Upregulation of Systemic Inflammatory Pathways Following Anterior Cruciate Ligament Injury Relates to Both Cartilage and Muscular Changes: A Pilot Study
ABSTRACT
In conjunction with cartilage breakdown, muscle maladaptation including atrophy and increased fibrosis have been observed in the quadriceps following anterior cruciate ligament (ACL) injury. Previously observed upregulated muscle-related proteins in the synovial fluid following ACL rupture allude to cellular communication between the joint and muscle. Therefore, the purpose of this study was to determine whether muscle-related analytes are differentially expressed in the serum. Sixteen patients with an acute ACL tear participated in this IRB-approved study. Serum was obtained at two different time points at a mean of 6 and 14 days post-injury, and serum was analyzed by a highly multiplexed assay of 1,300 proteins. Pathway analysis using DAVID was performed; genes included met three criteria: significant change between the two study time points using a paired t test, significant change between the two study time points using a Mann–Whitney non-parametric test, and significant Benjamini post hoc analysis. Twelve analytes significantly increased between time points. Proteins chitinase-3-like protein 1 (p = 0.01), insulin-like growth factor binding protein 1 (p = 0.01), insulin-like growth factor binding protein 5 (p = 0.02), renin (p = 0.004), and lymphotoxin alpha 1: beta 2 (p = 0.03) were significantly upregulated in serum following acute ACL injury. The current results confirm the inflammatory pattern previously seen in the synovial fluid thought to play a role in the progression of post-traumatic osteoarthritis after ACL injury, and this data also provides further insights into important communication between the joint and quadriceps group, whose function is important in long term health. © 2019 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 38:387-392, 2020
Traumatic anterior cruciate ligament (ACL) injury causes a deleterious cascade of events including a robust inflammatory response and cartilage breakdown characterized by increases in markers of cytokines and collagen catabolism that could lead to chondrocyte death.1-6 Along with increased pro-inflammatory markers and cartilage breakdown, another hallmark of ACL injury is persistent long-term quadriceps atrophy. Atrophy and weakness of this muscle group continues long after injury and even surgical intervention, causing the joint to remain unstable due to a lack of strong dynamic stabilization.7, 8
Changes in quadriceps morphology post-injury suggest that acute ACL injury affects not only the local environment within the synovial joint but the surrounding structures as well. Following injury, the quadriceps muscle shows increased fibrosis, decreased satellite cell number,9, 10 and a decreased fiber cross-sectional area, indicating atrophy. Furthermore, after ACL reconstruction, there are upregulations in common muscle growth regulators including myostatin,11 a widely studied signaling molecule of the transforming growth factor-beta superfamily that induces atrophy by activating the ubiquitin-proteasome pathway (UPP).11 What remains unknown is the effect of the inflammatory process on the quadriceps and its influence on changes in muscle. The differential muscle morphology post-injury is relevant because it lends evidence to the fact that the joint and muscle may communicate cellularly. This cross-talk between systems could be contributing to the persistent atrophy seen clinically and therefore may identify proteins vital to the interplay between the inflamed joint and quadriceps muscle.
Previously, a study looking at the proteomic response in the synovial fluid to ACL injury revealed upregulated muscle-related analytes after injury.12 Muscle-related analytes myoglobin and SMAD 2/3 are linked to muscle stress and cell growth but were an unexpected finding in the synovial fluid following acute ACL injury. Therefore, the purpose of this study was to determine whether muscle-related analytes are differentially expressed in the serum. We hypothesized that proteins related to muscle would be increased in the serum after ACL injury, providing a means of transport to the synovial fluid.
METHODS
Study Design
This study represents a secondary analysis of samples collected as part of a randomized clinical trial of patients with acute ACL rupture (clinicaltrials.gov: NCT01692756). This RCT aimed to determine the effectiveness of early anti-inflammatory treatment post ACL injury.4 Whereas the aim of this RCT was to determine the effect of intra-articular triamcinolone acetonide, none of the patients included in this subgroup analysis received a corticosteroid injection at either of the time points used in the current analyses. Subjects included in this proteomic analysis were a part of the placebo group who underwent blood collections via venipuncture, arthrocentesis and intra-articular saline injections (10 ml). Blood collection was performed on the day of initial presentation (mean = 6 days post-injury, range = 2–8 days) and again between 6 and 12 days after initial presentation (mean = 14 days post-injury, range = 8–20 days).
Patient Demographics
This study consisted of 16 patients (8 females, 8 males; mean age 18 years, range 15–26 years; mean body mass index 23, range 18–28 kg/m2) with acute ACL rupture. Inclusion criteria were as follows: isolated ACL injury determined by clinical exam (positive Lachman test with intra-articular effusion) that was corroborated using magnetic resonance imaging (MRI); age 14 years or older; skeletally mature with closed growth plates visualized by radiograph; no history of ipsilateral knee injury; ACL injury had to occur during sports activities; no clinical evidence of posterior cruciate ligament involvement; and no more than a grade 1 medial or lateral collateral ligament injury. Exclusion criteria were: injury occurrence more than 8 days before enrollment; previous knee surgery; history of intra-articular cortisone injection into either knee within three months of injury; or history of any inflammatory disorder.
ACL tear was confirmed intraoperatively at the time of reconstruction and pre-operative MRI ensured there were no concomitant ligamentous injuries. Patients were instructed to avoid any prescription or over the counter anti-inflammatory medications but were allowed to use rest and ice to manually control swelling. All patients received the same rehabilitation protocols which included home-based exercises focused on a range of motion and quadriceps muscle activation.13
Proteomic Analyses
Similar to analyses previously performed on synovial fluid samples,12 serum samples were collected for proteomic analysis with high throughput and highly multiplexed assay (SOMAscan version 3; SomaLogic, Inc., Boulder, CO). The technique uses aptamers, chemically modified oligonucleotides that recognize the three-dimensional structure of proteins with high specificity and high sensitivity. Each aptamer is tagged with a DNA sequence enabling quantification using a hybridization array. The assay converts the measurement of proteins into the measurement of the corresponding DNA. Data are recorded for each of the 1,317 proteins as relative fluorescent units (RFU). The coefficient of variation for the SOMAscan Assay is 5% (somalogic.com/somascan-assay-faqs). Proteomic analyses were performed by the Genome Technology Access Center, Washington University, St. Louis, Missouri.
Statistical Analysis
Paired t tests were used to determine differences between biomarker levels at the two preoperative time points for each analyte. Due to the small sample size, standardized response means (SRMs) were also calculated to potentially identify any trends within the data. SRM is the pre- to post change divided by the standard deviation of the change scores for that particular variable, and values of >0.8 are considered large.14, 15 In general, higher values of these proteins would reflect a state of greater inflammatory response and a potential propensity for osteoarthritic changes and progression. We also included an exploratory group of five analytes previously found to be upregulated in the synovial fluid that are related to skeletal muscle (myoglobin, SMAD 2/3, CDON, and fibronectin), three related to inflammation (interleukin 1β [IL-1β], transforming growth factor-β, and tumor necrosis factor-α) and one related to cartilage breakdown matrix metalloproteinase-1 (MMP-1).12
The Database for Annotation, Visualization and Integrated Discovery (DAVID Bioinformatics Resources, National Institute of Allergy and Infectious Diseases; NIH, Frederick, Maryland) was used to complete the pathway analysis. To be included in the post hoc pathway analysis, genes had to pass three different statistical tests. All of the genes had to have a significant change between the two study time points using a paired t test, a significant change between time points using a Mann–Whitney nonparametric test and significant Benjamini post hoc analysis. Upregulated pathways were identified using the Gene Functional Analysis Tool, with Benjamini post hoc analyses to account for multiple comparisons.16, 17 Non-parametric Spearman correlations were used to determine potential relationships between analytes that were significantly upregulated and a known marker of cartilage breakdown (MMP-1). Prior to running the correlations, a Grubbs test for outliers was used to determine the presence of any outliers within the data set. α was set at 0.05 a priori.
RESULTS
Analytes With the Largest Increase
There were 1,317 analytes measured in total.12 Of those, 12 demonstrated the largest changes in the serum within the first 2 weeks after injury (Table 1). Even with small sample size, moderate to large changes in analyte concentrations between time points (SRM ranges, −0.58 to 0.69) were observed. There were five analytes that significantly increased in concentration in the serum within the first 2 weeks after ACL injury and seven that significantly decreased (p < 0.05).
| Protein | Time 1 | Time 2 | p | SRM |
|---|---|---|---|---|
| Chitinase-3-like protein 1 | 5468.9 ± 3369.2 | 8102.8 ± 6508.8 | 0.015 | 0.69 |
| Insulin-like growth factor-binding protein 5 | 1697.6 ± 323.04 | 1992.9 ± 531.9 | 0.015 | 0.69 |
| Insulin-like growth factor-binding protein 1 | 659.6 ± 389.9 | 1070.6 ± 816.6 | 0.030 | 0.60 |
| Renin | 368.0 ± 109.6 | 435.1 ± 96.3 | 0.032 | 0.59 |
| Lymphotoxin alpha1: beta2 | 193.2 ± 52.8 | 238.3 ± 96.7 | 0.038 | 0.57 |
| Cathepsin Z | 3006.1 ± 751.1 | 2497.4 ± 778.8 | 0.034 | −0.58 |
| Fibrinogen | 32497.3 ± 12149.5 | 25956.5 ± 7428.3 | 0.024 | −0.63 |
| Carbonic anhydrase 6 | 10018.4 ± 4794.5 | 8828.9 ± 4425.8 | 0.005 | −0.63 |
| Fibrinogen gamma chain | 7449.6 ± 2530.2 | 5850.8 ± 1547.8 | 0.020 | −0.65 |
| Lipopolysaccharide-binding protein | 45316.3 ± 10568.0 | 36271.2 ± 12465.6 | 0.015 | −0.69 |
| C-reactive protein | 11677.4 ± 12578.4 | 5246.2 ± 6217.1 | 0.012 | −0.71 |
| Parathyroid hormone | 1169.3 ± 317.3 | 961.7 ± 226.7 | 0.007 | −0.79 |
- All values are expressed in relative fluorescent units (RFU; mean ± standard deviation).
A Grubbs test revealed one outlier within the MMP-1 group (p < 0.05), therefore that subject was excluded from the correlation. There was a moderate correlation (ρ = 0.64, p = 0.009) between the initial serum levels of MMP-1 and the change in insulin-like growth factor binding protein 5 (IGFBP-5; Fig. 1).
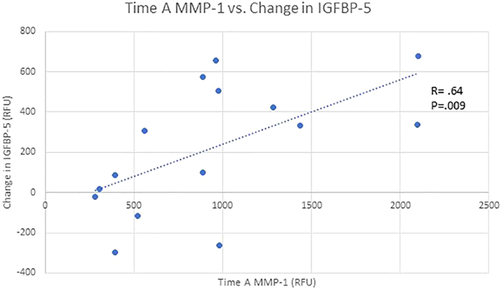
Scatter plot depicting a significant (p = 0.009) positive linear relationship between time A matrix metallopeptidase 1 (MMP-1) and the change in insulin-like growth factor binding protein 5 (IGFBP5). [Color figure can be viewed at wileyonlinelibrary.com]
Pathway Analysis
Four distinct pathways were identified using DAVID analysis. These included complement and coagulation cascades (p = 0.003, three analytes identified in this pathway), nuclear factor-κB (NF-κB) signaling pathway (p = 0.004, three analytes identified), platelet activation (p = 0.009, three analytes identified) and ghrelin (p = 0.05, two analytes identified). Analytes associated with each specific pathway can be found in Table 2.
| Pathway | Analytes |
|---|---|
| Complement and coagulation cascades | Fibrinogen alpha chain (FGA) |
| Fibrinogen beta chain (FGB) | |
| Fibrinogen gamma chain (FGG) | |
| NF-κB | Lipopolysaccharide binding protein (LBP) |
| Lymphotoxin alpha (LTA) | |
| Lymphotoxin beta (LTB) | |
| Platelet activation | Fibrinogen alpha chain (FGA) |
| Fibrinogen beta chain (FGB) | |
| Fibrinogen gamma chain (FGG) | |
| Ghrelin | Insulin-like growth factor-binding protein 1 (IGFBP1) |
| Insulin-like growth factor-binding protein 5 (IGFBP5) |
Analytes Previously Linked to Skeletal Muscle and Inflammation
There were no significant changes in any of the muscle-related analytes, inflammatory, or cartilage breakdown markers (Table 3). Of these analytes, fibronectin, myoglobin, MMP-1, and IL-1β decreased, whereas the others increased between the two study time points.
| Time 1 | Time 2 | p | SRM | |
|---|---|---|---|---|
| Muscle-related analytes | ||||
| Cell adhesion molecule-related/down-regulated by oncogenes | 5,028.82 ± 1293.72 | 5,240.1 ± 1,323.0 | 0.42 | 0.21 |
| Fibronectin | 1,1152.4 ± 4289.1 | 8,621.0 ± 5,605.3 | 0.15 | −0.38 |
| Myoglobin | 1,046.1 ± 368.8 | 889.1 ± 405.5 | 0.31 | −0.26 |
| Mothers against decapentaplegic homolog 2 | 983.3 ± 479.6 | 8,628.3 ± 2,7301.5 | 0.28 | 0.28 |
| Mothers against decapentaplegic homolog 3 | 2,997.6 ± 1625.0 | 3,258.0 ± 3,316.6 | 0.76 | 0.08 |
| Inflammatory-related analytes | ||||
| Interleukin-1β | 1,129.1 ± 571.0 | 9,189.0 ± 491.7 | 0.20 | −0.33 |
| Transforming growth factor β-1 | 1,509.0 ± 929.3 | 1,810.7 ± 1,707.0 | 0.46 | 0.19 |
| Tumor necrosis factor α | 388.9 ± 208.3 | 470.4 ± 422.4 | 0.38 | 0.23 |
| Cartilage-related analytes | ||||
| Interstitial collagenase | 937.2 ± 586.9 | 1,134.2 ± 977.2 | 0.67 | −0.11 |
- All values are expressed in relative fluorescent units (RFU; mean ± standard deviation).
DISCUSSION
The purpose of this study was to determine whether muscle-related analytes are differentially expressed in the serum.12 This would provide a potential mechanism of communication by which proteins may travel to the joint and influence the systemic environment. In a mouse model of knee osteoarthritis (OA), increases in inflammatory markers in the serum preceded increase in IGFBP-5 in the muscle and subsequent muscle wasting.18 Similarly, acute ACL injury initiates a biochemical cascade with increased pro-inflammatory cytokines and increased markers of cartilage breakdown within the joint.4 Castillero also found increase in inflammatory markers in the serum that preceded increase in IGFBP-5 in the muscle.18 The muscle-related analytes previously found in the synovial fluid12 were not significantly increased in the serum in the current study, possibly because they are downstream in the signaling pathways. However, IGFBP-5 was elevated in the serum, which may have implications for the long-term changes seen within the quadriceps muscle.
Intra-Articular Inflammation and Upregulation of IGFBP-5
IGFBP-5 may have important implications for skeletal muscle atrophy after ACL injury. Insulin growth factor (IGF) has important functions throughout the body, but most importantly, in this context, it is a potent skeletal muscle growth mediator19 and may regulate cartilage matrix synthesis.20 Insulin-like binding proteins (IGFBPs) regulate the function of IGF and IGFBP-5 mediates skeletal muscle differentiation and possibly hypertrophy.19, 21, 22 IGFBP-5 is the predominate growth factor binding protein expressed in muscle23 and can regulate IGF by binding to it with an affinity as high as binding with the IGF receptor. This then allows the binding proteins to influence IGFs,19 either by altering IGF actions21 or by switching on IGF-II.24 The regulation of IGFBP-5 is more elusive but studies point to regulation by key microRNAs25 like miR-140.26
The current study focused on the proteomic analysis after acute ACL injury, but there has been some research investigating the role of IGFBP-5 on skeletal muscle and cartilage in induced OA models. Microarray analysis in rats with monoiodoacetate induced OA showed upregulated gene clusters related to skeletal muscle development and specifically increase in IGFBP-5 in animals with cartilage damage.27 Rats with induced arthritis had significant upregulation in MuRF1, indicating muscle catabolism, in conjunction with increased levels of IGFBP-5 in the gastrocnemius muscle.28 Induced arthritis via adjuvant injection also showed significant increase in gastrocnemius IGFBP-5 two weeks after injection as well as a significant decrease in gastrocnemius muscle mass and serum IGF-1. Olney et al.20 found that chondrocytes from osteoarthritic knees had increased expression of IGFBP-5, theorizing it may play a role in cartilage breakdown. Our data support this finding and demonstrated a strong correlation between patient's initial levels of MMP-1, a cartilage breakdown marker, and changes in IGFBP-5 (Fig. 1). These positive correlations suggest a complex communication network that is triggered by intraarticular inflammation and cartilage changes, which then result in increased systemic inflammatory markers and finally changes in skeletal muscle.
IGFBP-5 and Muscle Atrophy
In animal models, elevated IGFBP-5 and inhibition of IGF has been consistently associated with atrophy. Stevenson et al.29 in 2003 found that in atrophied rat soleus, IGFBP-5 was elevated fourfold by day four of hindlimb unloading and remained elevated at 14 days. In a study of mouse muscle overload and atrophy, researchers found that the soleus muscle was atrophied by 20% after 8 days of unloading, with a twofold increase in IGFBP-5 and a decrease in IGF-1 messenger RNA (mRNA). After 8 days of overload, IGFBP-5 mRNA remained significantly lower in overloaded skeletal muscle than control muscles.30 Furthermore, overexpression of IGFBP-5 in a mouse model resulted in reduced muscle fiber hypertrophy as well as an overall lower body mass compared to control animals.22 Steves et al.31 demonstrated that the tibialis anterior muscles did not grow as expected following reloading after atrophy in IGF over-expressing mice, and reported significantly elevated IGFBP-5 levels 3 weeks after re-ambulation.
Although quantifying muscle tissue-specific IGFBP-5 levels was beyond the scope of this study, our results suggest that IGFBP-5 may be systemically upregulated in the first 2 weeks after ACL injury. Circulating levels of IGFBP-5 present a potential link between the traumatic joint injury and quadriceps atrophy. This serendipitous finding uncovers an interesting avenue for further investigation of the potential inhibition of IGF in the quadriceps by IGFBP-5 leading to the quadriceps atrophy seen clinically. There are many studies looking at the effects of IGFBP-5 in disuse atrophy and transgenic knockout,22, 24, 30, 31 but there is little evidence on its effects after traumatic joint injury. A more in-depth exploration into the communication between IGF and IGFBP-5 post-ACL injury would be necessary to define its effects on both the joint and musculature. In addition, future studies are necessary to determine if systemic changes in IGFBP-5 also result in changes to not only the quadriceps but other muscle groups as well (hamstrings, gastrocnemius, etc.).
Differences Between Serum and Synovial Fluid Proteomes
Proteomic changes in the serum did not mimic changes in synovial fluid reported previously, revealing fewer and much different analytes and upregulated pathways. Whereas this was not expected, the upregulated analytes in the serum related to either clotting or inflammation with the exception of the insulin-like growth factor-binding proteins. Pathways including complement and coagulation and platelet activation are to be expected due to hemarthrosis into the knee joint after injury.32 ACL injury creates an influx of pro-inflammatory markers, protein mediators and blood into the joint, which may perpetuate cartilage damage.33 As synovial fluid is an “ultrafiltrate” of plasma and not a static protein pool,34 contents continuously filter in and out of the joint. Under healthy conditions, there is flow of synovial fluid over the synovial interstitial space that turns over allowing in and out flux of transudate. During periods of trauma, the transudate becomes an exudate, a protein-filled fluid that resides extracellularly in the synovium.34
After ACL injury in particular, the length of time that hyaluronan is present in the joint decreases suggesting that there is a transport system for altered synovial fluid post-injury.35 Moreover, the molecular sieve theory postulates that hyaluronan chain length filters the synovial fluid only allowing molecules between 33 and 59 nm into the articular space,36 thus only allowing certain proteins to flow back and forth. Synovial fluid filtration post-injury may be a possible explanation as to why the analytes that were upregulated after injury in the synovial fluid were not the same as those upregulated in the serum. Persistent muscular dysfunction after ACL injury is undoubtedly a complex, multifactorial process but better understanding the communication between the intra- and extraarticular environments is important in identifying the underlying mechanisms and potential treatment targets.
Limitations
This pilot study of the proteomic response to ACL injury in human serum is not without limitations, particularly that an a priori power analysis was not used for sample size. This study included 16 subjects following acute, isolated ACL injury, which limits the conclusions that can be drawn and whether these results are generalizable to other populations. The progression of inflammation following ACL injury is multifaceted, and previous studies show that variables like body mass index, sex, age, and concomitant meniscus injury all effect this progression,37 and should be included in further analysis. These data provide insight into the systemic response after ACL injury and indicate that the effect in serum differs from that in synovial fluid. The present study used samples from two early time points after injury but demonstrates that proteomic analysis can be valuable in determining potential communicating factors between the intra- and extra-articular environments after ACL injury. Future studies focused on the influence of inflammatory and skeletal muscle markers on cartilage are necessary to determine the interplay between the local joint environment and surrounding structures.
CONCLUSIONS
ACL injury affects not only the knee joint but the surrounding musculature as well. The current proteomic analyses identified analytes that could have implications for inflammation, muscle atrophy, and cartilage health. The proteomic analysis of the serum provided insight into systemically upregulated analytes that could be important for communication between the inflamed joint and subsequent changes in muscle. Identifying key players in the communication between the intra- and extraarticular environments will be crucial to developing successful interventions for the prevention of muscle atrophy and cartilage breakdown. Further investigations will be necessary to elucidate the relationship of analytes like IGFBP-5, inflammation, and muscular changes after ACL injury.
AUTHORS’ CONTRIBUTION
All authors made substantial contributions to conception and design, analysis and interpretation of data, were involved in the preparation of the manuscript, have given final approval of the manuscript to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
This study received funding from the Arthritis Foundation of America. The research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Disease of the Nation Institutes of Health under Award Number 5K23AR060275. Data collection and study administration was supported by the University of Kentucky CTSA award (UL1TR000117).




