Comparison of meniscal allograft transplantation techniques using a preclinical canine model
Abstract
Meniscal allograft transplantation (MAT) can be a safe, effective treatment for meniscal deficiency resulting in knee dysfunction, leading to osteoarthritis (OA) without proper treatment with 5-year functional success rates (75%-90%). While different grafts and techniques have generally proven safe and effective, complications include shrinkage, extrusion, progression of joint pathology, and failure. The objective of this study was to assess the functional outcomes after MAT using three different clinically-relevant methods in a preclinical canine model. The study was designed to test the hypothesis that fresh meniscal-osteochondral allograft transplantation would be associated with significantly better function and joint health compared with fresh-viable or fresh-frozen meniscus-only allograft transplantations. Three months after meniscal release to induce meniscus-deficient medial compartment disease, research hounds (n = 12) underwent MAT using meniscus allografts harvested from matched dogs. Three MAT conditions (n = 4 each) were compared: frozen meniscus–fresh-frozen meniscal allograft with menisco-capsular suture repair; fresh meniscus–fresh viable meniscal allograft (Missouri Osteochondral Preservation System (MOPS)-preservation for 30 days) with menisco-tibial ligament repair; fresh menisco-tibial–fresh, viable meniscal-tibial-osteochondral allografts (MOPS-preservation for 30 days) with menisco-tibial ligament preservation and autogenous bone marrow aspirate concentrate on OCA bone. Assessment was performed up to 6 months after MAT. Pain, comfortable range of motion, imaging, and arthroscopic scores as well histological and cell viability findings were superior (P < .05) for the fresh menisco-tibial group compared with the two other groups. Novel meniscal preservation and implantation techniques with fresh, MOPS-preserved, viable meniscal-osteochondral allografts with menisco-tibial ligament preservation appears to be safe and effective for restoring knee function and joint health in this preclinical model. This has the potential to significantly improve outcomes after MAT.
1 INTRODUCTION
Meniscal deficiency is a major problem for millions of individuals worldwide.1, 2 Besides notable health care costs, time lost from work, related socioeconomic impact, and disability, significant loss of functional meniscal tissue results in knee dysfunction and inevitably leads to osteoarthritis (OA) if not effectively treated.1-6 Depending on age, patient-specific demands, and nature of meniscal pathology in terms of severity, location, and biologic and biomechanical characteristics, treatment options may include nonoperative management, meniscectomy, meniscal repair, and meniscal allograft transplantation (MAT).7-9
MAT can be a safe and effective treatment for meniscal deficiency with 5-year functional success rates from 75% to 90% reported.9, 10 The first description of MAT was in conjunction with osteochondral allograft in the 1970s and the first description of meniscus-only MAT was reported in 1984.9, 11, 12 Since then, clinical use of MAT has grown worldwide and been proven to be a reliable therapeutic option based on long-term follow-up.13-15 The majority of MAT procedures now are performed using size-matched fresh-frozen allografts distributed from an accredited tissue bank. Surgical techniques for implantation include soft-tissue, bone plug, and bone bridge methods for root fixation, followed by menisco-capsular suture repair.9, 16 While these grafts and techniques have proven safe and effective in general, complications including shrinkage, extrusion, progression of joint pathology, and failure persist9, 16, 17 that negatively influence outcomes, patient satisfaction, and long-term survivorship due to revision and removal (6%-27%) or conversion to total knee arthroplasty (19%).10, 18-21
In an attempt to mitigate these complications, our group has developed and validated a method for preservation of fresh, viable menisci that complies with the United States Food and Drug Administration (FDA) classification of a human cell and tissue product under Section 361 of the Public Health Services Act, as well as techniques for reconstruction or preservation of the menisco-tibial ligament and combined transplantation with fresh tibial osteochondral allograft (OCA).22-27 While safety and initial efficacy for this approach have been documented, comprehensive comparative data are necessary in order to directly compare the currently available MAT techniques.26, 27 Therefore, the objective of the present study was to assess the functional outcomes after MAT when performed using three different clinically-relevant methods in a preclinical canine model. The study was designed to test the hypothesis that fresh meniscal-osteochondral allograft transplantation would be associated with significantly better function and joint health compared with fresh-viable or fresh-frozen meniscus-only allograft transplantations because of the potential for addressing common complications based on tissue viability, preservation of key anatomical structures, and concurrent treatment of associated articular cartilage pathology.
2 METHODS
All procedures were approved by our institution's animal care and use committee (#9167). Twelve skeletally-mature purpose-bred research hounds (mean age = 1.6 ± 0.1, mean weight = 21.7 kg ± 1.5) were enrolled and pre-treatment assessments were performed including pain and function evaluations, measurement of knee range of motion, assessment of knee effusion, forcemat kinematics, and radiographic and arthroscopic grading, as described below. For induction of medial compartment OA, dogs were premedicated (dexmedetomidine: 5-10 µg/kg IV; morphine: 0.5 mg/kg IM), anesthetized (propofol: 4-8 mg/kg IV), and prepared for aseptic surgery of one knee using a validated arthroscopic meniscal release (MR) model.28, 29 The dogs were recovered from anesthesia and provided with an additional intramuscular morphine (0.5 mg/kg) within 6 hours of the preceding dose. All dogs were restricted to their kennels for 1 week and then provided with monitored daily out-of-kennel exercise, which included on leash walks by an individual handler for 15 min.
Two months after MR, the tibial plateaus with attached menisci were recovered from age- and breed- and size-matched purpose-bred research hounds (n = 12) from an unrelated IACUC-approved study. Recovered tissues were either fresh-frozen (n = 4) or preserved in the Missouri Osteochondral Preservation System (MOPS) (n = 8) for 30 days according to tissue bank protocols.
Three months after MR, the presence of medial compartment gonarthrosis in the operated knees was documented radiographically and arthroscopically based on joint space narrowing, subchondral sclerosis, effusion, focal synovitis, and extruded medial menisci with focal areas of partial- to full-thickness cartilage loss on the medial tibial plateau and medial femoral condyle. Assessments of pain and function, knee range of motion, effusion, and forcemat kinematics were performed and recorded.
-
Frozen meniscus (n = 4): Fresh-frozen meniscal allograft with menisco-capsular suture repair using a double bone plug technique with suspensory cortical fixation based on current clinical practice. The medial meniscus allograft was removed from the tibial plateau using sharp dissection for peri-meniscal soft tissues and a cannulated coring reamer system (Arthrex, Inc. Naples, FL) to create 6 mm diameter bone plugs to encompass each root. The posterior (caudal) bone plug was trimmed to a depth of 5 mm and the anterior (cranial) bone plug was trimmed to a depth of 7 mm. A #0 braided nonabsorbable suture (FiberWire; Arthrex) was used to create suspensory fixation for each bone plug. Recipient sockets were created at each root insertion site using a 6 mm diameter cannulated low-profile reamer (Arthrex) to a depth of 8 mm posteriorly and 10 mm anteriorly. Passing sutures were placed through the cannulation channels and the bone plugs were pulled into the respective socket. Once the bone plugs were seated and insertion depth was adjusted to optimize meniscal transplant positioning, tension, and tibial coverage, the suspensory sutures were tied together for cortical fixation. Simple interrupted sutures (2-0 PDS) were placed to secure the peripheral meniscus to joint capsule.16 Joint capsule, subcutaneous tissues, and skin were then closed routinely.
-
Fresh meniscus (n = 4): Fresh, viable 30-day MOPS-preserved meniscal allograft with menisco-tibial ligament reconstructed using a double bone plug technique with suspensory cortical fixation. The meniscal allograft was prepared in the same manner as described above except that the menisco-tibial (coronal) ligament was maintained with respect to its attachment around the full circumference of the meniscus by sharp dissection from the tibia. The recipient site was prepared in the same manner as described above. The MAT was implanted as described above, but prior to placing the menisco-capsular sutures, two horizontal mattress sutures (2-0 FiberWire; Arthrex) were placed in the menisco-tibial ligament and each was secured to the proximal tibia using suture anchors (2.4 mm PushLock; Arthrex) to attach the donor menisco-tibial ligament to the recipient tibia. Menisco-capsular sutures and closure of the surgical wound then proceeded as described above.
-
Fresh menisco-tibial (n = 4): Fresh, viable 30-day MOPS-preserved meniscal-tibial-osteochondral allografts with menisco-tibial ligament preservation, autogenous bone marrow aspirate concentrate applied to donor bone, and fixation using bioabsorbable implants. The dog's medial meniscus was completely resected to capsular junction and removed. An osteotome was used to make a sagittal osteotomy at the axial margin of the hemiplateau; this avoided damaging the cruciate ligaments. A sagittal saw was then used to resect the hemiplateau at a depth of 7 mm and matching the slope of the articular surface. The saw was used to create a slot at the anterior (cranial)-abaxial margin of the recipient site. The donor tissues were prepared by carefully dissecting periarticular soft tissues while preserving the meniscal roots and menisco-tibial (coronal) ligament. The donor tibia was then cut to match the dimensions of the resected hemiplateau while preserving the meniscus and its attachment and creating a tab to match the recipient slot. The menisco-tibial allograft was secured to the tibial using bioabsorbable nails (SmartNail; ConMed, Largo, FL). Menisco-capsular sutures and closure of the surgical wound then proceeded as described above.
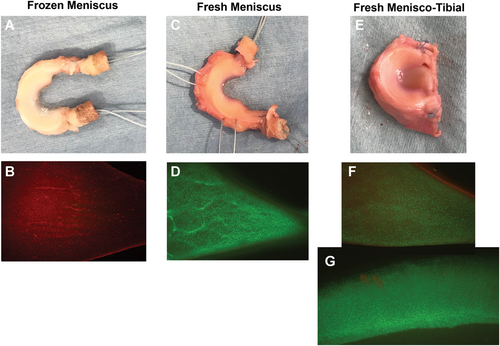
Allograft tissues that were not implanted were used to determine chondrocyte and menisco-fibrochondrocyte viability using a live-dead cell staining assay. Tissues were incubated with calcein AM (live cell stain) and ethidium homodimer (dead cell stain), and tissue viability images were taken using a fluorescent microscope at ×4 magnification (Olympus, Tokyo, Japan). For cartilage tissue, viable cell density (VCD) was determined and reported as a percentage of normal healthy canine cartilage (% VCD) as previously described.22, 24, 30, 31 The viability of the meniscus was determined by subjective assessment of staining by one observer blinded to storage treatment. Meniscal viability staining was assessed on a scale of 0 to 100, with 0 indicative of no viable cells stained in the tissue, and 100 indicative of viability staining similar to fresh canine control tissue.
Dogs were recovered from surgery and morphine (0.5 mg/kg IM) and carprofen (4.4 mg/kg subcutaneously) were provided. A soft-padded bandage was placed and maintained on the operated limbs for 1 week. The animals returned to their runs and given analgesics (an additional intramuscular morphine within 6 hours of the preceding dose, followed by Tramadol PO [2-7 mg/kg BID] for 3 days and carprofen PO [4.4 mg/kg SID] for 7 days) and then as needed based on physical parameters indicating the presence of pain. Cefpodoxime PO (5-10 mg/kg SID) was given for 10 days. All dogs were restricted to their kennels for 8 weeks and received monitored daily out-of-kennel exercise as previously described and maintained for 6 months post-implantation.
All dogs were evaluated by a board-certified veterinary surgeon blinded to treatment type and time point, and assessments were performed pre-MR, post-MR/OA/pre-MAT, and 1, 3, and 6 months after MAT. Assessments included comfortable knee range of motion (CROM), limb function (clinical lameness and limb kinetics), knee pain, and knee effusion, as previously described.32 Briefly, knee CROM was measured using a standard goniometer placed along the lateral axis of the tibia and the other arm placed along the lateral axis of the femur with the hinge point centered over the knee joint line. The knee was then manually extended to the highest angle the dog tolerated without showing resistance or pain to determine and record the extension angle (degrees). The knee was then manually flexed to the most acute angle the dog tolerated without showing resistance or pain to determine and record the flexion angle (degrees). The flexion angle was subtracted from the extension angle to determine CROM for each knee. Clinical lameness scores were determined for each dog based on visual examination of gait by a board-certified veterinary orthopedic surgeon using a 10 cm visual analog scale (VAS) and a validated grading system: 0—no observable lameness; 1—intermittent, mild weight-bearing lameness with little, if any, change in gait; 2—moderate weight-bearing lameness, obvious lameness with noticeable gait change; 3—Severe weight-bearing lameness, “toe-touching” only; 4—non-weight-bearing. Limb kinetics were performed as previously described.33-35 Briefly, kinetics assessment was performed by having a dedicated handler trot dogs across a pressure-sensing walkway (GAITFour, Haverton, PA) on-leash in each direction with the handler attempting to maintain a consistent velocity. Passes were included for analysis when the dogs walked at a steady pace with all four footfalls recorded for at least three gait cycles. At least three acceptable passes (3-5 gait cycles), with video documentation, were obtained for each dog at each time point. The software program was used to distinguish the paw print for each footfall, which were then identified manually as left front, right front, left hind, or right hind, accordingly. Mean percent body weight distribution (% BW) was determined for each limb using the three complete data sets based on total pressure index (TPI). The % TPI for the operated limb was chosen a priori as the measure to report and compare. Knee pain and knee effusion were assessed subjectively based on a VAS and recorded for each hindlimb of each dog.
Arthroscopic evaluation of each knee joint was then performed by a board-certified veterinary surgeon blinded to treatment type and time point to assess whole-joint pathology 1, 3, and 6 months after MAT, as described previously.36 Briefly, the entire joint was subjectively evaluated based on visualization and probing of suprapatellar, patellotrochlear, medial, and lateral femorotibial, and intercondylar notch structures to assess for synovitis and/or cartilage, meniscal, and/or ligament pathology, which was described and documented, if present. Specifically, synovial pathology was graded as normal, slight, mild, moderate, marked, or severe.36 Articular cartilage pathology was graded as smooth, slightly fibrillated, partial thickness lesions, deep lesions, or diffuse severe damage.36 Meniscal pathology was graded as none, fibrillation, incomplete tear, complete tear, or complete disruption.36
Craniocaudal (anteroposterior) and mediolateral digital radiographic views of surgically treated knees were obtained in a standardized fashion post-MR/OA/pre-MAT and 1, 3, and 6 months after MAT. The radiographs were evaluated by one board-certified veterinary radiologist blinded to treatment type and time point using a modified subjective system.37 Subjective findings related to relative joint space and osteochondral allograft integrity, positioning, and integration were also described.
Ultrasonographic examination was performed by one investigator trained in musculoskeletal ultrasonography and blinded to treatment type and time point using a portable ultrasound machine (Logiq i; GE Healthcare, Milwaukee, WI) with a 10 to 14 MHz linear array transducer 6 months after MAT. Each meniscus was evaluated for displacement, echogenicity, shape, and associated effusion as previously described.38, 39
Under general anesthesia, magnetic resonance imaging (MRI) of the affected knee was performed 6 months after MAT using a Toshiba Titan 3T (Canon Medical Systems, Inc, Tustin, CA). The sequences performed included axial, coronal, and sagittal 2-dimensional T2 (TR = 3800, TE = 84); sagittal T2 with fat saturation (T2FS) (TR = 4000, TE = 84); sagittal and coronal proton density (TR = 2500, TE = 12), and coronal short T1 inversion recovery (STIR) (TR = 3300, TE = 80), using 2 mm thickness and 2.2 mm spacing. A dedicated 8-channel phased array knee coil (Canon Medical Systems) was used for scanning. A board-certified veterinary radiologist, blinded to treatment, evaluated all MRIs to subjectively assess each MRI for meniscal pathology, including evidence of tears (horizontal, vertical, peripheral), shape and location of the meniscus, presence of cartilage pathology, and the presence and severity of bone marrow lesions.32
Dogs were humanely euthanatized 6 months after implantation and the knees evaluated by gross and histologic assessments of joint tissues, with sections of transplanted menisci and tibial plateaus also assessed for chondrocyte and menisco-fibrochondrocyte viability as described above.
Synovia, menisci, and bone samples were allowed to fix for at least 3 days in 10% neutral buffered formalin fixative. Medial and lateral menisci, the medial and lateral femoral condyles, and medial and lateral tibial condyles were each divided into three sections 2 to 3 mm thick, and then placed in 10% ethylenediaminetetraacetic acid decalcifying agent till softened (10 days for menisci and 4 weeks for bones). After decalcification was complete, menisci and bone sections were processed, embedded in paraffin, microtome sectioned (4-6 μm), and stained (hematoxylin and eosin [H&E] and toluidine blue). Synovial tissue was routinely processed, sectioned (4 μm), and stained (H&E). Histologic scoring of the joint tissues was performed by two board-certified veterinary pathologists, blinded to treatments, using the Osteoarthritis Research Society International histologic scoring system for canine OA for synovia, menisci, femoral condyles, and tibial plateaus, and a total score for each joint was derived by adding all category scores.36
Data were compared for statistically significant differences using one-way analysis of variance (ANOVA), one-way ANOVA on ranks, or repeated measures ANOVA based on data type and normality. Significance was set at P < .05 and displaying means with standard deviations.
3 RESULTS
All dogs completed surgeries and assessments as scheduled without complication. All MR knees showed arthroscopically confirmed extruded medial meniscus with focal areas of partial- to full-thickness cartilage loss on the medial tibial plateau and medial femoral condyle, and had radiographic signs of mild medial compartment gonarthrosis 3 months after MR.
Chondrocyte viability in the tibial OCAs was well above the minimum essential level of 70% VCD at the time of transplantation, which was maintained within transplanted tissues to the 6-month study endpoint. Menisco-fibrochondrocyte viability was significantly (P < .05) higher in MOPS-preserved meniscal allografts compared with frozen meniscal allografts at time of transplantation and at the 6-month study endpoint. (Table 1; Figure 1).
| Group | PreOp | PreOp | Endpoint | Endpoint MAT viability |
|---|---|---|---|---|
| OCA viability | MAT viability | OCA viability | ||
| Frozen | N/A | 0 ± 0a | N/A | 18.3 ± 11c |
| Meniscus | ||||
| Fresh | N/A | 88.6 ± 9b | N/A | 66.2 ± 14d |
| Meniscus | ||||
| Fresh menisco-tibial | 97.9 ± 10 | 90 ± 9b | 87.8 ± 16 | 88.3 ± 16d |
- Note: Also see Figure 1. Different superscript letters denote statistically significant differences within columns.
- Abbreviations: MAT, meniscal allograft transplantation; OCA, osteochondral allograft.
All dogs in each group were judged to have lameness, loss of function, increased effusion, and decreased range of motion when assessed 1 month after MAT. All dogs in each group showed significant (P < .05) improvements in all outcome measures over the 6-month post-MAT study period such that all regained functional use of the operated limbs by study endpoint.
No statistically significant differences in effusion or lameness scores were noted at any time point (1, 3, or 6 months postoperatively). However, function scores were higher in the frozen meniscus group compared with the fresh menisco-tibial group at 1-month post-MAT and were higher in the two meniscus-only groups compared with the fresh menisco-tibial group at 3 months post-MAT. Similarly, %-TPI was significantly (P < .05) higher in the frozen meniscus group compared with the fresh menisco-tibial group at 1-month post-MAT. No significant differences were noted among groups for function scores or % TPI were noted at study endpoint. (Table 2).
| Group | Pre | OA | 1 Mo | 3 Mo | 6 Mo | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| VAS | %TPI | VAS | %TPI | VAS | %TPI | VAS | %TPI | VAS | %TPI | |
| Frozen | 10 ± 0 | 22.8 ± 2 | 8.5 ± 0.5 | 18.5 ± 0.7 | 9a ± 0.8 | 18.8c ± 1.2 | 9.7e ± 0.6 | 21.9 ± 1.5 | 8.8 ± 0.6 | 19.8 ± 0.7 |
| Meniscus | ||||||||||
| Fresh | 8.2 ± 0.5 | 18.2 ± 1.7 | 7.1a,b ± 2.4 | 16.2c,d ± 4.8 | 9.5e ± 0.4 | 20.5 ± 2.3 | 8.6 ± 0.5 | 21.7 ± 1.4 | ||
| Meniscus | ||||||||||
| Fresh | 8 ± 0.7 | 17.6 ± 1.7 | 7b ± 1.9 | 13.8d ± 2.2 | 8.1 f ± 1.1 | 18.6 ± 2.3 | 8.9 ± 1.6 | 21.8 ± 2.7 | ||
| Menisco-tibial | ||||||||||
- Note: Different superscript letters denote statistically significant differences within columns.
- Abbreviations: MAT, meniscal allograft transplantation; MR, meniscal release; OA, osteoarthritis; VAS, visual analog scale.
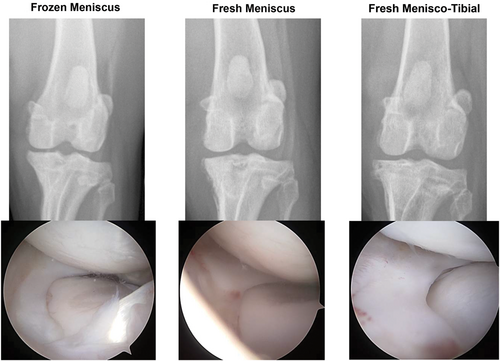
At study endpoint, knee pain was judged to be significantly (P < .05) lower in the fresh menisco-tibial group compared with the frozen meniscus group and CROM of the knee was significantly (P < .05) higher in the fresh menisco-tibial group compared with both meniscus-only groups. (Table 3) In addition, radiographic, and arthroscopic pathology scores were superior (P < .05) for the fresh menisco-tibial group compared with the two other groups (Table 4; Figure 2) at 6 months post-MAT.
| Group | Pre | OA | 1 Mo | 3 Mo | 6 Mo | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| VAS | ROM | VAS | ROM | VAS | ROM | VAS | ROM | VAS | ROM | |
| Frozen | 0 ± 0 | 108 ± 2 | 1.1 ± 0.9 | 99 ± 2.9 | 0.8 ± 0.8 | 98 ± 6.7 | 0.3 ± 0.5 | 102 ± 3.9 | 1.5a ± 0.6 | 102c ± 2.6 |
| Meniscus | ||||||||||
| Fresh | 2.3 ± 1.1 | 94 ± 5.3 | 2.5 ± 1.6 | 98 ± 8.8 | 0.4 ± 0.2 | 100 ± 7.8 | 0.8a,b ± 0.6 | 101c ± 7.3 | ||
| Meniscus | ||||||||||
| Fresh | 1.7 ± 0.9 | 91 ± 4.5 | 1.4 ± 0.5 | 99 ± 3.9 | 0.5 ± 0.5 | 101 ± 5.4 | 0.5b ± 0.5 | 107d ± 1-4 | ||
| Menisco-tibial | ||||||||||
- Note: Different superscript letters denote statistically significant differences within columns.
- Abbreviations: MAT, meniscal allograft transplantation; MR, meniscal release; OA, osteoarthritis; ROM, range of motion; VAS, visual analog scale.
| Radiographic pathology | Arthroscopic pathology | Meniscus pathology | Cartilage histopathology | Synovial histopathology | |
|---|---|---|---|---|---|
| Group | Score | Score | Score | Score | Score |
| Frozen | 8.9 ± 2a | 10.2 ± 3.1c | 12.2 ± 1.6e | 99 ± 22 g | 5 ± 1.7 |
| Meniscus | |||||
| Fresh | 8.4 ± 2a | 6.4 ± 2.5c | 7.6 ± 2.1 f | 101 ± 14g | 5.4 ± 2.7 |
| Meniscus | |||||
| Fresh | 2.3 ± 1.4b | 1.9 ± 1.9d | 7.6 ± 1.7 f | 44 ± 15h | 4.4 ± 1.5 |
| Menisco-tibial |
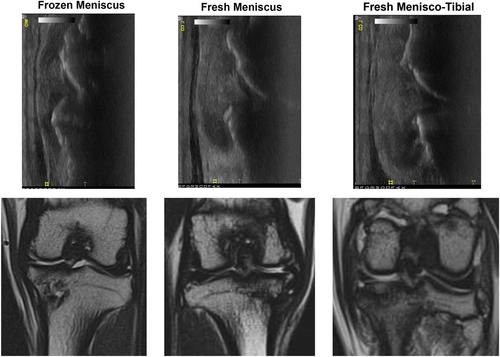
Assessments of ultrasonographic and MRI assessments at study endpoint corresponded well to each other and to the radiographic and arthroscopic imaging, showing evidence for all meniscal transplants being intact but with subjective differences among treatment groups (Figure 3). MRIs of the frozen meniscus group were consistent with relative shrinkage and subluxation/extrusion of meniscal transplants, focal articular cartilage defects of the medial tibial plateau and medial femoral condyle with associated bone marrow lesions, varying degrees of joint effusion, and mild to moderate synovitis. MRIs of the fresh meniscus group were consistent with normal-appearing size, shape, and position of meniscal transplants, focal articular cartilage defects of the medial tibial plateau and medial femoral condyle with associated bone marrow lesions, varying degrees of joint effusion, and mild to moderate synovitis. MRIs of the fresh menisco-tibial group were consistent with normal-appearing size, shape, and position of meniscal transplants, focal articular cartilage defects of the medial femoral condyle, integration, and incorporation of tibial osteochondral allografts with maintenance of articular cartilage architecture, varying degrees of joint effusion, and mild to moderate synovitis.
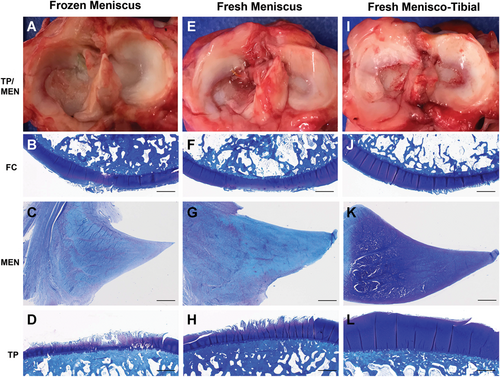
Gross and histologic assessments performed at the 6-month study endpoint (Figure 4) corresponded well with arthroscopic, ultrasonographic, and MRI. Scores revealed significant (P < .05) differences for medial meniscus histopathology severity and total articular cartilage histopathology severity, while synovial histopathology severity scores were not significantly different among treatments (Table 4). The frozen meniscus group was associated with more severe meniscal pathology compared with fresh-meniscus and fresh menisco-tibial groups based primarily on loss of cellularity with the majority of specimens being described as diffusely acellular (Figure 4). The fresh menisco-tibial group was associated with the least severe articular cartilage pathology based primarily on the absence of full-thickness cartilage loss on the medial tibial plateau (Figure 4). Synovial pathology was considered mild to moderate for all knees based on consistent findings of hypertrophy, hyperplasia, subintimal cell, and vascular ingrowth.
4 DISCUSSION
The results of the present study allow for the hypothesis to be accepted in that fresh meniscal-osteochondral allograft transplantations were associated with significantly better pain scores, CROM, and radiographic and arthroscopic measures of joint health, which corresponded well with MRI, ultrasonography, cell viability, and histological assessments when compared with fresh-viable or fresh-frozen meniscus-only allograft transplantations at the 6-month study endpoint in a preclinical canine model. The superior outcomes associated with fresh meniscal-osteochondral allograft transplantations are thought to be associated with their capabilities for addressing the major problems known to limit the efficacy of traditional MAT methods using fresh-frozen meniscal allografts. The use of fresh, MOPS-preserved grafts with high VCD appears to address the problems of graft shrinkage and failure due to tissue degeneration and/or necrosis. By transplanting menisci with a high population of viable menisco-fibrochondrocyte, the need for cellular repopulation of the allograft as required for fresh-frozen, non-viable meniscal transplants can be avoided.40, 41 This difference may allow for immediate extracellular matrix maintenance by the transplanted cells, preserving tissue integrity and material properties and mitigating shrinkage and failure. Preservation of the menisco-tibial ligament is designed to address the problem of graft extrusion. The importance of the menisco-tibial ligament has been documented and the evidence from the present study further supports preservation or reconstruction of this structure as important for meniscal biomechanics and function.25, 42, 43 The combined transplantation of meniscus with tibial osteochondral allograft allows for preservation of the menisco-tibial ligament while also addressing the tibial cartilage pathology. While MAT is contraindicated in the presence of advanced degenerative OA, focal articular cartilage defects are commonly encountered in conjunction with meniscus deficiency, especially those involving the associated tibial plateau.3, 6, 44 Fresh meniscal-osteochondral allograft transplantation with menisco-tibial ligament preservation allowed for resurfacing of affected tibial cartilage with intact hyaline cartilage. In addition, this technique addresses key sizing and fixation factors that have been reported to directly affect meniscal function, chondroprotection, and transplant survival.45-47 Finally, by replacing the damaged tibial plateau in conjunction with MAT, whole-joint pathology is more completely addressed, which in turn mitigates the progression of cartilage damage, degradation and loss, and the associated failure of MAT. Safety of fresh MOPS-preserved meniscal allografts was demonstrated in the present study based on the lack of untoward clinical, diagnostic imaging, gross and histologic findings related to inflammatory or immune responses. Lymphoid aggregates, plasma cells, or multinucleated giant cells were not features noted on histologic assessment of transplanted menisci, and synovial histopathology was considered mild to moderate for all knees based on consistent findings of synovial intimal lining cell hypertrophy, hyperplasia, and subintimal vascular ingrowth. Taken together, these novel meniscal preservation and implantation techniques appear to have the potential to safely and significantly improve MAT outcomes.
Importantly, significant improvements in lameness scoring, functional kinematics, and knee effusion after MAT were noted for all three treatment types, reflecting the reported benefits of addressing meniscus deficiency with meniscus allograft transplantation reported clinically and validating the preclinical canine model used.14 These findings accentuate the significant differences noted with regards to pain, CROM, diagnostic imaging, gross, and histologic assessments as well as cell viability results for the fresh menisco-tibial MAT treatment. Interestingly, the meniscus-only MAT groups showed early superiority to the menisco-tibial MAT group for several of the functional assessments, which is thought to be related to the less invasive nature of meniscus-only techniques. However, at the 6-month study endpoint when healing and graft integration had occurred, the menisco-tibial MAT group was associated with superior outcomes, most likely due to preserved meniscal integrity and function, treatment of tibial articular cartilage pathology, and avoidance of shrinkage, extrusion, failure, or progression of joint pathology.
The superiority of MOPS for preservation of OCAs has been previously reported.22, 31 In the present study, VCD at time of transplantation for OCAs was further verified and similarly high menisco-fibrochondrocyte viability was noted for all MOPS-preserved meniscal allografts. Importantly, cell viability was maintained in both fresh tissue types after transplantation through study endpoint. Use of OCAs with high chondrocyte viability at time of transplantation and maintenance of donor chondrocyte viability throughout the lifespan of the grafts has been associated with mitigation of complications and graft failures, as well as superior clinical outcomes.22, 26, 48 The results of the present study suggest that the associations between donor cell viability and complications and outcomes may apply to MATs as well.
The limitations of the present study must be considered when interpreting and applying the results, including the use of an animal model with a 6-month study endpoint, the evaluation of only two preservation methods, the use of only three of the numerous MAT techniques being used clinically, and the focus on the medial meniscus only. While the canine model cannot fully replicate the scenario surrounding MAT in human patients, the biology, biomechanics, and clinical aspects of canine meniscal health and disease compare favorably to those of humans.30, 42 In addition, the use of a model that included pre-existing meniscal and cartilage pathology and comprehensive and clinically relevant longitudinal outcome measures over a study period that meets the American Society for Testing and Materials and the FDA recommendations support the validity and translational applicability of the data.49, 50 While additional MAT preservation and surgical techniques could have been included, ethical and financial considerations for animal use, as well as commercial availability and clinical relevance, governed the methods chosen. Bone-bridge and all-soft-tissue MAT techniques have been employed with success, but have not been shown to be superior to bone plug techniques while having important disadvantages,8, 9, 13-18, 45, 51, 52 and to the authors’ knowledge, neither cryopreserved meniscal allografts nor those preserved using other fresh-preservation methods are validated for clinical use or are commercially available. Similarly, only medial MAT was evaluated in the present study based on ethical and financial considerations in conjunction with incidence and success rates reported for patients.8, 9, 13-16, 18, 51, 52
5 CONCLUSION
Fresh, MOPS-preserved, viable meniscal-osteochondral allografts with menisco-tibial ligament preservation were safe and effective for restoring joint health and optimizing pain levels and range of motion in this preclinical canine model. This meniscal allograft transplantation method may provide clinical advantages for patients with meniscal deficiency and associated articular cartilage pathology, warranting continued validation in clinical studies.
ACKNOWLEDGMENTS
This work was supported by The Assistant Secretary of Defense for Health Affairs endorsed by the Department of Defense, through the Peer Reviewed Orthopedic Research Program under Award No. W81XWH-18-1-0430. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
-
James L. Cook: IP royalties; paid consultant; paid presenter or speaker; research support.
-
James P. Stannard: Paid consultant; research support.
-
Cristi R. Cook: IP royalties; paid presenter or speaker; research support.
-
Aaron M. Stoker: IP royalties; other financial or material support.
-
Patrick A. Smith: IP royalties; paid consultant; paid presenter or speaker; research support.
DISCLOSURE STATEMENT
Authors James L. Cook and Aaron M. Stoker are patent holders in the Missouri Osteochondral Preservation System that is used in this study. Both authors also receive royalties from the Musculoskeletal Transplant Foundation, based on that patent.
AUTHOR CONTRIBUTIONS
All authors have read and approved the final submitted manuscript. The following is the author contribution: JLC, JPS, CRC, CCB, AJS, KK, PAS, and AMS: substantial contributions to research design, acquisition, analysis and interpretation of data; JLC, AJS, CRC, CCB: drafting the paper and revising it critically; JLC, AJS, JPS, CRC, CCB, KK, AMS, and PAS: approval of the submitted and final versions.




