Synovial mesenchymal stem cells promote the meniscus repair in a novel pig meniscus injury model
Abstract
Stem cell therapy has potential for the treatment of degenerative meniscus injuries; however, an optimal animal model has not been established. Basic and clinical research show that synovial mesenchymal stem cells (MSCs) promote meniscus repair. The purposes of this study were to create a novel meniscus injury model in microminipigs and to investigate the effectiveness of synovial MSCs on meniscus healing in this model. The posterior portion of the medial meniscus in microminipigs was punctuated 200 times with a 23G needle. Allogenic synovial MSC suspension was placed on the injury site for 10 min for transplantation. The meniscus was evaluated histologically and via sagittal magnetic resonance imaging (MRI), radial MRI reconstructed in three dimensional, and T2 mapping at 1 and 8 weeks. Proteoglycan content stained with safranin-o disappeared 1 week after treatment in both the MSC and control groups but increased at 8 weeks only in the MSC group. Histological scores at 8 weeks were significantly higher in the MSC group than in the control group (n = 6). At 8 weeks, the T2 values of the MSC group were significantly closer to those of a normal meniscus than were those of the control group. High signal intensity areas of the MRIs and positive areas stained with picrosirius red coincided with meniscal lesions. In conclusion, we created a novel meniscus injury model in microminipigs. Evaluation via histology, MRIs, and polarized microscopy showed that transplantation of synovial MSCs improved meniscus healing.
1 INTRODUCTION
Among orthopedic surgeons who treat the meniscus, interest in preserving the function of the meniscus has been increasing worldwide.1 Though the reoperation rate of meniscus repair is higher than that of meniscectomy,2 satisfactory mid- or long-term clinical results have been obtained after meniscus repair.2, 3 Previously, a good indication of meniscus repair was limited to vertical longitudinal meniscus injuries within the vascular area4; however, recent attempts to repair various types of meniscus injury also showed satisfactory clinical results for horizontal5 and radial tears.6 According to the 2016 ESSKA Meniscus Consensus, arthroscopic partial meniscectomy should not be the first line of treatment for the surgical management of degenerative meniscus injuries because there are no significant differences between the clinical results of partial meniscectomy and nonsurgical treatment at short-term follow-up,7 and it is well-known that partial meniscectomy results in osteoarthritis in the long term.8 However, meniscus repair of degenerative meniscus injuries is not mentioned in the consensus statement due to a lack of evidence on this treatment. To promote better healing of a torn meniscus, biological enhancement is recommended.9
Various animal models have been used to develop meniscus treatments, including a longitudinal tear model,10 a meniscus defect model,11 and a meniscus extrusion model.12 However, none of these models include a pathology that mimics a torn meniscus as observed in clinical situations. A torn meniscus with degeneration shows abnormal cell arrangement, including decreased cell density or diffuse hypercellularity and a loss of collagen fiber organization.13 To develop a new strategy for treating meniscus injuries with degeneration, a novel animal model is needed.
Recently, stem cell therapy has been used to treat meniscus pathology in clinical situations.14-16 Synovial mesenchymal stem cells (MSCs) are a particularly promising source of stem cells due to their high proliferative and chondrogenic potential,17, 18 and synovial MSCs are suitable for stem cell treatment of meniscus injuries because the developmental origin of the synovium is similar to that of the meniscus.19, 20 Synovial MSCs have been shown to enhance meniscus healing with a longitudinal injury to the avascular area in microminipig models,10 and transplantation of synovial MSCs onto a repaired meniscus with degenerative injuries improved clinical evaluation scores at 2 years in a human clinical study.14 The purposes of the present study were to create a meniscus injury model in microminipigs and to investigate the effectiveness of synovial MSCs on meniscus healing in this model.
2 MATERIALS AND METHODS
2.1 Animals
All animal care and experiments were conducted in accordance with the institutional guidelines of the Animal Committee of Tokyo Medical and Dental University. Seventeen 10-month-old, skeletally mature microminipigs were used (Fujimicra Corp.). Throughout the study, all microminipigs had free access to food and water and were kept in a postoperative care cage.
2.2 Preparation of synovial mesenchymal stem cells
Synovial MSCs were prepared as previously described.10 First, synovial tissue was harvested from the infrapatellar fat pad via an arthrotomy of the knee. Synovial tissue and the underlying connective tissue were digested with 3 mg/ml collagenase (Sigma-Aldrich) at 37°C for 3 h and then filtered through a 70 µm cell strainer (Greiner Bio-One GmbH). The nucleated cells were plated in 150-cm2 culture dishes (Nalge Nunc International); 5 × 105 cells/dish were plated in a complete medium (alpha-minimum essential medium [alpha-MEM] supplemented with 10% fetal bovine serum [FBS], 100 U/L penicillin, 100 mg/ml streptomycin, and 250 ng/ml amphotericin B [all from Thermo Fisher Scientific]). The plated cells were cultured for 14 days at 37°C, 5% CO2, and saturated humidity. The adherent cells were harvested with 0.25% trypsin-EDTA (Thermo Fisher Scientific) and cryopreserved as Passage 0. Aliquots of 2 × 106 cells in 2 ml of stock medium (alpha-MEM supplemented with 10% FBS and 5% dimethyl sulfoxide [Fujifilm Wako Pure Chemical Corporation]) were slowly frozen in a bio freezing vessel (Bicell, Japan Freezer) and cryopreserved at −80°C. To expand the cells, the frozen vials of cells were thawed, plated in 60-cm2 culture dishes at 1 × 106 cells/dish, and cultured for 4 days as Passage 1. Then the cells were re-plated in 150-cm2 culture dishes at 5 × 105 cells/dish and cultured for 14 days as Passage 2. Passage 2 cells were used for transplantation.
2.3 Surgery and transplantation of synovial MSCs
Under general anesthesia, both knees were operated at the same time using a medial parapatellar approach. A medial collateral ligament was cut, and the knee was maximally flexed and externally rotated to expose the entire medial meniscus. A meniscus injury was created by puncturing the posterior half of the medial meniscus 200 times with a 23 G needle, including 100 times in the vascular region and 100 times in the avascular region. We put the spatula beneath the meniscus to avoid injuring the cartilage and to ensure complete puncture of the meniscus from the femoral side to the tibial side. (Figure 1A). For the MSC group, before the knee joint was closed, 2.0 × 107 allogenic synovial MSCs were suspended on the injury site. The control group received only phosphate-buffered saline. Knees were then held stationary for 10 min. After the medial collateral ligament was repaired, the joint capsule and skin incisions were closed for each layer. The microminipigs were allowed to walk freely in their cages without any fixation devices on the knee joints. At 1 and 8 weeks after the surgery, the pigs were killed and the knees were evaluated (Figure 1B).
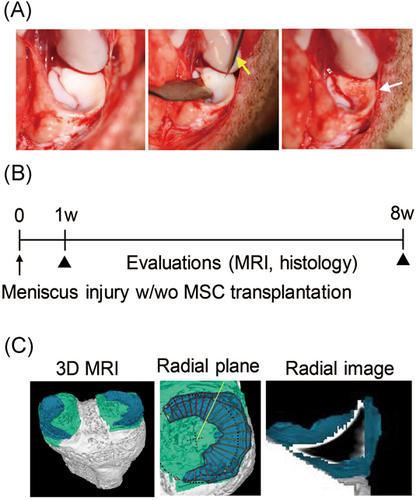
2.4 Magnetic resonance imaging analysis
Magnetic resonance imaging (MRI) were performed with an MR imaging system at 3.0T (Achieva 3.0TX; Philips) with 16-channel coils. Two images of the sagittal plane of the knee joint were obtained: a fat-suppressed spoiled gradient echo sequence image and a proton weighted image. Total scan duration was 7 min, 30 s and 7 min, 10 s, respectively. For both images, sagittal images were obtained at an in-plane resolution of 0.31 × 0.31 mm, a partition thickness of 0.30 mm (320 slices), and a field of view (head to tail × anterior to posterior) of 150 × 150 mm. Both images were used to reconstruct a 3D MRI image with our software21; then a radial slice of the meniscus was obtained (Figure 1C). For T2 mapping, the total scan duration was 9 min, 5 s, and the slice thickness was 1.0 mm. For three sections of the posterior portion of the meniscus, T2 values were obtained by averaging the entire area of the meniscus. Details of the MRI sequence are shown in Tables S1–3.
2.5 Macroscopic and histological analysis
The medial menisci were photographed using a Leica M165 FC stereoscopic microscope (Leica). For the histological examination, the medial menisci were fixed in 4% paraformaldehyde for 7 days, decalcified in a 20% EDTA solution for 21 days. We separated the meniscus into three regions—anterior, middle, and posterior—using a scalpel, followed by separating the posterior region of the meniscus into three pieces. We embedded the middle piece of the posterior region in paraffin wax. The area that we selected was entirely within the posterior half of the meniscus that we punctured. The specimens were sectioned along the radial plane at 5 μm. Sections were stained with safranin-o and fast green, with hematoxylin and eosin, and with picrosirius red (Picrosirius Red Stain Kit; Polysciences, Inc.). Before any staining, the sections were observed using polarizer (U-POT; Olympus Corp.) and analyzer (U-ANT; Olympus) for transmitted light microscopy (Olympus BX53). Histological pictures were taken via light microscopy. The medial menisci were evaluated using a histological scoring system for safranin-o staining, picrosirius red staining, and hematoxylin and eosin staining (Table S4).10 The scoring was performed by two orthopedic surgeons in a blind manner.
2.6 Immunohistochemistry
Types I and II collagen were visualized using diaminobenzidine staining. A mouse monoclonal antibody (ab90395; Abcam) was used for Type I collagen, and a rabbit polyclonal antibody (Kyowa Pharma Chemical) was used for Type II collagen. The sections were counterstained with hematoxylin.
2.7 Statistical analysis
Statistical analysis was performed with the Wilcoxon singed-rank test using GraphPad Prism 6 (GraphPad Software). p < .05 were considered to be statistically significant. All data were reported as the mean ± 95% confidence interval.
3 RESULTS
3.1 Histological analyses
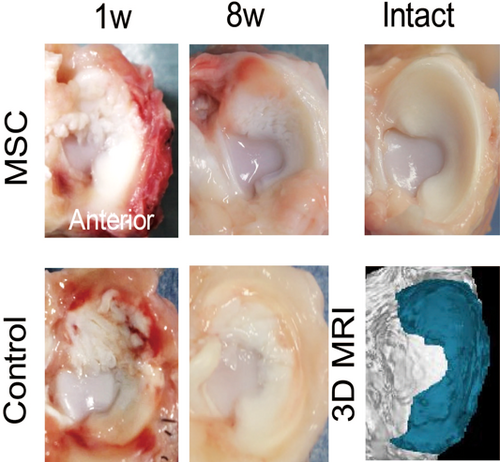
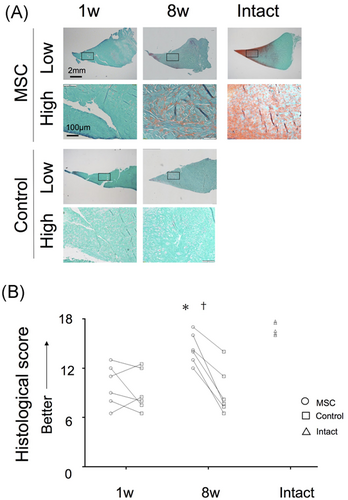
Macroscopically, in the both control and MSC groups, the meniscus surface was rough at 1 week and appeared smoother at 8 weeks (Figure 2). Histologically, the stainability of the proteoglycan content with safranin-o disappeared at 1 week in both groups and remained absent at 8 weeks in the control group; stainability was slightly increased in the MSC group at 8 weeks (Figure 3A). Histological scores were not different at 1 week but were significantly higher in the MSC group than in the control group at 8 weeks (Figure 3B). In addition, it was better in the MSC group at eight weeks than at one week. The expression of Types I and II collagen maintained in both groups at 1 week; at 8 weeks, it decreased in the control group while maintaining in the MSC group (Figure 4). Picrosirius red staining clearly showed obvious damage in both groups at 1 week; at 8 weeks, this was still the case in the control group but not in the MSC group.

3.2 MRI analyses
In the MRI of the sagittal slice, a full high-intensity area was observed in the control menisci at 1 week and a partial one at 8 weeks; the high-intensity area was smaller in the MSC group than in the control group at both 1 and 8 weeks (Figure 5A). The radial view of the reconstructed MRI showed similar results. In the sagittal view of MRI T2 mapping, extensive red areas in the menisci were observed in both groups at 1 week and in the control group but not the MSC group at 8 weeks. T2 values were not different for the groups at 1 week but were significantly shorter in the MSC group than in the control group at 8 weeks; the T2 values of the MSC group at 8 weeks were closer to those of an intact meniscus (Figure 5B; Table 1). In the polarized microscopic images, extensive low birefringence areas in the menisci were observed in both groups at 1 week; these decreased in the control group at 8 weeks and decreased more in the MSC group at 8 weeks (Figure 5A).
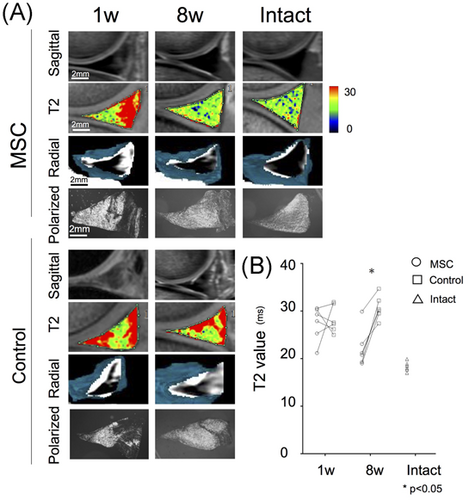
| 1 Week | 8 Weeks | |
|---|---|---|
| MSC group | 27.1 (±3.4) | 22.6 (±3.9) |
| Control group | 28.9 (±3.2) | 30.2 (±2.6) |
- Abbreviation: MSC, mesenchymal stem cell.
4 DISCUSSION
In this study, we created a novel meniscus injury model in microminipigs by repeatedly puncturing the posterior half of the medial meniscus with a needle. Eight weeks after synovial MSCs were transplanted onto the injured lesions, the treated menisci were better healed than those in the control group based on histological evaluation, immunohistochemical analysis, and MRI findings.
Histologically, the proteoglycan content decreased and the surface of the meniscus disrupted in both groups 1 week after repeated punctuation; this was similar to degenerated menisci of human cadaveric knees.13 In addition, the expression of Type II collagen decreased in the control group at 8 weeks, indicating that the quality of the meniscus also decreased in this model. In the polarized microscopic images, extensive low birefringence areas in the meniscus were observed in both groups at 1 week, indicating poor organization of collagen fibers. In the proton weighted MRI image, the entire meniscus showed high intensity, indicating meniscus degeneration.22 These findings indicate that this model mimics a degenerative meniscus injury and is useful for investigating the effectiveness of new treatments for this meniscus pathology.
For MRI analysis, we used a sagittal slice of the proton weighted image, a radial slice reconstructed with 3D MRI, and a sagittal slice of T2 mapping. A sagittal slice of an MRI depicts the anterior or posterior portion of the meniscus as a triangle shape but does not show the middle portion. On the other hand, a coronal slice of an MRI depicts the middle portion of the meniscus as a triangle shape but does not show the anterior or posterior portions. The radial slice was reconstructed using a 3D MRI software that we developed21; this software makes it possible to evaluate any portion of the meniscus using the same triangle shape. The histological sections of the meniscus were also sliced radially so that the radial slices of the MRI and the histological analysis were evaluated compatibly. In this study, we performed multiple MRI analyses, making it possible to compare MRI findings with histological and immunohistological findings.
In the MRI, the injured menisci showed decreased T2 intensity or high T1δ values23, 24; however, the mechanism by which the intensity of the meniscus decreased remained unclear. In this study, the high-intensity areas in the proton weighted images, the high T2 value areas in T2 mapping, and the low birefringence areas in polarized microscopy were consistent. However, these areas had no correlation with the expression of Types I or II collagens; this indicates that high intensity in an MRI is affected primarily by poor collagen alignment, regardless of the type of the collagen. As a different factor, glycosaminoglycan content may influence MRI intensity. Some studies have shown the correlation between T2 relaxation times and T1rho, meaning that there is a relationship of loss of proteoglycan and collagen fiber organization.25, 26 In our study, the meniscus in the MSC group at 8 weeks and the intact meniscus had better proteoglycan content than others; therefore, this may affect the T2 relaxation times.
After developing the model, we investigated the effect of synovial MSC transplantation on the healing of a torn meniscus. Eight weeks after transplantation of synovial MSCs, treated menisci were better healed and the stainability of safranin-o was better in the treatment group than in the control group. In addition, polarization microscopy indicated less damage in the organization of collagen fibers in the MSC group than in the control group. These findings indicate that transplanted MSCs increased the proteoglycan content and improved the organization of collagen fibers at the injury site. The decrease in high-intensity areas of the meniscus in the proton weighed images and the decrease in T2 values in the T2 mapping of the MSC group support this conclusion. Transplanted MSCs may have potential to secrete trophic factors, such as bone morphogenetic proteins,27 which could contribute to meniscus healing.
The current study has some limitations. First, we did not investigate the appearance of osteoarthritis with long-term follow-up. Meniscus pathology affects the gradual progression of osteoarthritis.28 However, the healing of the menisci in the MSC group may have a chondroprotective effect because the healed meniscus is more similar to an intact one in histology, immunohistological findings, and MRI imaging. The expression of Type II collagen was relatively lower after transplantation of MSCs compared with the intact meniscus, which may be due to the short-term follow-up in this study. With longer follow-up, remodeling of the collagen would be expected. Second, we did not identify where synovial MSCs survived after the transplantation. Previous studies have found that synovial MSCs attach to the injured lesion10 and the synovium.29 Similarly, in this model, MSCs had the possibility of attaching to the injured lesion and the synovium around the injured meniscus, which contributes to meniscus healing. Third, a histologically improved meniscus might also have improved function; however, we did not conduct a biomechanical analysis. To confirm the function of the meniscus after MSC transplantation, we should complete biomechanical testing. Finally, differences between microminipigs and humans suggest that the current results should not be directly applied in clinical situations.
In conclusion, we created a meniscus injury model in microminipigs and demonstrated that transplantation of synovial MSCs onto the meniscus lesion promoted meniscus healing.
ACKNOWLEDGMENTS
This work was supported by JSPS KAKENHI (Grant number: JP19K09643). The author would like to thank Ms. Mika Watanabe and Ms. Kimiko Takanashi for the management of our laboratory and Ms. Ellen Roider for English editing.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Nobutake Ozeki contributed to the conception and design, collection of data, analysis and interpretation of data, and manuscript writing. Yuji Kohno, Yoshihisa Kushida, and Naoto Watanabe contributed to the collection and interpretation of data. Mitsuru Mizuno, Hisako Katano, Jun Masumoto, and Hisako Katano contributed to the interpretation of data. Ichiro Sekiya contributed to the conception and design, financial support, manuscript writing, and final approval of manuscript.




