Lateral to medial fibro-adipogenic degeneration are greater in infraspinatus than supraspinatus following nerve and tendon injury of murine rotator cuff
Ayelet Dar and Frank A. Petrigliano are co-senior authors.
Abstract
Small animal models of massive tears of the rotator cuff (RC) were introduced a decade ago and have been extensively used to study the pathophysiology of chronically injured RC. Transection of rodent suprascapular nerve and RC tendon results in progressive muscle atrophy, fibrosis and fat accumulation and affect the infraspinatus and supraspinatus muscles similarly to that seen in the setting of massive RC tears in humans. The purpose of this study was to perform a comprehensive and detailed analysis of the kinetics of fibrotic scar and adipose tissue development comparing phenotypic differences between chronically injured infraspinatus and supraspinatus. Automatic mosaic imaging was used to create large image of whole infraspinatus or supraspinatus sectioned area for quantification of spatial heterogeneity of muscle damage. Pathologic changes advanced from the lateral site of transection to the medial region far from the transection site. A prominent, accelerated muscle fibrosis and fat accumulation was measured in injured infraspinatus compared to supraspinatus. Furthermore, adipose tissue occupied significantly larger area than that of fibrotic tissue in both muscles but was greater in infraspinatus within 6 weeks post induction of injury. Our findings confirm that infraspinatus is more susceptible to accelerated chronic degeneration and can be used to identify the physiological functions that distinguish between the response of infraspinatus and supraspinatus in the setting of massive tears. Whether these pathologic differences observed in mice are reflected in humans is one key aspect that awaits clarification.
1 INTRODUCTION
Massive tears of the rotator cuff (RC) represent a significant clinical challenge in the management of shoulder pain and dysfunction.1 Chronic tears can lead to impaired shoulder function, activity-related pain, and decreased quality of life.2-5 Progressive muscle atrophy, fat accumulation, and development of fibrosis in the chronically injured muscle correlate with tear size, patient age, and the duration of the tear and are known to contribute to the high surgical failure rate seen in these larger tendon tears.6
Given the significance of fibro-adipogenic degeneration as it relates to prognosis, an imaging-based evaluation of muscle quality was developed by Goutallier and colleagues.7 This five-stage classification has been extrapolated to sagittal MRI imaging for evaluation and determination of disease course.8 Using this classification system, observations regarding natural progression of fibro-adipogenic degeneration have been made, most importantly as it relates to timing of progression from intermediate infiltration to more severe degeneration.9 Their review of 1688 imaging cases found that the time to onset of intermediate fibro-adipogenic degeneration (Stage I–II) to more severe degeneration (Stage III–IV) varied between the muscles that were torn.9 When evaluating supraspinatus tears, progression to severe degeneration took four years, while infraspinatus tears progressed within 3–6 years depending on the age of the patient.9 This information can help guide management, as severe degeneration leads to poor surgical outcomes. Additionally, increased severity of final tear type was associated with greater risk of pain and degeneration of both infraspinatus and supraspinatus.10 Therefore, understanding factors affecting disease progression will help providers tailor the treatment of large tears.
Experimental RC tears in rats and mice involving the transection of supraspinatus and infraspinatus tendons (TT) along with the suprascapular nerve (DN) have replicated the marked degenerative fibrosis and fat accumulation found in these patients.11-13 The rodent model of massive RC tears has been used by researchers to study the pathological progression of the disease and to improve their understanding of the cellular response13-15 to injury, the molecular pathways that are responsible for it16-18 and test therapeutic approaches to attenuate the progression of muscle degeneration.19-23
One of the most commonly used assays for analysis of development of damage in injured RC is muscle histology. However, so far, data was gained from images limited to a small representative section area of mixed longitudinal and axial cuts. Although such analysis clearly demonstrates that the injured muscles become increasingly scarred and massively accumulates adipose tissue, it is impossible to use randomly selected areas to determine the spatial heterogeneity of fibrosis and fatty degeneration as well as the direction and kinetics of damage spreading. For the purpose of such analysis the entire cross-sectional area of multiple sections of the muscle should be histologically analyzed at various time points post-TTDN.
Another aspect of injury assessment is the relative contribution of each of the muscles, supraspinatus and infraspinatus, to the total cumulative damage of the RC. Previous studies clearly demonstrate that the damage progresses in both muscles and increased fatty degeneration was measured in infraspinatus compared to supraspinatus at end-stage of massive muscle degeneration at 6 weeks and 8 weeks post-TTDN.11 However, regional differences in damage progression in supraspinatus and infraspinatus have not been well-characterized to date. The purpose of this study was to provide a comprehensive and detailed analysis of the kinetics of fibrotic scars and adipose tissue development, comparing phenotypic differences between chronically injured infraspinatus and supraspinatus.
2 METHODS
2.1 Mice
Mice (female C57/BL6, 8 week-old) were randomly divided into four groups of four mice: non-injured, 5 days post-TTDN, 2 weeks post-TTDN, and 6 weeks post-TTDN. Mice were killed at the indicated time points and supraspinatus and infraspinatus were each harvested separately for histological evaluation. All experiments were approved by the Institutional Animal Care and Use Committee of the University of California, Los Angeles.
2.2 Mouse RC injury
We induced massive RC tears in mice as previously described.13 In brief, we anesthetized the mice with 2% isoflurane and oxygen, administered buprenorphine for analgesia, and sterilely prepared and draped the right shoulder. A 1-cm longitudinal skin incision was made over the right glenohumeral joint to access the deltoid fibers, which were then split directly posterior to the deltoid tuberosity longitudinally to reach the supraspinatus and infraspinatus tendons. These tendons were isolated and sharply dissected from their insertions on the greater tuberosity using a no. 11 blade scalpel for the tenotomy procedures; additionally, the distal 5-mm of each tendon was resected to prevent scar formation to the humerus. Next, the suprascapular nerve was identified before entering the supraspinatus through a 5-mm incision in the trapezius musculature anterior to the lateral scapula and cut for the denervation procedure. Last, a 5-0 Vicryl (Ethicon) suture was used to close the deltoid muscle and skin.
2.3 Histology
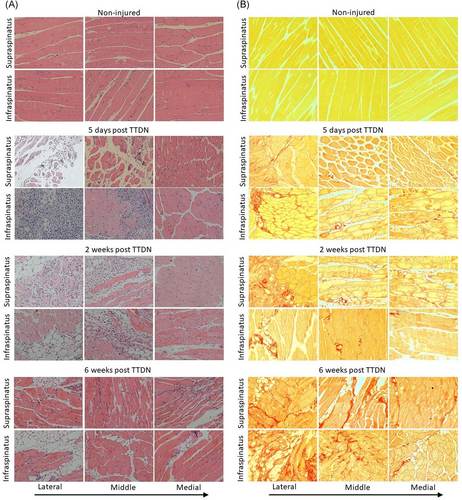
Infraspinatus and supraspinatus muscles were separately fixed in 4% formalin, embedded in paraffin and sectioned axially. Sections were stained with hematoxylin and eosin (H&E) for general tissue structure analysis, measurement of myofiber cross-section20 or picrosirius red staining of collagen for assessment of developing fibrosis according to manufacturer instructions (Abcam). Images were acquired on an Axio Imager 2 light microscope (Zeiss) and automatic mosaic imaging was used to create large image of whole infraspinatus or supraspinatus sectioned area. Areas of adipose containing tissue were selected in images of H&E stained RC sections and measured by Photoshop CC 2018 19.1.7.3. Percentage of the area of adipose tissue was calculated by dividing the total area of adipose tissue by the total area of the tissue section. Fibrosis was quantified by picrosirius red staining and red pixel intensity measurement by Photoshop. Percentage of fibrosis was calculated by dividing the number of red pixels measured in the entire area of the mosaic (including these contributed by large blood vessels and nerves) by the number of pixels of total tissue area. Mosaic microscopy derived images of entire RC area (n = 4 mice per group and n = 6 mosaics per each muscle type/tested region) were used for quantification of fibrosis or fat accumulation at each time point. Three investigators (non-blinded, without repetitions) performed measurements of muscle degeneration of mice that were randomly chosen from each of the four tested groups (non-injured, 5 days, 2 weeks, and 6 weeks post-TTDN). Data were then pooled for statistical analysis
2.4 Statistical analysis
All data are presented as mean + SEM. One-way ANOVA was used to compare mean values among study groups (SPSS Version R24.0).
3 RESULTS
3.1 Microscopic mosaic acquisition as an approach for comparing the progression of supraspinatus and infraspinatus damage
Following tendon transection (Figure 1A) at the lateral side and nerve transection (Figure 1B) of both supraspinatus and infraspinatus, each muscle was harvested separately at 5 days, 2 weeks, and 6 weeks post-TTDN for histological examination (Figure 1C). We have previously demonstrated that each time point is correlated to a distinct stage of RC remodeling post-TTDN, 1. Pro-fibro-adipogenesis stage at 5 days post-TTDN, 2. Intermediate-stage of fibro-adipogenesis at 2 weeks post-TTDN, and 3. End-stage fibro-adipogenesis at 6 weeks post-TTDN.13, 14, 20 However, these analyses were based on capturing field-of-view images for small areas of tissue sections and therefore could not determine the anatomic direction and rate of injury progression. Additionally, no prior measurements were made to differentiate the relative contribution of each muscle to the total damage that was measured at each stage of RC degeneration. For the purpose of this analysis, an automatic mosaic acquisition was used to create a larger image from a series of smaller images individually acquired systematically across each muscle section. This microscopic technique enables generation of mosaics that allow wide-field view of the entire cross section of supraspinatus or infraspinatus for analysis of morphology and fat accumulation (Figure 1D) as well as fibrotic collagen deposition (Figure 1E). Collagen expression was detected in non-fibrotic healthy muscle in perimysium residing, large blood vessels (Figure 1F) and in RC nerves (Figure 1G). The size and number of areas stained positive for picrosirius red progressively increased post-TTDN but not that of nerves within 6 weeks post-TTDN (Figure 1H). To determine the kinetics and direction of injury progression, the entire muscle section was divided into three regions, wherein the lateral region is close to the site of tendon and nerve transection and is followed by the middle and medial regions along the lateral to medial axis (Figure 1C).
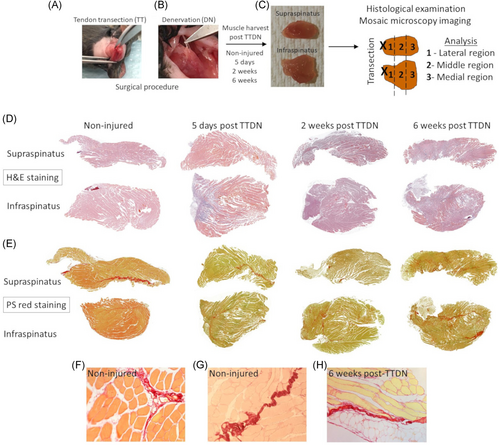
3.2 Spreading of fibrosis and fat within supraspinatus
Mosaic images of the entire section of supraspinatus enabled quantification of the percentage of fibrosis and adipose tissue in the lateral, middle, and medial aspects of the muscle at 5 days, 2 weeks, and 6 weeks post-TTDN in comparison to non-injured supraspinatus (Figure 2). Fibrotic injury progressed most quickly in the lateral supraspinatus, showing a marked increase at 5 days post-TTDN when compared to non-injured controls (Figure 2A,B) but not in the middle or medial region of supraspinatus. By 2 weeks post-TTDN, each of the three regions of supraspinatus had substantially elevated fibrosis as compared to non-injured controls (Figure 2A,B). Greatest increase in development of fibrosis was measured within 6 weeks post-TTDN in each section of supraspinatus (Figure 2A,B). Comparison of fibrotic damage between sections at 5 days post-TTDN showed substantial elevation in lateral sections and very modest elevations in middle and medial sections (Figure 2A,B). At 2 weeks post-TTDN, fibrosis injury was inversely related to distance from injury with lateral showing greater damage than middle and medial sections and middle sections showing increased fibrosis relative to medial sections (Figure 2B). By 6 weeks post-TTDN, differences in lateral and middle fibrosis elevation were not statistically significant but were both significantly elevated as compared to the medial section (Figure 2B). The early elevation in lateral supraspinatus fibrosis relative to middle and medial supraspinatus, as well as increase in middle supraspinatus at 2 weeks and 6 weeks compared to medial supraspinatus likely reflects the location of injury in the tendon of supraspinatus. However, substantial increases in fibrosis in all sections of supraspinatus at 6 weeks imply that damage progresses in a lateral to medial fashion, from the site of tendon transection into the muscle belly.
Adipocytes were rarely visualized in non-injured mice (Figures 2A and 2C). At 5 days post-TTDN, adipose tissue was detected only in lateral region of supraspinatus (Figures 2A and 2C). At 2 weeks post-TTDN, there was an increase in fat tissue accumulation in the lateral and middle regions of supraspinatus, which significantly increased at 6 weeks post-TTDN (Figures 2A and 2C). At 6 weeks post-TTDN, there was fat tissue accumulation in the medial region of supraspinatus as well.
At each time point, the relative area occupied by adipose tissue was the largest in the lateral region, intermediate in the middle region and the smallest in the medial region.
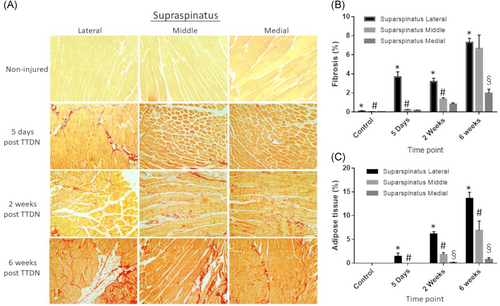
3.3 Spreading of fibrosis and fat within infraspinatus
Fibrosis in infraspinatus was measured by location within the muscle as above. There was no significant fibrosis detected in non-injured infraspinatus. As with supraspinatus, fibrotic damage was most rapid and pronounced in the lateral infraspinatus, with significant increase by 5 days post-TTDN when compared to non-injured controls (Figures 3A,B). Again, similarly to supraspinatus, 2 weeks post-TTDN showed substantial elevation of fibrotic tissue in lateral, middle, and medial regions of infraspinatus as compared to controls (Figures 3A,B). Further, significant increase in fibrosis was measured at 6 weeks post-TTDN in each location when compared to non-injured controls, 5 days post-TTDN, and 2 weeks post-TTDN (Figure 3B). When comparing regions within a single time period, 5 days post-TTDN showed marked elevation of fibrosis in the lateral region with modest increases in middle and medial regions (Figure 3B). At 2 weeks post-TTDN, relative levels of fibrosis were inversely related to distance from injury, with lateral sections showing more fibrosis than both middle and medial sections, and middle sections showing increased fibrosis compared to medial sections (Figure 3B). By 6 weeks post-TTDN, lateral sections again were inversely related to distance from injury with lateral sections showing increased fibrosis relative to middle and medial sections, and middle sections showing increase relative to medial sections (Figure 3B). In line with the progression of fibrosis within supraspinatus, fibrosis increased with time and was most pronounced closer to the site of injury (lateral sections) within infraspinatus.
In infraspinatus, adipocytes were also rarely visualized in non-injured mice (Figures 3A and 3C). At 5 days post-TTDN, there was adipose tissue in the lateral and middle regions of infraspinatus (Figures 3A and 3C; p < .005). At 2 weeks post-TTDN, an increase in fat tissue accumulation was detected in the lateral and middle regions of infraspinatus, which significantly increased at 6 weeks post-TTDN (Figures 3A and 3C; p < .005). Similar to supraspinatus, at 6 weeks post-TTDN, there was non-negligible fat tissue accumulation in the medial region of infraspinatus muscle.
At each time point, the relative area occupied by adipose tissue was the largest in the lateral region, intermediate in the middle region and the smallest in the medial region.
3.4 Higher development of fibrosis and greater fat accumulation in infraspinatus relative to supraspinatus
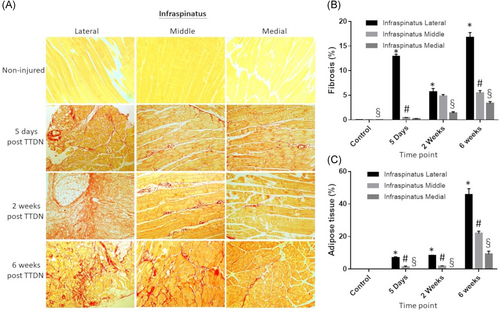
Adipogenesis was compared between supraspinatus and infraspinatus muscles by region of muscle. As stated above, there was no adipose tissue detected in supraspinatus or infraspinatus of non-injured mice (Figures 4A and 5A–C). At 5 days post-TTDN, adipose tissue was significantly greater in lateral and middle regions of infraspinatus compared to supraspinatus (Figures 4A and 5A,B). Medial regions of both muscles showed no adipose tissue (Figures 4A and 5C). At 2 weeks post-TTDN, fat accumulation showed slight elevation in the lateral region of infraspinatus compared to supraspinatus, similar levels in the middle regions of both infraspinatus and supraspinatus, and negligible levels in the medial region of infraspinatus and supraspinatus (Figures 4A and 5A–C). At 6 weeks post-TTDN, there was significantly greater adipose tissue percentage in all regions of infraspinatus compared to supraspinatus (Figures 4A and 5A–C).
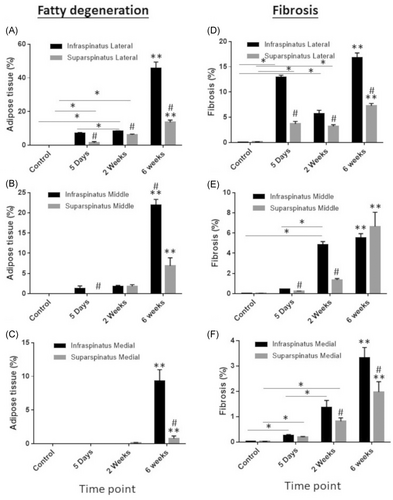
Fibrosis was also compared between supraspinatus and infraspinatus at each timepoint and location within the muscle. Neither supraspinatus nor infraspinatus showed fibrosis in non-injured mice (Figures 4B and 5D–F). Comparison of fibrosis in the lateral supraspinatus and lateral infraspinatus showed significantly greater fibrosis in infraspinatus relative to supraspinatus at 5 days post-TTDN, 2 weeks post-TTDN, and 6 weeks post-TTDN (Figures 4B and 5D). Medial regions of supraspinatus and infraspinatus showed no significant difference in fibrosis at 5 days post-TTDN (Figures 4B and 5E). However, at 2 weeks post-TTDN fibrosis in the middle region of infraspinatus was significantly greater than in the middle region of supraspinatus (Figures 4B and 5E). At 6 weeks post-TTDN, middle supraspinatus showed greater levels of fibrosis compared to middle infraspinatus (Figures 4B and 5E). Medial sections of supraspinatus and infraspinatus showed no significant difference in fibrosis at 5 days post-TTDN (Figures 4B and 5F). At 2 weeks post-TTDN, fibrosis was greater in the medial infraspinatus than in the medial supraspinatus (Figures 4B and 5F). Further, by 6 weeks post-TTDN, fibrosis was greater in the medial infraspinatus when compared to the medial supraspinatus (Figures 4B and 5F).
3.5 Adipose tissue occupies more area than fibrosis in both muscles and is greater in infraspinatus
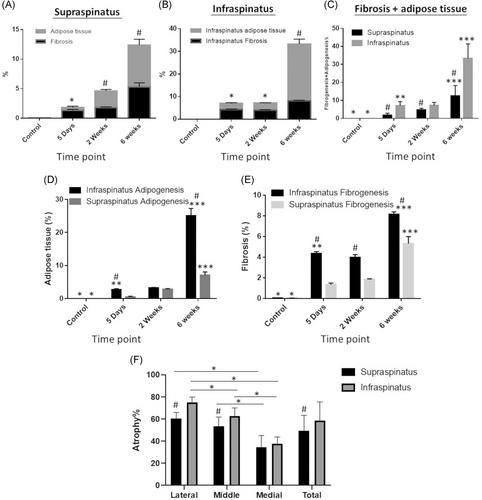
Only when transection of the suprascapular nerve is combined with tendon transection mice develop severe fatty accumulation at end stage of muscle degradation within 6 weeks post-TTDN. However, no prior measurements were performed for quantification of the relative area occupied by each type of damaged tissue in the course of muscle degenerative remodeling and to determine whether the extent of each damage type differs between supraspinatus and infraspinatus. In injured supraspinatus, fibrosis occupied more area than adipose tissue at early stage (5 days post-TTDN) of muscle degeneration, wherein at intermediate (2 weeks post-TTDN) and late (6 weeks post-TTDN) stages of muscle degeneration adipose tissue occupied significantly greater area than fibrosis (Figure 6A; p < .05 and Figure S1). Similarly, development of fibrosis was greater than adipogenesis in infraspinatus at 5 days post-TTDN (Figure 6B, p < .05 and Figure S1). However, infraspinatus fatty degeneration was markedly accelerated within 2 weeks post-TTDN (Figure 6B, p < .05 and Figure S1) and robustly developed within 6 weeks post-TTDN (Figure 6B, p < .05 and Figure S1). Fibrosis increased in both muscles at 6 weeks post-TTDN in comparison to its level at earlier time points (p < .00001, Figures 6A,B and 6E and Figure S1) and was significantly greater in infraspinatus compared to supraspinatus (p < .00001, Figures 6E and S1). Adipose tissue developed in both muscles within 5 days post-TTDN but further accumulated only in supraspinatus between 5 days and 2 weeks post-TTDN (p < .0001, Figures 6D and S1). The most prominent increase in fat accumulation was measured at 6 weeks post-TTDN in both muscles and was greater in infraspinatus (7.8-fold increase, Figures 6B and 6D and S1) compared to supraspinatus (2.5-fold increase, Figures 6A and 6D and S1). Greater total damaged area (fibrosis and fat tissue) was measured in infraspinatus compared to supraspinatus (33.2 ± 8% compared to 12.4 ± 6%, p < .00001), of which fat tissue occupied more area than fibrosis in both muscles and was markedly greater in infraspinatus (25.2 ± 8%) compared to supraspinatus (7.1 ± 3%).
3.6 Greater atrophy in infraspinatus relative to supraspinatus at end-stage of muscle degeneration
Atrophy was compared between supraspinatus and infraspinatus muscles by region of muscle as well as entire muscle area at 6 weeks post-TTDN, the time point in which severe chronic tissue damage is evident. The average myofiber cross-section diameter in non-injured RC muscles was 82 ± 3.4 µm, (N = 3 mice) and was set as the threshold diameter of non-atrophic myofibers. Coinciding with fibro-adipogenic changes (Figure 5), atrophy advanced from the lateral site of transection to the medial region far from the transection site in both infraspinatus and supraspinatus with significantly greater muscle wasting in lateral and medial regions (Figure 6F; p < .001). By 6 weeks post-TTDN, average rate of atrophy was markedly higher in the lateral (p < .00005) and middle (p < .05) regions of infraspinatus than corresponding regions of supraspinatus. Similar rate of atrophy was measured in the medial region of both muscles (Figure 6F). Greater total muscle atrophy was measured in infraspinatus compared to supraspinatus (Figure 6F, 58.5 ± 17% compared to 49.5 ± 13%; p < .05),
4 DISCUSSION
The mouse RC tears model is widely used to investigate the pathophysiology of this chronic muscle disease, however, little is known about differences in the response to injury between mouse supraspinatus and infraspinatus and whether it can be used to predict and reflect disease progression in humans. Our detailed analyses of damage progression throughout the entirety of each injured RC muscle revealed marked variance between supraspinatus and infraspinatus with an overall tendency of greater degeneration observed in infraspinatus. Our findings are in agreement with published data showing that adipocytes could be detected in rat infraspinatus but not in supraspinatus at 6 weeks post-TTDN.11
We show here for the first time that differences in damage progression between supraspinatus and infraspinatus should be taken under consideration to ensure correct design of data analysis in animal RC tears model studies. The kinetics of development of muscle damage, either fibrosis or fatty degeneration, not only markedly differ between supraspinatus and infraspinatus but also varies between distinct regions of each type of injured RC muscle. Hence, it is crucial to avoid a type of analysis that is based on random sampling to assess effect of any treatment on muscle degeneration. To avoid faulty comparison that will lead to false conclusions, each untreated RC muscle, supraspinatus or infraspinatus, should be compared to its treated counterpart and comparison should be made between matched tested areas. Further comprehensive characterization of progressive RC degeneration combined with additional research techniques such as flow cytometry, MRI, and single-cell RNA sequencing will reveal whether differences in cell frequency between RC muscles (e.g., fibro-adipogenic progenitors, endothelial and satellite cells) can be correlated to the differential progression of RC degeneration.
Although collagen content was significantly higher in the infraspinatus than supraspinatus within 5 days post-TTDN, both muscles exhibit prominent reduction in collagen content and similar levels of mild fibrosis at 2 weeks post-TTDN. Our previous reports,14, 20 further supported herein, show that attempts for muscle regeneration appear successful within 2 seeks post-TTDN but fail within 6 weeks post-TTDN, wherein chronically injured RC is severely damaged. Therefore, greater collagen content in both muscles at 5 days post-TTDN should be associated with myofiber necrosis, marked decrease in collagen content at 2 weeks should be linked to attempts of muscle repair that fail and are accompanied be massive increase in collagen content at 6 weeks post-TTDN due to progressive muscle degeneration.
It has been reported in some human studies that infraspinatus demonstrates more severe tissue degeneration than supraspinatus.7, 24 It has even been found that isolated supraspinatus tears can lead to tissue degeneration of infraspinatus in humans.25 Since the suprascapular nerve is at risk of compression distal to supraspinatus at the spinoglenoid notch when supraspinatus retracts after a massive tear, authors of these human studies have posited that this compression of the nerve at the spinoglenoid notch, which would only influence infraspinatus, is likely the cause of accelerated infraspinatus degeneration in these patients.24, 26, 27
However, in our mouse model, the suprascapular nerve is transected completely before the nerve innervates either supraspinatus or infraspinatus. Therefore, at least in the mouse model, there must be factors beyond suprascapular nerve injury that influence this differential rate of tissue degeneration between supraspinatus and infraspinatus.
Although innervated by the same nerve in both mice and humans, supraspinatus and infraspinatus perform fundamentally different functions on the glenohumeral joint. The supraspinatus is primarily a humeral head depressor, stabilizing the joint while the larger muscles surrounding the shoulder, such as the deltoid, pectoralis major, and latissimus dorsi, provide primary movement. The infraspinatus, on the other hand, primarily externally rotates the shoulder joint, in addition to providing axial plane force coupling and stability. That these two muscles have different functions may potentially be related to the differential degenerative remodeling seen in our study as well as in human studies.
Not only does the finding of lateral to medial progression of degenerative changes have important implications for the performance and analysis of animal RC tears model studies, it also has significant implications for the understanding of human rotator cuff disease. To the best of our collective knowledge, this lateral to medial progression of rotator cuff tissue degenerative is a novel finding that has not been discussed in the human literature. If true in humans as well, this phenomenon would have significant surgical treatment implications – since the rotator cuff often retracts medially after a massive tear and therefore the lateral muscle belly is seen in relatively a more medial position on imaging modalities, the extent of volumetric fatty degeneration and fibrosis may be routinely overestimated on the traditional T1 sagittal MRI slices at the level of the glenoid used for degenerative grading. This overestimation, potentially, could dissuade surgeons from performing rotator cuff repair and bias them toward salvage options like tendon transfers or reverse shoulder arthroplasty. Future studies are needed to confirm this finding in humans.
ACKNOWLEDGMENTS
This study was supported by Orthopaedic Research and Education Foundation (#16-065), the UCLA David Geffen School of Medicine, Department of Orthopaedic Surgery and The Ministry of Science and Technology of China (2020YFC2002800). G.W. was supported by China Scholarship Council.
AUTHOR CONTRIBUTIONS
Genbin Wu performed mouse surgeries and imaging, analyzed data, and wrote sections of the manuscript. Vivian J. Hu, Daniel J. McClintick, Jonathan D. Gatto, and Temidayo Aderibigbe assisted with data analysis and wrote sections of the manuscript. Andrew R. Jensen reviewed and wrote sections of the manuscript. Ayelet Dar and Frank A. Petrigliano monitored project design, manuscript review, revision, and approval. All authors have read and approved the final submitted manuscript.




