulder elevation between the selective capsulorrhaphy models compared to the “None” model, with mean absolute difference typically less than 2° (see Table 3). An exception to this was the head touch task, where the error in shoulder elevation was larger (i.e., ∼3°–4°) in the anteroinferior, posteroinferior, posterosuperior, total anterior, and total posterior conditions (see Table 3). This was driven by a reduction in shoulder elevation throughout the entirety of the task (see Figure 5). The elevation angle was consistent across all tasks and capsulorrhaphy conditions, with all mean absolute differences under 0.75° (see Table 3). The consistency of shoulder axial rotation angles was task dependent. Shoulder axial rotation angles were consistent across the capsulorrhaphy conditions in the forward reach task, with all mean absolute differences under 0.42° (see Table 3). Larger mean absolute differences (i.e., ∼1°–2°) were observed for shoulder axial rotation angles across the majority of capsulorrhaphy conditions for the head touch task, with the exception of the total posterior condition (4.50°) (see Table 3)—driven by a reduction in shoulder internal rotation during the task (see Figure 5). Small mean absolute differences (i.e., <0.7°) were observed for shoulder axial rotation angles across the anteroinferior, anterosuperior, and total anterior conditions for the upward reach task; while larger errors (i.e., ∼2°–5°) were observed across the remaining capsulorrhaphy conditions (see Table 3).
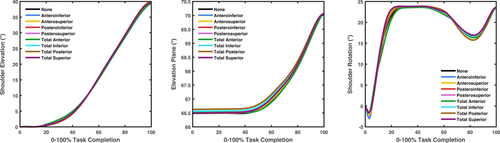

| Capsulorrhaphy model | Upward reach | Forward reach | Head touch | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Shoulder elevation | Elevation angle | Axial rotation | Shoulder elevation | Elevation angle | Axial rotation | Shoulder elevation | Elevation angle | Axial rotation | |
| Anteroinferior | 1.26 | 0.16 | 0.58 | 0.18 | 0.04 | 0.24 | 3.58 | 0.02 | 1.63 |
| Anterosuperior | 1.00 | 0.07 | 0.67 | 0.08 | 0.02 | 0.18 | 0.24 | 0.01 | 0.12 |
| Posteroinferior | 1.19 | 0.75 | 2.32 | 0.27 | 0.10 | 0.39 | 3.70 | 0.23 | 1.62 |
| Posterosuperior | 1.11 | 0.34 | 3.36 | 0.26 | 0.06 | 0.42 | 3.45 | 0.30 | 1.18 |
| Total anterior | 1.31 | 0.22 | 0.37 | 0.17 | 0.03 | 0.42 | 3.91 | 0.03 | 1.47 |
| Total inferior | 1.50 | 0.63 | 2.73 | 0.27 | 0.11 | 0.35 | 1.69 | 0.53 | 1.65 |
| Total posterior | 1.62 | 0.65 | 5.23 | 0.40 | 0.17 | 0.39 | 4.18 | 0.07 | 4.50 |
| Total superior | 1.47 | 0.25 | 3.52 | 0.26 | 0.06 | 0.41 | 0.05 | 0.03 | 0.79 |
3.4 Muscle cost
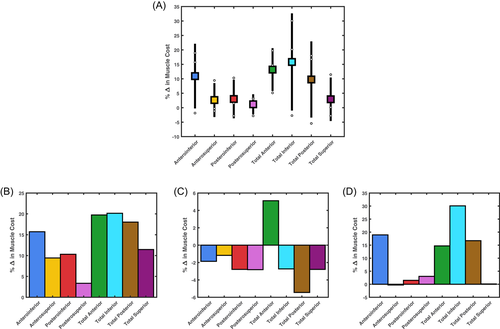
Across all tasks on average, total muscle cost relative to the “None” condition increased under the selective capsulorrhaphy conditions. The largest increases in relative average total muscle cost came under the total inferior (15.83 ± 16.82%), total anterior (13.19 ± 7.43%), anteroinferior (10.93 ± 11.20%), and total posterior (9.77 ± 13.19%) conditions (see Figure 6A). The inspection of individual tasks revealed variable changes in total muscle cost. An increase in relative total muscle cost was observed across all conditions for the upward reach task—with the greatest increases under the total inferior (20.15%), total anterior (19.74%), total posterior (18.03%), and anteroinferior (15.72%) conditions (see Figure 6B). Total anterior was the only condition to increase relative total muscle cost (5.11%) during the forward reach task—with a decrease observed in all other conditions (see Figure 6C). The changes in relative total muscle cost for the forward reach task were smaller compared to the other two tasks. The largest increases in relative total muscle cost were observed under the total inferior (30.07%), anteroinferior (18.94%), total posterior (16.72%), and total anterior (14.71%) conditions in the head touch task—with the remaining conditions recording minimal change (i.e., <5%) (see Figure 6D).
The anterior deltoid (25.65 ± 3.69% relative contribution to change in muscle cost across simulated capsulorrhaphy conditions), lower trapezius (18.65 ± 7.55%), and lower serratus anterior (15.16 ± 3.92%) were the primary contributors to the increase in total muscle cost during the upward reach task—with smaller contributions made by the upper pectoralis major (6.71 ± 3.98%), supraspinatus (4.63 ± 1.62%), subscapularis (3.48 ± 2.44%), and upper trapezius (3.47 ± 1.05%) (see Figure S1). A relatively consistent contribution from a number of muscles generated the increase in total muscle cost under the total anterior condition during the forward reach task (see Figure S2). The lower serratus anterior was the primary contributor to the decrease in total muscle cost under the remaining selective capsulorrhaphy conditions (−19.69 ± 8.80%), with smaller contributions made by the anterior (−7.64 ± 3.13%) and posterior (−6.50 ± 3.52%) deltoids, teres minor (−5.63 ± 7.60%), infraspinatus (−5.55 ± 7.68%), and middle serratus anterior (−4.24 ± 1.33%) (see Figure S2). The lower trapezius (13.78 ± 16.24%), anterior deltoid (9.30 ± 11.22%), lower serratus anterior (7.88 ± 12.00%), middle deltoid (7.07 ± 17.63%), and supraspinatus (5.67 ± 3.52%) were the primary contributors to increased total muscle cost during the head touch task—with smaller contributions made by the infraspinatus (3.09 ± 5.39%) and subscapularis (2.46 ± 8.30%) (see Figure S3).
3.5 Performance time
Performance times remained consistent (i.e., within 0.05 s) across the capsulorrhaphy conditions and all tasks (see Table 4).
| Capsulorrhaphy model | Upward reach | Forward reach | Head touch |
|---|---|---|---|
| None | 0.701 | 0.548 | 0.674 |
| Anteroinferior | 0.653 | 0.548 | 0.622 |
| Anterosuperior | 0.664 | 0.548 | 0.672 |
| Posteroinferior | 0.658 | 0.549 | 0.635 |
| Posterosuperior | 0.674 | 0.548 | 0.642 |
| Total anterior | 0.648 | 0.547 | 0.621 |
| Total inferior | 0.657 | 0.549 | 0.645 |
| Total posterior | 0.656 | 0.548 | 0.629 |
| Total superior | 0.656 | 0.548 | 0.674 |
4 DISCUSSION
This predictive simulation study assessed the impact of selective glenohumeral capsulorrhaphy on shoulder kinematics, muscular effort, and performance during upper limb movements. Contrary to our hypotheses, shoulder joint kinematics and task performance remained relatively stable during the simulated tasks. A trend for increased muscular effort (typical range of 5%–30%) under the selective capsulorrhaphy conditions was observed; however, this response was dependent on the task and selective capsulorrhaphy condition.
The selective capsulorrhaphy conditions had a minimal impact on movement strategies and task performance time. While kinematics and performance time did fluctuate, most changes were relatively small (i.e., 1°–2° and 0.05 s). With the increases in muscle activation and forces observed, the performance output of the tasks was maintained. Our study simulated the average reduction in the range of motion following selective capsulorrhaphy.1 Both larger and smaller effects on a range of motion were observed across the individual cadaveric specimen by Gerber et al.1—with large ranges (e.g., over 50°) observed between the worst- and best-case scenarios. Greater changes in movement and task performance may occur under scenarios where passive range of motion is severely hampered. The models and code (https://simtk.org/projects/gh-caps-sims) provided with this paper can be used for replication experiments to assess the impact of variable reductions in passive range of motion.
Certain capsulorrhaphy conditions induced slightly larger changes in shoulder joint kinematics during specific tasks. First, a small reduction in shoulder elevation was observed during the head touch task under the anteroinferior, posteroinferior, posterosuperior, total anterior, total inferior, and total posterior conditions—with this most evident towards the end of the task at the highest degrees of elevation. Second, the total posterior condition varied from the remaining conditions for the degree of shoulder internal rotation during the head touch task—demonstrating reduced internal rotation, particularly during the first half of the task. These results align with the specific impacts on the range of motion these capsulorrhaphy conditions incur. Anteroinferior, posteroinferior, posterosuperior, total anterior, total inferior, and total posterior conditions generate the largest reductions for elevation range of motion in the abduction.1 Similarly, the total posterior condition incurs the largest reductions in shoulder internal rotation range of motion at lower degrees of shoulder elevation (i.e., between 0° and 45°).1 Larger passive-resistive forces were experienced by our models under these capsulorrhaphy conditions when generating abduction and internal rotation movements at lower elevation angles, respectively. The head touch task was performed at a lower elevation plane angle compared to the reaching tasks—signifying the arm was raised more towards the frontal versus sagittal plane. This is likely why shoulder elevation and internal rotation were more readily altered under the aforementioned capsulorrhaphy conditions during the head touch, but not other, tasks.
In general, muscle cost (i.e., “activity” or “effort”) increased under the capsulorrhaphy conditions. Compared to the “None” model, all capsulorrhaphy conditions incurred an increase in passive resistance to shoulder motion (see Figures S10–S12). It is logical that under these conditions the muscles increased their activation to produce more force to counter the additional resistance—and hence an increase in cost is observed. This was the case in our study, whereby greater activation and forces were observed in the specific muscles responsible for elevating muscle cost (see Figures S4–S9). This was predominantly observed in the prime movers (e.g., anterior and middle deltoids for the overhead [i.e., upward reach and head touch] tasks) and was the major factor in increasing muscle cost. The minimal changes in kinematics meant that muscles remained operating at similar lengths relative to optimal. Similarly, we did not observe dramatic changes in muscle contraction velocity.
The increase in total muscle cost under the majority of capsulorrhaphy conditions highlights an increased load on the muscles, which could lead to fatigue or muscle damage.10 This may present a problem for individuals who more readily repeat these tasks in their daily activities (e.g., manual handling workers), or for those who perform tasks with shoulder movement that nears full range of motion (e.g., overhead lifting). Elevated muscle loading early after surgery could also present a risk to the joint capsule repair. Inadequate muscle function stemming from fatigue or damage may not maintain the glenohumeral joint center of rotation during movement, risking instability, and damage to the surgically repaired capsule or overloading the rotator cuff tendons.30, 31 Rehabilitation following glenohumeral capsulorrhaphy must consider these factors, and include a focus on strengthening the muscles primarily responsible for movements commonly performed by the individual. It may also be advisable to avoid shoulder postures near full range of motion that could significantly elevate muscle loads early on in recovery when some muscle weakness may present.
Changes in muscle costs were relatively smaller for the forward reach compared to the other tasks. This is likely due to the smaller joint angle ranges achieved during the forward reach task. Shoulder elevation and rotation during the forward reach peaked at approximately 40° and 24°, respectively, versus larger peaks for shoulder elevation (i.e., 90°–100°) and rotation (i.e., 30°–40°) in the other tasks. The passive structures at the glenohumeral joint are responsible for restricting glenohumeral motion at end-range humeral elevation.32 Our findings replicate this, whereby the model forces simulating passive restraints produced more resistive force closer to end-range shoulder elevation—and the largest effects were observed in tasks where shoulder elevation was closer to this point (i.e., ∼90°—100°). While an increase in passive resistance would be experienced at lower degrees of shoulder elevation, this did not appear large enough to generate substantial (or any) increases in total muscle cost. In conjunction with targeted rehabilitation therapy, individuals who have undergone glenohumeral capsulorrhaphy may minimize muscle stress by limiting activities that involve high degrees of shoulder elevation. This is, however, a temporary solution as patients will eventually require a full range of motion to function in daily activities. Rehabilitation strategies that focus on muscle strength and control33, 34 are supported by our results. Strengthening the major shoulder muscles is recommended to counter the additional passive resistance and limit potential increases in muscle cost.
Total anterior capsulorrhaphy was the only condition to increase muscle cost relative to the “None” model across all tasks. All of the simulated movement tasks in the present study were performed with an elevation angle greater than zero (i.e., arm in front of the body) and internal axial rotation. Total anterior capsulorrhaphy induces the largest reductions in shoulder flexion and internal rotation range of motion,1 and, hence, additional resistance to these motions was present under this condition. The anteroinferior, and total anterior, inferior, and posterior conditions incurred the largest increases in total muscle cost in the upward reach and head touch tasks. Again, these capsulorrhaphy conditions demonstrate the largest impacts on a range of motion in both shoulder elevation and internal rotation.1 Our study supports a relationship between the range of motion deficit induced by the glenohumeral capsulorrhaphy and an increase in muscle cost. Individuals who experience substantial range of motion loss following glenohumeral capsulorrhaphy may be at greater risk of musculoskeletal issues induced by increased muscle loads. Certain selective capsulorrhaphy conditions also have a prominent effect on limiting external rotation (e.g., total superior) and abduction (e.g., total inferior) range of motion.1 While we did not test any tasks that included abduction and external rotation, increases in muscle cost could also be observed in tasks incorporating these movements.
Our study demonstrates how musculoskeletal modeling and predictive simulation can be used to assess changes in neuromuscular function and movement during upper limb tasks following musculoskeletal system changes. We employed a generic musculoskeletal model, literature-based values for the reductions in shoulder range of motion, and simulated task performance based on a generic set of goals. A similar approach could be taken in a patient-specific manner, whereby: (i) a patient-specific model of the upper limb; (ii) patient-specific ranges of motion, with expected or measured losses following surgery; and (iii) kinematic data measured from the patient during functional tasks relative to their daily performance could be used. This approach may be useful in providing a patient-specific understanding of how an already performed or planned glenohumeral capsulorrhaphy procedure may impact their upper limb function and could be used to design rehabilitation plans that target the specific muscles that may see an increased load. Patient-specific musculoskeletal modeling and simulation could provide added value across a wide spectrum of patients. However, efforts must be made to ensure this is easily accessible and can be efficiently integrated into clinical practice.
There are certain limitations relating to the modeling approaches used in our study that need to be considered. First, we locked the motion of the trunk to ensure that the desired movement was achieved by the upper limb alone. This was necessary to ensure that adaptations to counter the additional passive resistance were achieved by the shoulder muscles—however, it may not represent adaptive movement strategies following glenohumeral capsulorrhaphy. In the face of added passive resistance at the shoulder, an individual may use the trunk to a greater extent (i.e., leaning) to achieve reaching or overhead movements. This has been observed in individuals with shoulder pain during a fatiguing reaching task—whereby those with pain tend to move their center of mass more (i.e., suggesting greater trunk involvement).35 Greater trunk involvement would likely reduce muscular involvement at the shoulder, reducing muscle cost, but could increase the moment arm of the trunk relative to the spine—suggesting a likely tradeoff in shifting load from the shoulder to the spine with this strategy. Future predictive simulations would need to consider appropriate cost functions to accurately model trunk involvement during upper limb movements. Second, we did not include a constraint on the resultant glenohumeral joint reaction vector as has been done in previous studies.20, 36-38 The goal of these constraints is to ensure the simulated muscle activity includes appropriate rotator cuff function to maintain glenohumeral joint stability.20, 36-38 Rotator cuff forces may, therefore, have been underestimated in our study. However, this remained consistent across the simulated capsulorrhaphy conditions, and, hence, we believe our estimates of changes in muscle cost are still appropriate. Future modeling studies, particularly those with an interest in glenohumeral (in)stability and/or rotator cuff function, should still consider this inclusion. Third, we used a single “participant” design with an “optimized” movement strategy. Differential effects on shoulder kinematics and muscle function may occur with different baseline movement strategies. For example, an individual who performs the functional tasks differently to what we modeled may need to adapt their kinematic strategy in a different manner to adjust to additional passive resistance. Future predictive simulation studies could include participant-specific movement strategies as a baseline condition and observe how a model adapts under these initial conditions.
5 CONCLUSION
Our predictive simulation study found that shoulder kinematics and performance times did not dramatically change during upper limb movements under various glenohumeral capsulorrhaphy conditions. Despite the lack of kinematic and performance changes, glenohumeral capsulorrhaphy generally resulted in an increase in total muscle cost. Larger increases in muscle cost were observed under the simulated total inferior, total anterior, anteroinferior, and total posterior conditions. Changes in muscle cost were, however, task dependent. The largest increases in muscle cost were observed in tasks that required shoulder kinematics closer to the end range of motion. Our results highlight the need for appropriate guided and targeted rehabilitation following glenohumeral capsulorrhaphy to account for the elevated demands placed on the muscle, particularly when a significant range of motion loss presents.
ACKNOWLEDGMENT
This study was supported by a Deakin University Postdoctoral Research Fellowship Grant (held by ASF).
CONFLICT OF INTERESTS
Richard S. Page receives institutional educational support from De Puy Synthes. Other authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Aaron S. Fox and Richard S. Page conceived the study concept. Aaron S. Fox analyzed the data. Aaron S. Fox, Jason Bonacci, Stephen D. Gill, and Richard S. Page interpreted the data. Aaron S. Fox and Richard S. Page drafted the manuscript. All authors revised the manuscript for intellectual content and approved the final version.