Feasibility of a self-assembling peptide hydrogel scaffold for meniscal defect: An in vivo study in a rabbit model
Scientific editing by Matthew Allen.
Abstract
The inner avascular zone of the meniscus has limited healing capacity as the area is poorly vascularized. Although peptide hydrogels have been reported to regenerate bone and cartilage, their effect on meniscus regeneration remains unknown. We tested whether the self-assembling peptide hydrogel scaffold KI24RGDS stays in the meniscal lesion and facilitates meniscal repair and regeneration in an induced rabbit meniscal defect model. Full-thickness (2.0 mm diameter) cylindrical defects were introduced into the inner avascular zones of the anterior portions of the medial menisci of rabbit knees (n = 40). Right knee defects were left empty (control group) while the left knee defects were transplanted with peptide hydrogel (KI24RGDS group). Macroscopic meniscus scores were significantly higher in the KI24RGDS group than in the control group at 2, 4, and 8 weeks after surgery. Histological examinations including quantitative and qualitative scores indicated that compared with the control group, the reparative tissue in the meniscus was significantly enhanced in the KI24RGDS group at 2, 4, 8, and 12 weeks after surgery. Immunohistochemical staining showed that the reparative tissue induced by KI24RGDS at 12 weeks postimplantation was positive for Type I and II collagen. KI24RGDS is highly biocompatible and biodegradable, with strong stiffness, and a three dimensional structure mimicking native extracellular matrix and RGDS sequences that enhance cell adhesion and proliferation. This in vivo study demonstrated that KI24RGDS remained in the meniscal lesion and facilitated the repair and regeneration in a rabbit meniscal defect model.
1 INTRODUCTION
Meniscal tear is the most common intra-articular knee injury and may lead to osteoarthritis.1 As the meniscus is poorly vascularized, its inner portion of the meniscus has limited healing capacity. Consequently, meniscal tear are difficult to treat.2 Although multiple meniscal repair techniques and scaffolds have been developed,3, 4 the clinical outcome of meniscal repair remains unsatisfactory.5, 6 Therefore, new approaches are required for meniscal repair and regeneration.
Significant progress in tissue engineering has generated useful biomaterials.7 Self-assembling peptide hydrogel scaffold (SAPS) is candidate for meniscal regeneration.8 Several studies showed that SAPS stimulates the regeneration of bone,9, 10 cartilage,11, 12 nerve,13 and other tissues.14 The meniscus is a component of the knee joint that is filled with synovial fluid and subject to excessive load bearing. SAPS must therefore maintain their structure while bear the load under such conditions. SAPS is typically composed of alternating hydrophilic and hydrophobic amino acid residues that spontaneously self-assemble into nanofibers and form hydrogels.8 The mechanical properties of SAPS vary with amino acid sequence, pH, temperature, amino acid charge, hydrophilicity, hydrophobicity, and so on.8, 15 Modulating these factors precisely controls SAPS hydrogelation. SAPS stiffness has been consistently improved over the decades. Nonetheless, increasing it to the viscosity required for load-bearing joints remains a challenge.8 Therefore, no in vivo study on the use of SAPS for meniscal regeneration has been conducted yet.
We had improved SAPS stiffness and developed KI24RGDS (IKIKIKIKIKRGDSKIKIKIKIKI; where K = lysine, I = isoleucine, R = arginine, G = glycine, D = aspartic acid, and S = serine), a β-hairpin peptide with the amino acid sequence, RGDS.16-18 KI24RGDS is an injectable SAPS viscous enough to remain in the meniscal lesion. RGDS interacts with cell surface integrin receptors, participates in cell adhesion, behavior, and signaling, and is the minimum motif required for cell adhesion to a surface.19
The purpose of this study was to determine whether KI24RGDS stays in a meniscal defect and facilitates meniscal repair and regeneration in a rabbit model. Our in vivo study tests the feasibility of SAPS for meniscal scaffold.
2 MATERIALS AND METHODS
2.1 KI24RGDS synthesis
The KI24RGDS peptide was synthesized on Alko-PEG resin using a standard manual Fmoc-protocol with a 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM) activation procedure. N-α-(9-fluorenylmethoxycarbonyl)-l-isoleucine-p-methoxybenzyl alcohol polyethylene glycol resin (Fmoc-Ile-Alko-PEG-Resin; Watanabe Chemical Industries Ltd) (0.83 g) was placed in a polypropylene column (PD-10; GE Healthcare Company) and washed three times with N,N-dimethylformamide (DMF) and methanol. The resin was swollen with 25% (wt/vol) dimethyl sulfoxide/DMF for 30 min. The mixture was then reacted with 20% (wt/vol) piperidine/DMF for 25 min to deprotect the Fmoc group. Then 0.6 mmol Fmoc-protecting amino acid, 0.06 mmol N-methylmorphiline, and 1.2 mmol DMT-MM were added to the resin. The condensation reaction was run for 120 min. The resin was washed three times with DMF and methanol for 1 min each time. The Fmoc group deprotection and condensation reactions were repeated to synthesize the (Ile-Lys(Boc))5-Arg(Pbf)-Gly-Asp(OBtu)-Ser(Btu)-(Lys(Boc)-Ile)5-Alko-PEG-Resin. Finally, peptide protecting group deprotection and resin cleavage were conducted under acidic conditions. The protected peptide-Alko-PEG-Resin and the cleavage mixture (8.50 ml trifluoroacetic acid (TFA), 0.50 ml thioanisole, 0.50 ml pure water, 0.25 ml 1,2-ethanedithiol, and 0.75 g phenol) were stirred for 240 min. The crude peptide was dialyzed with a membrane with a molecular weight cutoff range of 100–500 Da. The crude KI24RGDS peptide was purified and analyzed by high-performance liquid chromatography (HPLC 8020 System; Tosoh Corp). Preparative HPLC (0.1% (wt/vol) TFA/MilliQ water; 0.1% (wt/vol) TFA/acetonitrile was performed for resin purification with COSMOSIL Protein-R (C-18). All Fmoc amino acids were acquired from Watanabe Chemical Industries Ltd. All other chemicals were ordered through Wako Pure Chemical Industries Ltd, and used without further purification. The KI24RGDS peptide (m/z: 2844.9 [M + H]+, calcd.:2844.6) was identified by matrix-assisted laser desorption/ionization mass spectrometry (Microflex LRF System, Bruker Corp) using α-cyano-4-hydroxycinnamic acid as a matrix.
2.2 Preparation of peptide hydrogel sample
To prepare a KI24RGDS hydrogel sample, pure KI24RGDS peptide was dissolved in MilliQ water to make a peptide stock solution (6.0% [wt/vol]). Then an equal volume of phosphate-buffered saline (PBS) (pH 7.4; 2X, 6X, and 10X) was added to adjust the peptide concentration, pH, and ionic strength to the final target conditions (3.0% [wt/vol]; pH 7.4). The KI24RGDS hydrogel (in 1X, 3X, and 5PBS PBS) was studied under simulated physiological and high-salinity conditions. The latter allowed the physical properties of the hydrogel to be controlled. The relative effects of variable salinity on shear thinning and healing were assessed.
2.3 Scanning electron microscopy and physical properties of KI24RGDS
Peptide hydrogels were vacuum freeze-dried, mounted on aluminum scanning electron microscopy (SEM) slides with carbon tape, and sputter-coated with gold for 1 min. Hydrogel morphology was examined by SEM (JSM-6700 FE-SEM; JEOL Ltd) at an accelerating voltage of 10 kV.
All rheological studies were performed on a HAAKE MARS 40 rheometer (Thermo Fisher Scientific) on an 8 mm parallel plate and 0.5 mm gap distance at 25°C. The mechanical properties of the hydrogels were studied by oscillatory shear rheology. The storage modulus (G’) (elastic component) and the loss modulus (G”) (viscous component) were measured as functions of oscillation frequency and strain.
2.4 Experimental animal preparation
The design of the present study was approved by the Animal Research Committee of our institution (No. 30122). Twenty Japanese white rabbits (age: 6 months; weight range: 3.0–3.4 kg) were obtained from Oriental Bio Service (Kyoto). Both the knees of all animals were used in this study.
2.5 Surgical procedure
Rabbits were anesthetized by intravenous injection of a mixture of 2 mg kg−1 midazolam (Dormicum; Astellas Pharma), 0.1 mg kg−1 medetomidine hydrochloride (Domitor; Nippon Zenyaku Kogyo), and 0.4 mg kg−1 butorphanol (Vetorphale; Meiji Seika Pharma) and by subcutaneous injection of 1% (wt/vol) lidocaine hydrochloride (Xylocaine; AstraZeneca). Surgery was performed under sterile conditions as described previously.20 A medial parapatellar procedure was used to expose the knee joint. The medial meniscus was dislocated by pulling it anteriorly. Reproducible full-thickness, 2.0-mm-diameter cylindrical defects were introduced by biopsy punch (Kai Industries) into the avascular inner two-thirds of the anterior portion of the medial meniscus (Figure 1). The cylindrical defect in 20 right knees was left empty (control group), while the defect in 20 left knees was transplanted with KI24RGDS (KI24RGDS group). In the latter group, the cylindrical defect was completely filled with KI24RGDS synthesized in 5X PBS. After surgery, the rabbits were returned to their cages, allowed to move freely. The rabbits were euthanized at 2, 4, 8, and 12 weeks after surgery (n = 5).
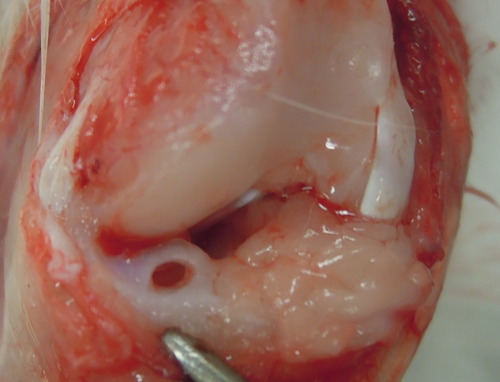
2.6 Macroscopic evaluation
Two, 4, 8, and 12 weeks post-surgery, the femoral articular cartilage surface was photographed. All medial menisci were collected and photographed before fixation. Macroscopic meniscus evaluation was graded according to the modified semiquantitative scoring system.21 The thinnest parts of the reparative tissues were evaluated as follows; four points: full thickness, complete healing; three points: incomplete healing up to >2/3 of the height of the meniscal defect; two points: from < 2/3 to >1/3 of the height of the meniscal defect; one point: from <1/3 of the height of the meniscus to >full-thickness defect; zero: full-thickness defect. The scoring system for macroscopic articular cartilage changes without India ink in accordance with Osteoarthritis Research Society International was used to assess the cartilage degeneration by the effect of KI24RGDS or meniscal defect on the medial femoral condyle of each knee (score range: 0–8).22, 23
2.7 Histological analysis
Each specimen was sliced into 6 μm sections in the radial plane and then stained with hematoxylin–eosin (H&E) and safranin O. The reparative tissue was evaluated quantitatively and qualitatively. For quantitative reparative tissue assessment, the occupation ratios of the reparative tissue in the defect were evaluated using sections from its central portion.24, 25 The average occupation ratios of the three sections for each sample were calculated. The areas of the reparative tissue (R) and the entire defect (D) were analyzed in ImageJ v. 1.52a (National Institutes of Health).26 R:D represents the quantity of tissue regeneration (Figure 2) which was calculated as follows: 
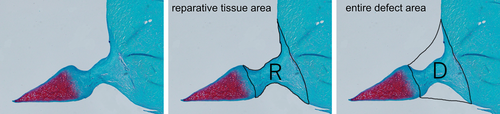
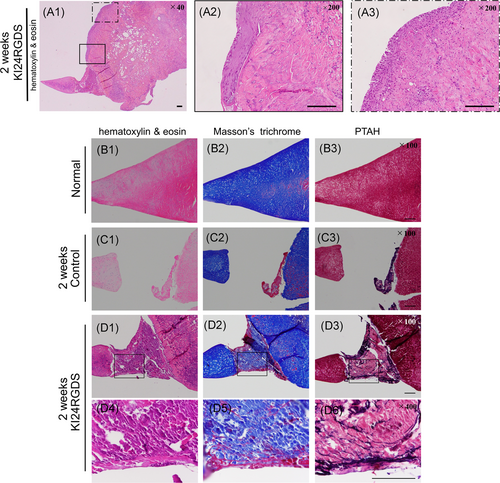
For qualitative reparative tissue assessment, tissue sections were histologically evaluated with the scoring system developed by Ishida (score range: 0–6).27 Reparative tissues are rated by bonding, the presence of fibrochondrocytes, and positive safranin O staining. Both quantitative and qualitative scores were independently assessed by two orthopedic surgeons blinded to the treatments. In addition to H&E and safranin O, additional Masson's trichrome and phosphotungstic acid hematoxylin (PTAH) stainings were performed to evaluate cell migration 2 weeks post-surgery. Picrosirius red stain samples were observed under polarized light microscopy (Olympus BX-50; Olympus Corp) for all tissue sections at 12 weeks after surgery. The results of this assessment were compared with those obtained by immunohistochemical analysis.
2.8 Immunohistochemical analysis
Immunohistochemical staining for anti-Type I collagen antibody (BD Bioscience, Franklin Lakes) and anti-Type II collagen antibody (Developmental Studies Hybridoma Bank) was performed on all tissue sections at 12 weeks after surgery. The sections were washed with PBS and then blocked with skim milk at room temperature (20°C) for 10 min. The sections were incubated overnight at 4°C with anti-Type I collagen antibody diluted to 1:100 and anti-Type II collagen antibody diluted to 1:10. The sections were washed with PBS and incubated with biotinylated antirabbit immunoglobulin G (Vector Laboratories) against Type I collagen and biotinylated anti-mouse immunoglobulin G (Vector Laboratories) against Type II collagen (secondary antibodies) at 20°C for 60 min. They were then incubated with streptavidin-horseradish peroxidase conjugate (Vector Laboratories) at 20°C for 10 min and reacted with a diaminobenzene chromogen or substrate kit (ScyTek Laboratories) for 5 min. The slides were counterstained with H&E. All tissues sections were simultaneously subjected to immunohistochemical staining.
2.9 Synovitis evaluation
The degree of synovitis surrounding the meniscus was histologically evaluated at all evaluation timepoints by semi-quantitative synovitis scoring, described by Krenn.28
2.10 Statistical analysis
All data were analyzed in JMP Pro v. 12.0.1 (SAS Institute). All data are means ± standard deviation. The Mann–Whitney U test was performed to compare the KI24RGDS and control group means at each evaluation timepoint. p < .05 was considered statistically significant. The inter-rater reliability for the macroscopic meniscus score and the Ishida score was assessed by two independent blinded observers for all samples.
3 RESULTS
3.1 Scanning electron microscopy and physical properties of KI24RGDS
Gross observation of KI24RGDS injected by syringe is shown in Figure 3A. It is viscous enough to stay at the site of injection. The SEM image of KI24RGDS is shown in Figure 3B. The hydrogel is highly porous and was formed from a nanofiber network. The SEM images show the three dimensional (3D) nanoscale structure. Peptide hydrogels with relatively larger amounts of high-aspect-ratio nanostructures appeared to have comparatively more fiber-like structures. The storage moduli were greater than the loss moduli for all materials forming SAPS hydrogels.
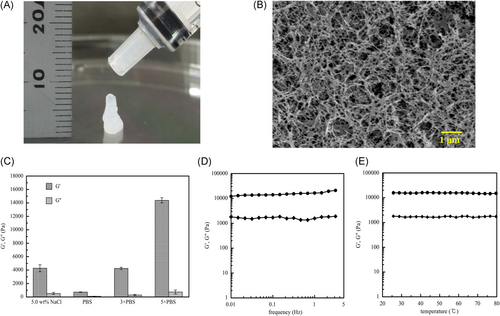
The storage and loss moduli (G’ and G”, respectively) of the peptide hydrogel under various conditions are summarized in Figure 3C. G’ apparently depended on the salt concentration of the peptide hydrogels. The medium with 5X PBS had the highest G’. However, aqueous solubility decreased with increasing PBS concentration.
The mechanical properties of the hydrogel were relatively independent of oscillation frequency (sinusoidal shear deformation in the sample and measure the resultant stress response). They stayed in the linear elastic region up to 3% strain with minimal change in the storage modulus (Figure 3D). However, they were dependent on hydrogel and salt concentration. Figure 3E shows that the mechanical properties of the hydrogels were highly stable at body temperature. Thus, they exhibit gel behavior in an in vivo environment.
3.2 Macroscopic findings
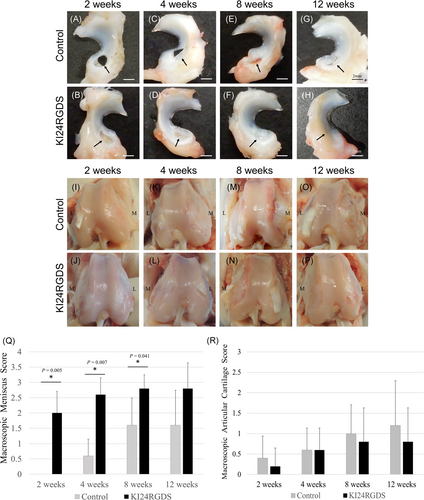
There was no evidence of complications including infection. Representative macrophotographs of reparative tissue in the defect at the anterior portion of the medial meniscus after surgery are shown in Figure 4A–H. The macroscopic differences in menisci between the control and KI24RGDS group were evident. In the control group, the defect was nearly vacant after 2 weeks after surgery. Thereafter, the reparative tissue gradually increased at 4 and 8 weeks after surgery but the defect never fully regenerated. It was still visible even at 12 weeks after surgery. In the KI24RGDS group, the defect was filled with soft tissue at 2 weeks after surgery. This new growth was considered reparative tissue because it was macroscopically different from the peptide hydrogel. Reparative tissue grew steadily at 4 and 8 weeks after surgery. By 12 weeks, the defect had almost disappeared. The KI24RGDS group had significantly higher macroscopic meniscus scores than the control group at 2, 4, and 8 weeks after surgery. The inter-rater reliability for the macroscopic meniscus score was 0.94. The representative macrophotographs of the surface of the femur at after surgery are shown in Figure 4I–P. The macroscopic articular cartilage scores in both groups showed no severe cartilage degeneration (Figure 4R).
3.3 Histological findings
Representative microphotographs of H&E and safranin O staining after surgery are shown in Figure 5A–D. In the KI24RGDS group, histological analysis at 2 weeks after surgery showed that peptide hydrogel was observed in the meniscal defect and that cell migration and proliferation from the peripheral tissue was observed (Figures 5B,D(1–2)). These cells that migrated along the surface of meniscus did not form newly reparative tissue bounding to the inner and outer native meniscus. Histological inspection of another knee at 2 weeks after surgery revealed that the meniscal defect was nearly filled with KI24RGDS. Moreover, cells had migrated along the KI24RGDS surface from the surrounding tissue and penetrated and partially replaced it (Figure 6A1–3). Furthermore, Masson's Trichrome and PTAH staining showed that the KI24RGDS group had reticulated fibrin within KI24RGDS, filling the defect, while the control group had some fibrin only around the migrating cells (Figure 6B–D(1–3)). At 4 weeks after surgery, the reparative tissue attached to inner and outer edges of the native meniscal tissue. The boundary between newly formed tissue and native meniscus was not seen. At 8 weeks, very little peptide hydrogel remained in the defect (Figure 5B,D(5,6)) and by 12 weeks, the defect was almost entirely filled with reparative tissue. The reparative tissue at 12 weeks after surgery contained oval-shaped cells and rich extracellular matrix (ECM) components, which stained red with safranin O (Figure 5B,D(7,8)).
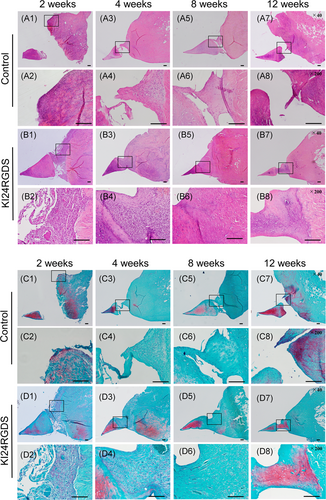
In the control group, only a few cells derived from peripheral tissue were observed at the outer edge of the defect at 2 weeks after surgery (Figure 5A,C(1,2)). At 4 and 8 weeks after surgery, the cells gradually migrated into the defect (Figure 5A,C(3–6)). At 12 weeks after surgery, the number of cells migrating from peripheral tissue increased but the defect was only partially filled with reparative tissue (Figure 5A,C(7,8)).
The R:D ratios for the KI24RGDS group were significantly higher than those for the control group at all timepoints (Figure 7A; 2 weeks: p = .009, 0.015 ± 0.013 [control] vs. 0.666 ± 0.084 [KI24RGDS]; 4 weeks: p = .016, 0.398 ± 0.164 [control] vs. 0.785 ± 0.141 [KI24RGDS]; 8 weeks: p = .016, 0.564 ± 0.157 [control] vs. 0.875 ± 0.080 [KI24RGDS]; 12 weeks: p = .009, 0.434 ± 0.187 [control] vs. 0.821 ± 0.110 [KI24RGDS]).
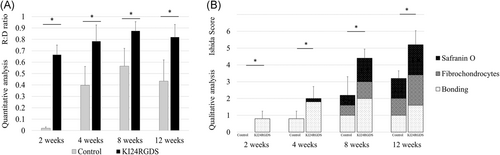
The Ishida scores for the KI24RGDS group were significantly higher than those for the control group at all timepoints (Figure 7B; 2 weeks: p = .014, 0.000 ± 0.000 [control] vs. 0.800 ± 0.447 [KI24RGDS]; 4 weeks: p = .018, 0.800 ± 0.447 [control] vs. 2.000 ± 0.707 [KI24RGDS]; 8 weeks: p = .007, 2.200 ± 1.095 [control] vs. 4.400 ± 0.548 [KI24RGDS]; 12 weeks: p = .009, 3.200 ± 0.447 [control] vs. 5.200 ± 0.837 [KI24RGDS]). The inter-rater reliability for the Ishida score was 0.92. The KI24RGDS group had the sub-score of bonding at 2 weeks after surgery. The average score of each component in the KI24RGDS group was higher than that in the control group at 8 and 12 weeks after surgery.
3.4 Picrosirius red and immunohistochemical staining
Picrosirius red staining samples viewed under polarized light microscopy and immunohistochemical staining samples at 12 weeks after surgery are shown in Figure 8. In picrosirius red staining, the defect in the control group was not repaired and fibers were unstructured, whereas the collagen fibers of the reparative tissue in the KI24RGDS improved in their organization and orientation. (Figure 8A,B(2)).
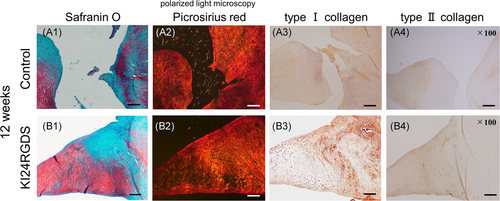
In immunohistochemical staining, the outer menisci in both groups were negative for Type II collagen (Figure 8B4). Although the reparative tissue in the control was negative for Type II collagen, the inner meniscus including the implantation site in the KI24RGDS was positive for Type II collagen. There was apparent difference in Type I and II collagen between the control and KI24RGDS groups (Figure 8A,B(3,4)).
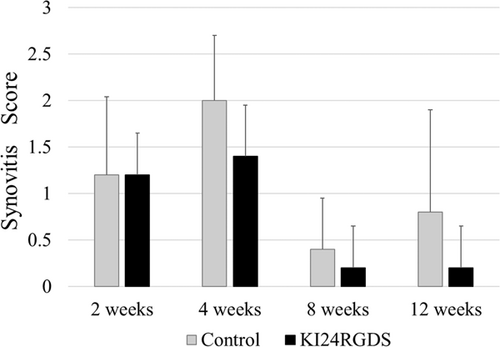
3.5 Synovitis score
As per synovitis scoring results, there was no case with high-grade synovitis in both groups at time-points evaluated. Moreover, although some cases with low-grade synovitis were observed at 2 and 4 weeks after surgery in both groups, there was no significant difference between them (Figure 9).
4 DISCUSSION
The present in vivo study examined whether the KI24RGDS remains in the meniscal defect and facilitates repair and regeneration in a rabbit model. The most important finding of the study was that SAPS, KI24RGDS has feasibility of facilitating meniscal repair and regeneration by serving as a scaffold. Macroscopic and histological evaluations indicated that KI24RGDS significantly increased meniscal reparative tissue production compared with the control group. Quantitative and qualitative analyses demonstrated that the KI24RGDS group had significantly higher scores than the control group at all evaluation timepoints. Various histological scoring systems are available for meniscal tissue analysis. Nevertheless, the R:D ratio is often used to measure reparative tissue in tissue engineering.24, 25 The qualitative analytical method developed by Ishida, is preferred for comprehensive meniscal reparative tissue evaluation.27, 29 In the KI24RGDS group, however, the volume of SAPS remaining in the defect at 2 and 4 weeks after surgery may have negatively impacted its R:D ratio and Ishida score. At 8 and 12 weeks after surgery, only very small amounts of SAPS were visible in the reparative tissue and probably had negligible effect on the quantitative and qualitative analyses.
The inner meniscus is fibrocartilage tissue and contains more Type II collagen than the outer meniscus.1 The reparative tissue in the KI24RGDS at 12 weeks after surgery was positive for Type I and II collagen in immunohistochemical staining. Furthermore, the regenerated tissues contained fibrochondrocytes and ECM rich in proteoglycan stained with safranin 0.
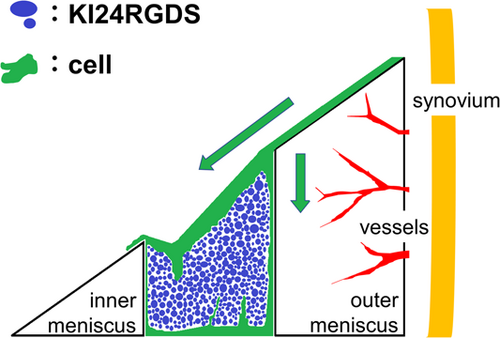
There was histological evidence (Figure 6) to suggest that in the KI24RGDS group at 2 weeks after surgery, KI24RGDS served as a scaffold. As KI24RGDS was not implanted together with cells, the reparative cells replacing KI24RGDS must originate from the surrounding tissues. Cells from the surrounding tissue migrated along the surface of the meniscus and penetrated into the KI24RGDS. Masson's trichrome and PTAH staining showed that capillary fibers were infiltrated into the peptide hydrogel, suggesting that growth factors incorporate the hydrogel and may be the basis of meniscal regeneration. On the base of these histological analysis, we deduced that the cells that had migrated from surrounding tissue to and proliferated within the high porous 3D structures of KI24RGDS had secreted chemotactic or mitogenic signals, leading to better meniscal repair and regeneration relative to the control group. As such, we proposed a model, represented in a schematic diagram of a meniscal scaffolds (Figure 10).
Evaluation of the mechanical properties of KI24RGDS showed that increasing the salt concentration in the peptide hydrogels also increased the storage modulus (G’). KI24RGDS synthesized using 5X PBS had a higher storage modulus (G’) than loss modulus (G’’) at all frequencies and temperatures. Thus, KI24RGDS exhibits gel behavior in an in vivo environment. As the elastic modulus of native human meniscus (radial samples) is about 10 MPa,30 the storage modulus of KI24RGDS synthesized using 5X PBS was about 15 KPa.
Hydrogels are preferred for meniscal tissue engineering studies because they fill meniscal lesions of various shapes and provide 3D support and scaffolding.31 Nevertheless, hydrogel alone is inadequate at promoting meniscal healing.31 Therefore, hydrogels have been co-applied with cells and bioactive molecules to stimulate regeneration.31, 32 Mesenchymal stem cells (MSCs) are pluripotent stem cells that are found in various tissues including bone marrow, adipose tissue, blood, and synovium.33-35 MSCs are often usedin research on meniscal repair and regeneration, and have the potential to promote meniscal healing.36 However, cell-based therapy is associated with risks, such as contamination, infection, and unknown factors.33 In contrast, cell-free therapy requires no cell culture and is safer and easier for clinical application than cell-based therapy.34 Cell-free scaffolds have been studied to realize cell-free therapy for meniscus and could be categorized into four groups; ECM components, synthetic polymers, tissue-derived materials, and hydrogel scaffolds.2 Cell-free meniscal scaffolds available in clinical use are CMI (Ivy Sports Medicine) and Actifit (Orteq Sport Medicine). CMI is composed of purified Type I collagen isolated from bovine Achilles tendon, and is categorized to ECM component scaffolds. Actifit is made of polycaprolactone and polyurethane, and is categorized to synthetic polymer scaffolds. Both meniscal scaffolds are good treatment options for patients with knee pain after partial meniscectomy.5, 37
SAPS is a promising candidate for hydrogel scaffolds.38 Hydrogels are classified according to the polymers whence they originate (natural or synthetic).39 Alginate is the most widely used hydrogel matrix. It is derived from the cells walls of brown algae.31, 40 In contrast, SAPS is synthetic and may be less likely to cause contamination or infection than natural materials. SAPS is also reproducible; its matrix composition is fairly consistent across production batches. Moreover, it is composed of peptides that are already naturally found in the human body. Thus, SAPS is highly biocompatible and biodegradable. In this study, according to the macroscopic articular cartilage and the synovitis score, KI24RGDS did not cause significant adverse effects on the articular cartilage and synovium.
SAPS forms 3D structures consisting of approximately 10 nm fibers long with pore diameters ranging from 5 to 200 nm. These dimensions resemble those of the ECM.8, 15, 41 On the other hand, hydrogels made of conventional polymers consist of fibers 10–50 µm long.8 As various SAPS have the aforementioned advantages, some of them have been produced with different functional motifs including facilitating the regeneration of bone, cartilage, nerve, and other tissues.9-14
The SAPS known as RADA16 (PuraMatrix; 3-D Matrix) is commercially available and has numerous applications in tissue engineering.8, 9, 12, 13 Nevertheless, it has not been tested for meniscal regeneration as its mechanical properties are unsuitable for this purpose.8, 42 In the present study, KI24RGDS was synthesized by increasing the PBS concentration so that the SAPS would remain in the meniscal defect. KI24RGDS has alternating “K” (lysine) and “I” (isoleucine) residues with its RGDS sequence as the turn peptide. Previous studies demonstrated that the RGDS sequence enhances cell differentiation and proliferation.19, 43, 44 This could be further enhanced by coimplantation of KI24RGDS with cells and growth factors, which can synergistically facilitate meniscal regeneration relative to the use of biomaterial alone.2
There were several limitations to this study. First, young rabbits have a much greater healing potential than adult humans. Therefore, the results obtained for the rabbit model of the present cannot be extrapolated to human.45 Second, the experimentally induced meniscal defect consisted of a cylindrical hole with a diameter of 2.0 mm into the anterior portions. This cylindrical defect structure markedly differs in types of meniscal tear and common sites observed clinically in humans and anterior portion of the meniscus would not be heavily loaded when rabbits sit with knee in hyperflexion. As the experimental procedure to posterior portion of the meniscus requires resection of soft tissues involving medial collateral ligament, cylindrical defects into the anterior portions have often been used in meniscal tissue engineering research. Although the most commonly used cylindrical defect in rabbit was 1.5 mm in diameter,27 we created a 2.0-mm-diameter defect to distinguish the effects of SAPS from spontaneous meniscal healing. In certain individuals, the KI24RGDS partially flowed out of the defect and reparative tissue was not fully regenerated there. This leakage might lead to KI24RGDS dissolution in the synovial fluid, which could cause the effects on other structures of the knee joint. SAPS stiffness requires improvement to ensure that the material does not exude from the meniscal lesion and more effectively activates regeneration there. Third, we did not evaluate the mechanical strength of the reparative tissue. However, histological analysis and immunohistochemical staining confirmed that the reparative tissue was composed of fibrocartilaginous cells.
Further research on the safety, efficacy, and biomechanics of SAPS must be performed before initiating clinical trials in humans. However, we confirm that KI24RGDS is a promising material for meniscal repair and regeneration. In the future, arthroscopic SAPS injection could be an efficacious and safe clinical treatment for meniscal lesion.
5 CONCLUSION
This in vivo study demonstrated that KI24RGDS remained in the meniscal lesion and facilitated the repair and regeneration of a rabbit meniscal defect model. KI24RGDS is highly biocompatible and biodegradable, has strong stiffness, and is safe.
ACKNOWLEDGMENTS
The authors thank Yoshie Seki for technical assistance with the laboratory research. Financial support for this study was provided by JSPS KAKENHI (grant no. 16K01400) and the Private University Research Branding Project.
AUTHOR CONTRIBUTIONS
Shuhei Otsuki, Yoshinori Okamoto, Yoshiaki Hirano, Yoshinobu Hirose, and Masashi Neo designed study planning. Jo Aoyama and Yoshiaki Hirano developed KI24RGDS and analysis the rheology of KI24RGDS. Nobuhiro Okuno mainly drafted manuscript. Nobuhiro Okuno, Kosuke Nakagawa, Tomohiko Murakami, and Kuniaki Ikeda participated animal surgeries and interpretation of data. All authors have read and approved the final submitted manuscript.




