Characterization of endotoxins on orthopaedic fixation screws, using physicochemical surface analyses
ABSTRACT
The objective of this study was to determine if surface analysis techniques could be used to detect endotoxin on stainless steel malleolus screws. New malleolus screws were compared to ones that had been coated in purified lipopolysaccharide (LPS) or Artificial Test Soil (ATS) containing lipopolysaccharide. X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), and time-of flight secondary ion mass spectrometry (TOF-SIMS) were used to assess the fixation screws surface. Organic material was visualized on the LPS and ATS-LPS inoculated screws but not on the new unsoiled screws. This was further supported by the peaks observed at masses between 40 and 100 D in TOF-SIMS spectra of the LPS and ATS-LPS inoculated screws. After deconvolution of N1s high resolution XPS spectra, the LPS inoculated screws showed amide groups whereas the ATS-LPS inoculated screws showed predominantly nitroso groups (C-NO). Our data demonstrate that surface analysis can be used to detect organic residuals present on fixation screws. The XPS data confirmed that LPS reacted predominantly with positively charged surface metallic ions (Fe and Cr), whereas proteins reacted with the surface oxide layer of fixation screws, forming C-NO groups. The application of these surface analysis techniques will be helpful in determining if the reprocessing of such items results in an accumulation of organic material that might lead to aseptic loosening, when implanted. © 2016 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 35:240–247, 2017.
Orthopedic surgery utilizes implants that range from complex joints to more basic fracture fixation devices, such as plates, pins, wires and screws. Metals (Ti-6Al-4V, Co-Cr-Mo, and 316L stainless steel) are the materials most frequently used for such devices. Implantable devices, regardless of their composition or complexity, are intended to be single-patient, single-use items. Unlike implantable joints, implantable fracture fixation devices, such as plates, wires and screws, in a range of sizes, are often part of the surgical instrument tray set and, as such, get reprocessed every time the instrument tray set is reprocessed.1 Some of these items are infrequently used and this may mean they are reprocessed hundreds of times before they are implanted. As reported by Alfa,1 the manufacturer's use instructions are unclear as to whether validation has been done, regarding leaving these items within the tray set during repeated reprocessing. As reported by Chun-Chi,2 repeated steam sterilization causes damage to the surface of 316L stainless steel wires. Changes in the surface passivation layer of an implant can prevent successful osseointegration.3, 4
The occurrence of infections is one of the most common reasons for revision arthroplasty,5 and aseptic loosening accounts for almost half of first time implant failures.6 Aseptic loosening is defined as the loosening of an implant in the absence of clinical or microbiological signs of infection, and does not exclude the presence of subclinical levels of bacteria. A single Escherichia coli has more than two million lipopolysaccharide (LPS) molecules per cell.7 The published literature3, 7-16 has documented that organic residuals, such as LPS on stainless steel wires implanted into animals, will result in inflammation that can lead to aseptic loosening.
As shown in the schematic molecular structure in Figure S1, LPS consists of toxic composites of hydrophobic fatty acids and hydrophilic carbohydrate domains, released by Gram-negative bacteria. Although the Lipid A and inner core components of LPS are almost always present, the polysaccharide chain varies for different genera of Gram-negative bacteria. Once released, the Lipid A component of endotoxin elicits a dosage-dependent stimulation of various inflammatory cytokines and signal-transduction pathways, leading to an array of symptoms.12, 13 These symptoms range from a simple fever to septic shock and death. Bonsignore et al.14 reported that LPS was found in sterile craniofacial and wrist implants, provided by the manufacturer, and the amount of LPS differed not only among lots but also within the same lot of implants. Others studies15, 16 documented that LPS can be extracted (using sterile reverse osmosis water) from surgical instruments after being processed through a washer-disinfector. These authors suggested that the LPS may have come from the final rinse water, due to biofilm formation in the deionized water. This suggests that implants in surgical instrument sets that are processed through washer-disinfectors could also be inadvertently contaminated with LPS or other organic residuals. As LPS molecules are heat-stable up to a temperature of 180°C, the possibility of residual LPS (or other organic material), on implantable plates, screws and wires, is of great concern, as it may lead to aseptic loosening of the implant due to the host inflammatory response.7
Despite various in vitro and in vivo research studies, there has been little progress in determining which techniques can be used to assess the current status of organic residuals on implantable items that are found in orthopedic tray sets. A recent report17 has documented that the cleaning and sterilization of soiled titanium disks change their surface properties. Those authors used SEM, XPS, contact angle measurements and profilometry to assess the surfaces of the titanium disks. However, to the present authors' knowledge, there have been no similar studies to assess organic residuals on stainless steel fixation fragment screws.
The objective of this study was to determine if it was possible to use surface analysis techniques to identify organic residuals on stainless steel fixation screws intentionally contaminated with LPS or other organic material.
MATERIALS AND METHODS
Fixation Screws
The orthopedic screws (Synthes Canada Ltd, Mississauga, Ontario, Canada) used in the study were new 4.5 × 70 mm malleolar screws (MS), made of 316 L stainless steel. The composition of these screws was 2.8% molybdenum, 14% nickel, 18% chromium, <2% carbon, with the balance consisting of iron.
Organic Materials
The LPS used as an organic challenge was from E. coli O127:B8 and was obtained from Sigma (St. Louis, MO). It was used as a 25 µg/ml solution. The second organic challenge consisted of Artificial Test Soil (ATS) that contained 25 µg/ml LPS (AT-LPS). It is an organic test soil that has been well characterized, and has a protein level of about 157 µg/cm2 when a 10 µl drop is applied to a surface (Alfa et al.).18
Inoculation of Fixation Screws With Organic Material
All screws were immersed in either LPS or ATS-LPS, blotted on a paper towel and then allowed to dry at room temperature overnight. An identical new MS that had not been exposed to any organic material was used for comparative purposes.
METHODS
X-Ray Photoelectron Spectroscopy (XPS)
The XPS analyses were performed using a VG ESCALAB 3 MK II; Al Kα radiation (hν = 1486.6 eV, with an instrument resolution of 0.85 eV) was used, at a pressure below 1 × 10−9 torr. Screws were fixed into 1 × 2 cm sample holders. The elements detected were observed using both survey and high-resolution, spectra, with element-dependent probe depths of ∼ 4–5 nm. The XPS binding energy (BE) values were charge-corrected to that of uncharged adventitious carbon at 285.0 eV. This analysis method gives the energy distribution of electrons emitted as a result of the irradiation of the biomaterial with the incident X-rays. Their analysis gives qualitative (elements present) and quantitative (the relative concentration of each spectral peak component) information. The Avantage (ThermoScientific, Inc., Waltham, MA) software supplied with the instrument was used to analyse the XPS results, with full width at half maximum (FWHM) peak widths held constant at C = 1.6 eV, O = 1.8 eV, and N = 1.7 eV.
Scanning Electronic Microscopy (SEM)
Photomicrographs were obtained with a JEOL JSM-7600TFE microscope. The orthopedic screws were placed on an amorphous carbon ribbon and inserted into the instrument. Images were obtained at a 2 keV accelerating voltage.
Time-of-Flight Secondary Ion Mass Spectrometry (TOF-SIMS)
Positive and negative ion spectra, obtained with a TOF-SIMS (ION-TOF IV: Münster, Germany), using a 15 kV Bi+ primary ion source, were acquired at masses up to 500 D, while maintaining the primary ion dose at less than 1012 ions/cm2 to ensure static conditions. The positive ion spectra were calibrated to the H+, C+, CH+, CH2+, CH3+, C2H5+, and C3H5+ peaks, and the negative ion spectra were calibrated to the C−, C2−, CH−, C2H−, C3−, and C3H− peaks, before data analysis. Sample spectra were taken over an area 50 × 50 μm2, with an emission current of 1.0 μA in bunch mode, rastered in random mode, and presented as 128 by 128 pixels.
RESULTS
SEM
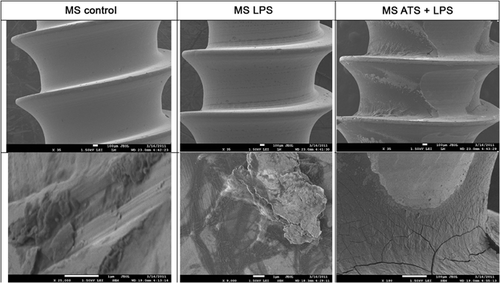
Figure 1 shows the SEM analysis of the surface of an untreated MS compared to that of one coated with LPS and one coated with ATS-LPS. The ATS-LPS-exposed MS shows the greatest amount of debris with the LPS-coated MS showing much lower levels. The unsoiled MS has some debris, which appears to be more like dust than organic residuals. Since these are new MS, this likely represents material deposited during the manufacturing process.
XPS
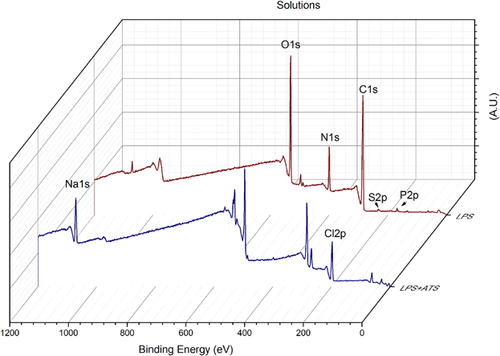
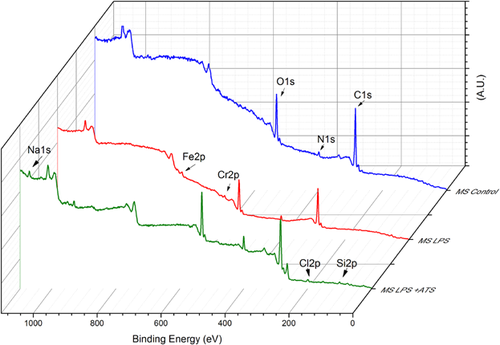
Figure 2 displays the XPS survey spectra for the two organic materials (LPS and ATS + LPS), and Figure 3 shows the spectra for the three experimental conditions. The atomic composition analysis of the organic materials and the surface of the contaminated orthopedic screws is given in Table 1. LPS has C, O, and N as major components, and traces of Na, S and P. However, ATS + LPS has a different composition: C, Cl, O, and Na being the major constituents, with N, Fe, and P less than 1%. It is important to notice that the 316 L chemical composition, mentioned previously, does not correspond with what was found by XPS, even for the MS with no soiling. Although the XPS spectrum for the LPS-exposed MS is almost identical to that with no soiling, the ATS-LPS-exposed MS shows additional elements, such as Na and Cl, as expected. It is well documented that the surface carbon (>60%) in all samples results from the deposition of atmospheric hydrocarbon. Atmospheric contamination forms a shell that is a few nanometers thick over the surface of the screw. The intensity of electrons detected from the metallic elements that are under this shell is, therefore, reduced. LPS solution seems be able to reduce the hydrocarbon atmospheric contamination shell, because this condition displays the highest concentrations of Fe and Cr from the MS alloy. The ATS-LPS provides an additional layer of material, resulting in a significant decrease in the Fe and Cr levels detected. Only ATS-LPS-exposed MS has Si, Na, Cl, and Cu on its surface. This likely represents the components of ATS, as these atomic components were not found on unsoiled MS or those having only LPS soiling.
| Organic Materials | % of Atomic Component on the Malleolar Screw Surface | ||||
|---|---|---|---|---|---|
| Atomic Component | LPS | ATS + LPS | No Soiling | LPS | ATS + LPS |
| C | 66.67 | 61.21 | 70.92 | 67.25 | 63.39 |
| O | 20.50 | 13.10 | 24.19 | 24.92 | 21.95 |
| N | 10.66 | 0.85 | 2.81 | 4.82 | 7.96 |
| Si | 0 | 0 | 0 | 0 | 2.09 |
| Na | 0.60 | 6.30 | 0 | 0 | 2.27 |
| Cl | 0 | 18.52 | 0 | 0 | 1.11 |
| Fe | 0 | 0.18 | 0.46 | 1.47 | 0.21 |
| Cr | 0 | 0 | 1.06 | 1.5 | 0.35 |
| S | 1.21 | 0 | 0 | 0 | 0 |
| P | 0.43 | 0.18 | 0 | 0 | 0 |
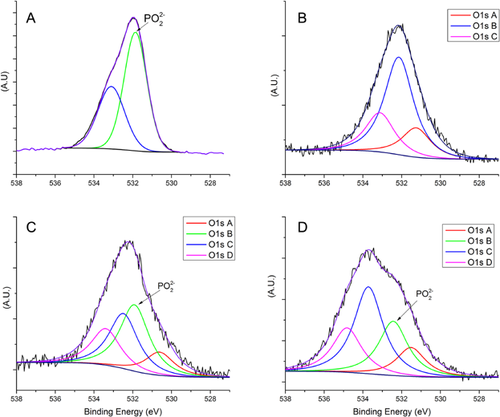
According to the molecular structure proposed by the supplier (Fig. S1), LPS contains phosphate atoms in its Lipid A component, and these should be detectable by XPS when the organic solutions are analyzed. The LPS-exposed MS should show a phosphate peak at 134 eV; however, this peak was not present in the XPS survey spectra. This may be explained by the low level of phosphate, compared to those of carbon or oxygen, or by its low sensitivity. On the other hand, the deconvolution of the O1s XPS high resolution peak, shown in Figure 4, provides evidence that supports the presence of LPS on these samples. The O1s peak in the LPS solution (Fig. 4A) has two peaks at 531.7 eV (related to OP) and 533.1 eV (related to OC) whereas, for the unsoiled control MS (Fig. 4B), three O1s chemical states were found: O1sA at 531.2 eV originated from metallic oxides, O1sB at 532.2 eV from CO and O1sC at 533.1 eV from C-OH. However, for LPS-exposed MS (Fig. 4C), four chemical states were found compared to unsoiled MS, including a new peak located at 531.7 eV, where PO2− and PO3− ions are found. The presence of these ions is confirmed by TOF-SIMS (Fig. 5). Further evidence comes from the ratio between OP and OC in LPS; it is 5:4, the same as the ratio found between peaks O1sB (10.4%) and O1sC (8.6%) in Figure 4C. Also, O-metal (i.e., OFe and OCr) bonding was predominantly observed for LPS-soiled MS, and appears to be due to the relative thinness of the adventitious hydrocarbon layer, which does not completely attenuate the photoelectrons from the underlying surface.
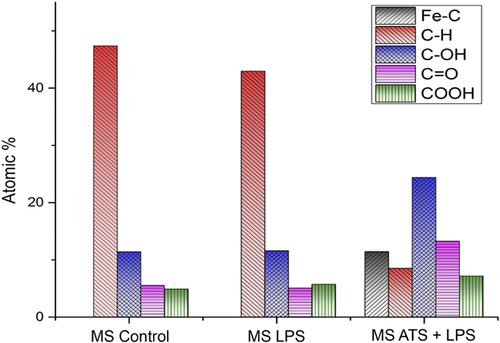
The C1s and XPS peaks were deconvoluted and the results are shown in Figure 6. For the unsoiled MS, the majority of the C1s is found to exist as CC and CH, at 285 eV, and can be attributed to the uncharged carbon shell, as mention earlier. LPS-soiled MS has a carbon composition at the surface that is very close to the unsoiled MS, whereas, for the ATS + LPS-soiled MS, the C1s was found predominantly as oxidized forms, including: COH, CO, and COOH. Figure 6 also shows an unexpected peak at 283.8 eV, attributed to carbon linked to a metallic atom, only found on the surface of ATS-LPS-soiled MS. This metallic atom may be Fe, not originating from the steel itself, but from the hemoglobin in blood or serum, both of which make up 15% of the ATS composition.
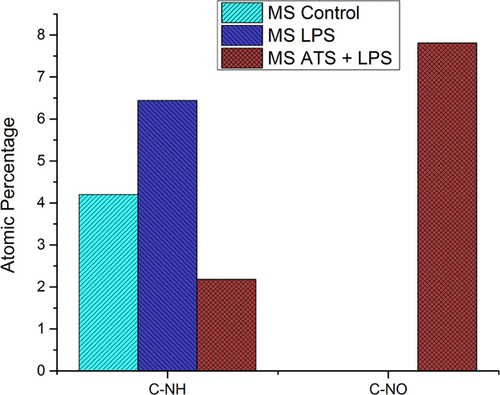
N1s peak deconvolution is showed in Figure 7. The unsoiled MS and LPS soiled MS display only amide groups in their outermost layer. Although ATS-LPS-soiled MS shows nitroso compounds (CNO-), indicating that the proteins present in ATS-LPS may have reacted with the oxide layer on the surface of the MS.
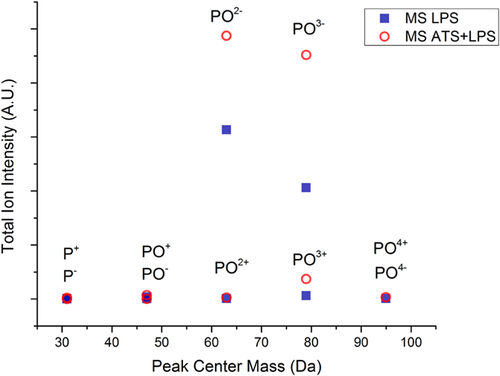
TOF-SIMS
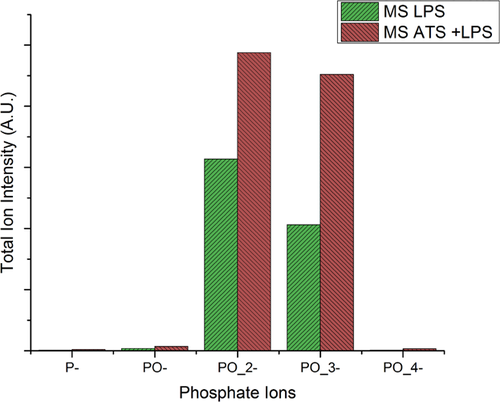
TOF-SIMS is very powerful tool to analyse the surface at depths of a nanometer at most, and was used to detect the presence of phosphorus-containing fragments originating from the LPS molecule on LPS soiled MS and ATS + LPS soiled MS. Contrary to XPS that was not able to expose a P peak, TOF-SIMS analyses shows presence of PO−, PO2−, PO3−, and PO4− fragments. Figure 8 displays the various peaks from both positive and negative SIMS. P ions are more detectable in negative spectra because of their greater ionization probability. While the peak intensity of PO2− appears to be greater than that of PO3−, this may be due to the greater ionization probability of the former, rather than its greater concentration.
DISCUSSION
The ability to detect endotoxin on orthopaedic screws is fundamental for patient safety. After all, a visibly clean stainless steel item is not necessarily clean enough for implantation. Among possible organic contaminants, endotoxin (i.e., LPS) is potentially the most likely to exist as a detrimental residue on implanted screws, plates or wires. As demonstrated in our results and those of others,4, 9, 11 LPS can adhere to stainless steel and other implantable materials.19
The US Food and Drug Administration (FDA) has elaborated protocols to test for LPS contamination of medical devices. The FDA recommends immersion of the device in LPS-free water for at least 1 h at room temperature then testing of the elution fluid for endotoxins.7, 20 The eluted level cannot exceed 0.5 EU/ml and for devices in contact with cerebrospinal fluid the limit is 0.06 EU/ml.21 The limulus amebocyte lysate (LAL) is another assay for LPS detection. This assay is based on a cell lysate of the horseshoe crab Limulus polyphenus that coagulates in the presence of very low levels of endotoxins.22 The FDA guidelines have received some criticism7, 9, 20, 23 due to the fact, for example, that it is not easy to elute LPS from the test item. One study showed that only 40–70% of inoculated LPS was recovered from devices following a very stringent extraction protocol in ethanol.24
Alternative methods have been proposed to detect LPS. One study tested the presence of LPS on sterile orthopaedic implants by direct immersion of these devises into the LAL assay.14 The results of this study showed not only previous LPS contamination in brand new sterile implants, but also that there was a 25-fold difference in LPS attachment between implants within the same lot. However, in this study, the authors used only small implants, due to the extremely costly LAL assay reagents. Lipscomb et al.24 tested 260 surgical instruments, from nine primary care units, and used 4,6-diamidino-2-phenylindole dihydrochloride to detect LPS and proteinaceous contaminations. Their results were negative for LPS, but 60% showed a high degree of protein soiling (0.4–4.2 μg protein/mm2). A recent study, using a combination of LAL and a spectrometric technique, succeeded in detecting and quantifying LPS in mouse plasma; a high performance liquid chromatograph tandem mass spectrometer (HPLC/MS/MS) was used to identify the presence of the Lipid A portion.25 For orthopaedic devices, this technique is limited to detecting only the eluted LPS.
The techniques proposed by our group, employing nanoscale surface analyses tools, such as XPS and TOF-SIMS, can be used to detect LPS residuals on orthopaedic devices, at the very least as a complementary technique. Our results were conclusive in detecting LPS surface contamination as an O1s XPS high resolution spectral peak, in conjunction with information from TOF-SIMS. The major advantages of our technique over LAL are first, that it is more sensitive, because it can detect LPS in the range of nano- (1 × 10−9) to picomoles (1 × 10−12).25 Second, no LPS elution is needed; the orthopaedic device has its surface directly analysed. Third, it can be used for small or large devices. Fourth, the speed of performing the testing and the lower cost of the analyses.26 However, it is important to keep in mind that our two techniques are interdependent: XPS is a quantitative analysis technique that probes the first 4–10 nm of the surface depth, whereas TOF-SIMS is a qualitative technique27 that probes only the first few layers of atoms. For instance, the amount of carbon, from either the LPS molecule or from the atmosphere contamination shell, is extremely high, compared to phosphorus that is present only in the inner core and Lipid A portion of the LPS molecule. Thus, the less sensitive XPS technique probes several nanometers while the more sensitive TOF-SIMS probes several atomic layers of the near surface. These differences, in sensitivity and depth probed, may explain why phosphate was not detected by XPS survey spectra but was detected by TOF-SIMS.
The immediate benefit of our results will be the possibility of using surface analyses to assess implantable items within clinical instrument tray sets after reprocessing (i.e., after patient-use, cleaning and steam sterilization). Such items within the instrument tray set are indirectly exposed to organic materials such as detergents, water-derived organisms and endotoxin during cleaning. Although they are subsequently steam sterilized, the inflammatory capability of residual LPS remains intact. This reprocessing cycle can be repeated hundreds of times and the risk of LPS contamination of surgical instruments post-cleaning remains real, as demonstrated by Alfa et al.1,28 However, to date, there have been no studies that had evaluated clinically used instrument tray sets to determine if residual LPS is found on implantable items.
CONCLUSION
In summary, our results have shown that, by deconvoluting the high resolution XPS O1s peak, it was possible to detect the presence of LPS on the surface of malleolus screws soiled with organic materials. XPS and TOF-SIMS were shown to be useful tools to evaluate the surface residuals on implantable screws. The utilization of these two techniques will lead to clinical studies to assess the risk of LPS and other organic residuals on implantable screws, plates and wires after multiple rounds of reprocessing.
AUTHORS’ CONTRIBUTIONS
R. França: Wrote the manuscript and performed the experiments. M. Alfa: Concept, experimental design, and supervised the experiments. N. Olson: Designed and performed the experiments. L'H. Yahia: Proofread and contributed substantially to the manuscript. E. Sacher: Experimental design and proofreading of the manuscript. All authors meet the criteria for authorship and all authors have read and approved the final submitted manuscript.




