Use of ultra-high molecular weight polycaprolactone scaffolds for ACL reconstruction
ABSTRACT
Previously, we reported on the implantation of electrospun polycaprolactone (PCL) grafts for use in ACL tissue engineering in a small animal model. In the present study, we hypothesized that grafts fabricated from ultra-high molecular weight polycaprolactone (UHMWPCL) would have similarly favorable biologic properties but superior mechanical properties as compared to grafts fabricated from PCL. Two forms of polycaprolactone were obtained (UHMWPCL, MW = 500 kD, and PCL, MW = 80 kD) and electrospun into scaffolds that were used to perform ACL reconstruction in 7–8 week old male Lewis rats. The following groups were examined: UHMWPCL, PCL, flexor digitorum longus (FDL) allograft, native ACL, as well as sham surgery in which the ACL was transsected. At 16 weeks post-operatively, biomechanical testing, histology, and immunohistochemistry (IHC) were performed. Analysis of cellularity indicated that there was no significant difference among the UHMWPCL, PCL, and FDL allograft groups. Quantification of birefringence from picrosirius red staining demonstrated significantly more aligned collagen fibers in the allograft than the PCL group, but no difference between the UHMWPCL and allograft groups. The peak load to failure of the UHMWPCL grafts was significantly higher than PCL, and not significantly different from FDL allograft. This in vivo study establishes the superiority of the higher molecular weight version of polycaprolactone over PCL as a scaffold material for ACL reconstruction. By 16 weeks after implantation, the UHMWPCL grafts were not significantly different from the FDL allografts in terms of cellularity, peak load to failure, stiffness, and collagen fiber alignment. © 2015 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 34:828–835, 2016.
Anterior cruciate ligament (ACL) rupture is a common surgical problem, as its intra-articular location limits the ACL's ability to heal. Current surgical strategies employ autograft or allograft tendon for ligament replacement. Though high success rates can be achieved with both of these options, serious complications can be associated with these grafts.1 The harvest of autograft tissue is associated with donor site morbidity including weakness, decreased range-of-motion, and chronic anterior knee pain. Also, autograft supply is limited, particularly in cases of re-rupture or multi-ligamentous injuries. The use of allograft tissue is limited by graft availability and is also associated with the risks of pathogen transmission and adverse inflammatory response.2 Synthetic non-degradable grafts were developed in the 1970s and 1980s but were discontinued from use in the U.S. due to problems such as premature graft rupture, foreign body reactions, osteolysis, and synovitis.3
Due to limitations of existing graft options and to recent advances in biology, engineering, and regenerative medicine, there has been increasing interest in a tissue engineered solution for ACL grafting.4 Current tissue engineering strategies employ synthetic materials that degrade in the body to allow for host tissue ingrowth while avoiding the limitations associated with implantation of non-degradable materials.4 Polycaprolactone (PCL) is a material that is currently utilized for a wide variety of medical5 and tissue engineering applications.2, 6-13 Favorable biocompatibility, relatively long in vivo half-life, degradation time, adequate mechanical strength, and high elasticity contribute to the popularity of this polymer in tissue engineering.4 Our laboratory developed a synthetic scaffold by electrospinning PCL to produce an aligned microfiber scaffold for ACL reconstruction. Previously we demonstrated that when implanted into rat knees, this scaffold was biocompatible, integrated with native tissue, and had increasing mechanical strength over time, to the range expected of autografts and allografts by 16 weeks post-operatively.14
One concern regarding the use of the PCL scaffold, however, is that the initial mechanical properties of the graft may not be sufficient. The peak load to failure of the scaffold previously studied was found to be 7.7 N, as compared to 56.7 N in the native ACL.15 While the mechanical properties by the 16 week post-operative time point improved substantially, it would be more ideal to have a stronger graft initially to potentially allow for more initial knee stability. Thus, ultra-high molecular weight polycaprolactone (UHMWPCL, molecular weight of 500 kDa) was obtained and evaluated as a potential scaffold material for ACL tissue engineering.
In this paper, we will describe the comparison of electrospun polycaprolactone grafts as previously reported with an ultra-high molecular weight polycaprolactone graft, using a rat model of ACL reconstruction. We hypothesized that grafts fabricated from UHMWPCL would have similarly favorable biologic properties but with superior mechanical properties as compared to grafts fabricated from PCL.
MATERIALS AND METHODS
Electrospinning PCL and UHMWPCL
Medical grade ester terminated poly(-caprolactone) in granule was obtained (Lactel Absorbable Polymers, Birmingham, AL) in two forms; ultra-high high molecular weight ([UHMWPCL], MW = 500 kDa) and a standard molecular weight ([PCL], MW = 80 kDa). These polymers were then dissolved 10% w/w in 1,1,1,3,3,3-hexafluoro-2-propanol (Sigma–Aldrich, St. Louis, MO). The PCL solution was electrospun around a lathe mandrel (30 mm diameter) rotating at a speed of 3450 RPM, using a 20 kV voltage source and a constant infusion rate of 2.5 ml/h for a total of 0.5 ml per scaffold. The UHMWPCL solution was electrospun around a lathe mandrel rotating at a speed of 1,725 RPM, using a 25 kV voltage source and a constant infusion rate of 0.70 ml/h for a total of 0.5 ml per scaffold. The differences in electrospinning parameters were previously optimized and were different between groups in order to account for the increased viscosity of the UHMWPCL.
In Vitro Degradation
Electrospun samples of PCL and UHMWPCL with an initial weight of 10 mg were immersed in 300 μl phosphate buffered saline at pH 7.4 and incubated at 37C for 4, 7, 14, 28, and 42 days. To measure degradation, samples were removed, rinsed twice in deionized water, dried overnight, and weighed. Three samples per group were used per time point.
Graft Design
Electrospun mats were laser cut using a VersaLaser Cutter 2.3 (Scottsdale, AZ) to dimensions of 1.5 mm × 35 mm × 150 μm with 150 μm diameter holes at 15% pore area. As previously described, holes were laser cut in the mats to increase porosity on a macroscopic level, to complement the porosity on the microscopic level, as this has been shown to promote vascularization and infiltration of cells vital to tissue regeneration.8 The scaffolds were then plasma etched (Harrick Plasma PDC-001 plasma cleaner, Ithaca, NY) to induce hydrophilicity,16 and bathed in 70% EtOH. Collagen coating using a 8:1:2.5 sterile solution of Purecol (Advanced Biomatrix, San Diego, CA), 10× PBS, and 0.1N NaOH diluted 1:9 in 1× PBS at 4°C. After 24 h of drying, four scaffold layers were stacked and affixed to one another using 5-0 vicryl sutures. This resulted in a construct that was 0.6 mm thick.
Animal Model and Operative Reconstruction
Prior to live animal surgery, ex vivo testing of the PCL and UHMWPCL scaffolds was performed in order to assess baseline graft properties at the time of implantation, n = 6.
All animal procedures were approved by the institutional animal use committee (PCC #2013-111423). Thirty-eight 7–8 week old male Lewis rats (250 ± 50 g, Charles River, Wilmington, MA) were used in the present study. The rats were housed under the care of the Veterinary Medical Unit at our institution. Rats were housed two per cage preoperatively. Postoperatively, rats were housed individually for 2 weeks and were then returned to social housing. Rats were not restricted in weight bearing or immobilized in any manner. Animals had ad libitum access to food and water and had standard bedding, daily light/dark cycles, and daily wellness checks by the veterinary staff.
ACL reconstruction was performed using the electrospun polymer graft on the left hind limb, with 10 rats in each of the following graft groups: PCL and UHMWPCL. In addition, 10 rats underwent ACL reconstruction with flexor digitorum longus (FDL) allografts (Allo). Rats were randomized into each group. Of the 10 rats in each graft group, four were sacrificed for histology and six were sacrificed for biomechanical testing at 16 weeks postoperatively. An additional four rats underwent sham surgery in which the ACL was transected but not reconstructed, and these specimens were examined via histology at 16 weeks. At the time of sacrifice, the contralateral native ACLs were used as controls for 10 rats (n = 4 for histology and n = 6 for mechanical testing). Thus, five groups were examined at 16 weeks post-operatively: (i) PCL grafts (ii) UHMWPCL grafts (iii) FDL allografts (iv) sham and (v) native ACLs (Table 1).
| Group | Intervention | N | Week 16 |
|---|---|---|---|
| I | UHMWPCL Scaffolds | 10 | Histology (4) Mechanical Testing (6) |
| II | PCL Scaffolds | 10 | Histology (4) Mechanical Testing (6) |
| III | FDL Allograft (Positive Control) | 10 | Histology (4) Mechanical Testing (6) |
| IV | Sham (Negative Control) | 4 | Histology (4) |
| V | Native ACL* | 10 | Mechanical Testing (6) Histology (4) |
- * Native ACLs were harvested from the healthy contralateral hind limbs of animals that were sacrificed following ACL reconstruction at week 16, and thus these 10 samples do not represent additional animals.
An adaptation of a previously established rat ACL reconstruction model17, 18 was performed. The procedure was performed in the morning in an animal operative suite. Anesthesia was induced by inhalation of 2% isoflurane with 2 L/min oxygen, delivered by inhalation chamber. Throughout the surgical procedure, the rats were maintained under anesthesia with 2% isoflurane in 2 L/min oxygen, delivered via flow-by with a nose cone. Rats were then injected with buprenorphine (0.03 mg/kg, Buprenex) and a single dose of ampicillin (25 mg/kg) subcutaneously. Medication doses and route of administration were those recommended by the veterinarians. Fur was clipped from the surgical site, and the area was prepped with three alternating scrubs of chlorhexidine and 70% ethanol. The rats were then placed in a supine position, on a heated pad to prevent hypothermia. The left hind limb was placed in extension, and draped in a sterile manner. A longitudinal cutaneous incision was made along the medial aspect of the lower extremity, centered at the level of the knee. The fascia was incised, and the incision was retracted laterally to expose the anterior knee. The patella, along with the attached quadriceps and patellar tendons, was dislocated laterally, exposing the femoral condyle. The anterior cruciate ligament was severed. The knee was flexed to approximately 60° and a 1.6 mm Kirchner wire was used to drill tunnels through the femoral and tibial footprints of the ACL. Both sides of the grafts were affixed to a 1.2 mm Keith needle used to pass the graft through the bone tunnels across the joint space, replacing the native ACL. With the graft under manual tension, 4-0 vicryl was used to suture the ends of the scaffold to the periosteum and soft tissue, and excess graft was trimmed from both ends. A layered closure was performed. Postoperatively, the rats were injected with 0.3 mg/kg buprenorphine every 12 h for 72 h. At the appropriate time postoperatively, rats were euthanized by carbon dioxide inhalation.
Histology/Immunohistochemistry
For histological analysis, the knee of each animal was fixed in 4% paraformaldehyde at 25°C for 48 h. Fixed knee harvests were then decalcified with Immunocal reagent. (Decal, Tallman, NY). Specimens were hand sectioned using a razor blade in the sagittal plane such that the graft was longitudinally bisected. Then, samples were submitted to a core laboratory, where the cut surface was placed flush against the paraffin mold, ensuring that the ACL replacement was properly oriented, and then sectioned. Additionally, slides were visually inspected prior to staining to ensure that the graft was clearly visible on the section. Representative sections were stained with hematoxylin & eosin (H&E) and these slides were digitized by the core facility. Four intra-articular images at 20× magnification, with an n = 3 animals per group, were analyzed. These images were used for manual quantification of cells per high powered field (hpf).
IHC
Colorimetric staining of expression of CD68 and type I collagen was performed on paraffin-embedded sections de-waxed with xylene washes and rehydrated. Ficin (Sigma–Aldrich) was applied for 20 min at room temperature for antigen retrieval. Sections were permeabilized in a 0.025% Triton X wash (Sigma) and were then blocked at 25°C for 1 h in a solution of 4% normal goat serum and 0.4% bovine serum albumin in tris-buffer saline/Tween20. Sections were incubated overnight at 4°C with primary antibody solutions of (i) 1:200 dilution of rabbit anti-rat type I collagen (Millipore, Billerica, MA), or (ii) 1:300 dilution of rabbit anti-rat CD68 (Abcam, Cambridge, MA), followed by 30 min incubation at 25°C with biotinylated goat anti-rabbit IgG secondary antibody and conjugation of peroxidase substrate (VECTASTAIN Elite ABC Kit, Vector Laboratories, Burlingame, CA) for 30 min at 25°C. DAB peroxidase substrate (Vector Laboratories, Burlingame, CA) was then added until desired stain intensity was visible under light microscopy.
Picrosirius Red Staining and Quantification
Samples were sectioned and mounted by a core laboratory as described above. Paraffin embedded slides were de-waxed with xylene, followed by rehydration. Slides were stained for fibrillar types I and III collagen using Picrosirius Red Stain Kit (Polysciences Inc., Warrington, PA). Images were taken under polarized light with a DMLB Leica Microscope (McBain Systems, Chatsworth, CA). Image analysis was performed to quantify the area fraction of birifrengence. Four intra-articular images at 20× magnification were analyzed per animal, with an n = 3 animals per group. The percent area of birefringent pixels was determined by converting images to black and white, where the threshold was set to convert all birefringent pixels to black and all non-birefringent pixels to white. The area fraction of black pixels was determined using ImageJ software (National Institutes of Health, Bethesda, MD) and reported as mean ± standard error per group.
Biomechanical Testing
For surgical samples, all soft tissue except the graft was removed from the harvested knees. The femur and tibia were secured with wires and potted in polymethylmethacrylate (PMMA) and tested with the knee in 20° flexion. The graft was kept moist during dissection and potting by regular and frequent spraying of normal saline. The femur-graft-tibia complex was mounted onto an Instron tensile tester (Model 5564, Norwood, MA) with a 1 kN load cell. The graft was pre-tensioned to 2 N and then tested to failure at a strain rate of 0.5 mm/s. The data was used to generate load-displacement curves. The peak and slope of the linear portion of the curve were used to determine failure load and stiffness, respectively, for the four groups: (i) PCL grafts at 16 weeks postop, (ii) UHMWPCL grafts at 16 weeks postop, (iii) FDL allografts at 16 weeks postop, and (iv) native ACLs, (n = 6).
Statistical Analysis
Mechanical testing values are reported as mean ± standard error of the mean. Two-way ANOVA with Bonferroni correction was used to assess differences between groups. Differences between time points were verified using unpaired t-tests. Statistical significance was achieved if p < 0.05.
RESULTS
Degradation
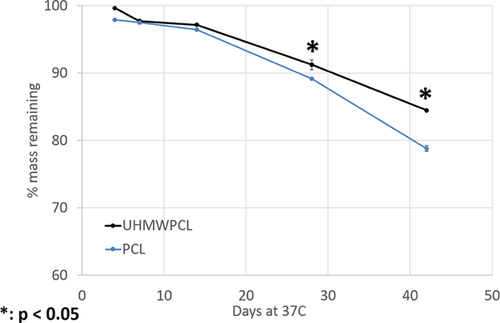
Both the PCL and UHMWPCL scaffolds degraded over time in vitro. The PCL degraded at a significantly faster rate than the UHMWPCL. At 42 days, PCL scaffolds had lost 21.2% of their original mass as compared to 15.5% in the UHMWPCL scaffolds (Fig. 1).
Histology
Hematoxylin and eosin staining demonstrated bony integration at both the femoral and tibial ends of the graft. The grafts were infiltrated with abundant cells, and the neo-ligament appeared to be well aligned (Fig. 2). There was no remaining polymer observed, indicating that the scaffolds had been fully resorbed by 16 weeks. On H&E staining, no degradation products were observed in the neighboring tissues. As the main degradation product of polycaprolactone is lactic acid, we would not expect to see any degradation products on histology. For comparison, an image of H&E stained scaffold prior to implantation is available (Supplementary Figure SA). Image analysis of cellularity demonstrated that there was no significant difference in cells in the UHMWPCL grafts (716 ± 51 cells/hpf), PCL grafts (723 ± 61 cells/hpf), and allografts (611 ± 20 cells/hpf). In comparison, native ACL had significantly lower cellularity than all the grafts at 300 ± 12 cells/hpf.
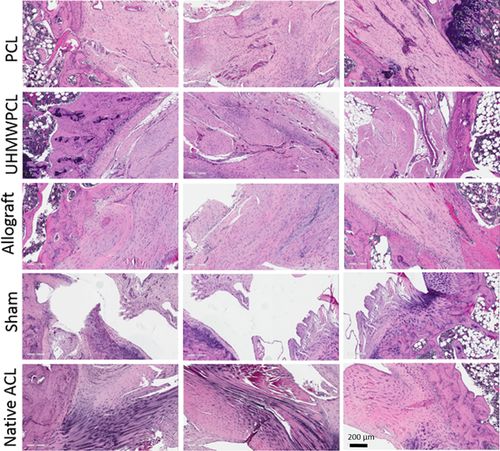
Sham surgery was performed to control for the potential healing effects of performing the arthrotomy and drilling the bone tunnels in the process of ACL healing. In the sham group, the severed ACL did not heal (Fig. 2).
Immunohistochemistry for CD68 (or ED1), a glycoprotein specifically expressed on cells of the macrophage/monocyte lineage, demonstrated minimal staining across all samples, without grossly observable differences among the graft groups (data not shown).
Collagen Deposition
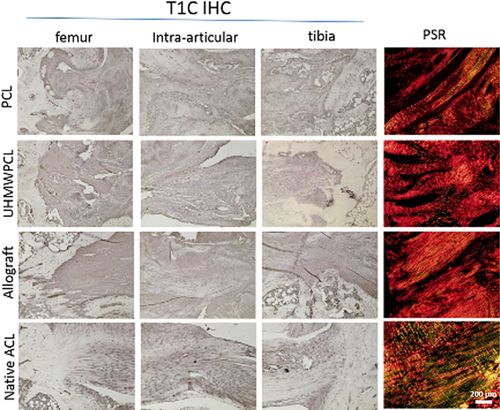
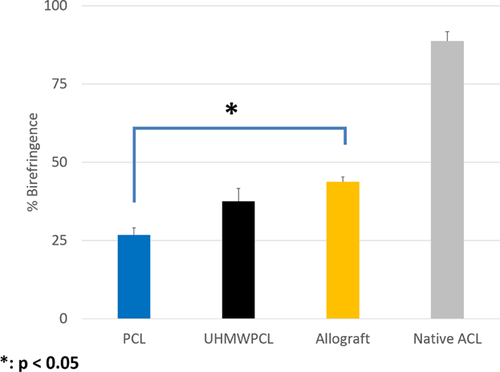
Immunohistochemistry for Type I Collagen demonstrated positive staining across all graft groups (Fig. 3), without grossly observable differences among these groups. To further examine collagen deposition on the tissue-engineered grafts, picrosirius red staining was performed, and the percent area birefringence was calculated to quantify the amount of aligned collagen observed. Under polarized light, positive birefringent staining was observed in all graft groups, signifying the presence of aligned collagen fibers. Qualitatively, there was more staining in the native ACL than in the graft groups. Using image analysis, the percent area of intra-articular sections that were birefringent was quantified (Fig. 4). At week 16, it was found that the allograft group (43.7 ± 1.6% birefringence) demonstrated significantly more birefringence than the PCL group (26.7 ± 2.3%), but there was no significant difference between the allograft and the UHMWPCL graft (37.5 ± 4.1%). In comparison, the native ACL demonstrated 88.7 ± 2.6% birefringence.
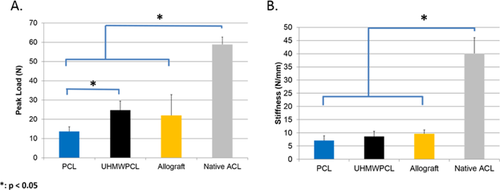
Biomechanical Analysis of PCL Grafts
Biomechanical properties of the graft or native ACL were assessed in all groups other than sham. All tested samples failed at the mid-substance. Using load-displacement curves generated from tensile testing, failure load and stiffness were computed for each group (Fig. 5). At the time of implantation, the UHMWPCL grafts had 2.4 times the peak load to failure of the PCL grafts. At 16 weeks post-implantation, the UHMWPCL group had significantly higher peak load (24.6 ± 4.7 N) as compared to the PCL group (13.6 ± 2.4 N). There was no statistically significant difference between the FDL allograft (22.0 ± 20.7 N) and either the UHMWPCL or PCL groups in terms of peak load. However, the native ACL was found to have a peak load of 58.8 ± 3.8 N, which was significantly higher than all three graft groups. In terms of stiffness, there was no significant difference at 16 weeks among the UHMWPCL graft (8.6 ± 2.0 N/mm), PCL graft (7.1 ± 1.8 N/mm), and FDL allografts (9.6 ± 1.4 N/mm), although they were all less stiff than the native ACLs (40.1 ± 6.0 N/mm). Therefore, by 16 weeks postoperatively, UMHWPCL grafts achieved 41.9% of the peak load and 21.3% of the stiffness of healthy native ACL healthy native ACL.
DISCUSSION
In this study, we compared the in vivo efficacy of electrospun UHMWPCL grafts to lower molecular weight PCL grafts and FDL allograft in a rat model of ACL reconstruction. The FDL allograft is currently the most well-described and well-established model of ACL reconstruction in rats.17, 18 The rat model used in this study represents a modification of a model for autologous ACL reconstruction first described by Rodeo and colleagues.17, 18 Overall, our UHMWPCL grafts achieved 41.9% of the peak load of a healthy native ACL at 16 weeks post-implantation. This is very promising as it is comparable to the mechanical properties of allograft ACL reconstruction. In comparison to other tissue engineering attempts, cell-seeded collagen constructs implanted in a rabbit completely resorbed by 8 weeks, preventing mechanical testing,19 a decellularized semitendinosus tendon graft that was repopulated with fibroblasts demonstrated 32.2% of the peak load of native ACL at 8 weeks postoperatively,20 and a silk-based scaffold seeded with MSCs resulted in a peak load that was 18.7% that of native ACL at 24 weeks postoperatively.21 Using a stacked bone and ligament monolayer construct, one group demonstrated 52% of the tangent modulus and 95% of the geometric stiffness of native ACL in an ovine model at 6 months.17 Specific to rat models of ACL reconstruction, we reported a peak load of 13.3 N and stiffness of 16.0 N/mm for collagen coated PCL grafts 12 weeks after implantation in Sprague–Dawley rats.22 Packer et al. reported a peak load of approximately 7 N and a stiffness of approximately 3.5 N/mm 2 weeks after undergoing ACL reconstruction with FDL autograft.23 Fu et al. found that Sprague–Dawley rats reconstructed with FDL autograft had approximately 25–30% the stiffness and peak load of native ACL after 6 weeks.24 Based on available data from medial collateral ligament and rotator cuff healing in rats,25-29 scar tissue is organized by 4–12 weeks post-injury. Thus, at 16 weeks, we would fully expect collagen to be organized, as was observed in this study. While we know that the UHMWPCL grafts had an increase in mechanical properties between the time of implantation and 16 weeks postoperatively, no intermediate time points were examined in an effort to be judicious about animal use.
A primary advantage of the scaffolds described in this study over early engineering attempts with materials such as Gore-Tex® and Dacron® is that they are biodegradable. Thus, with easily resorbable breakdown products such as lactic acid, there would not be any of the residual particles that resulted in the multitude of chronic complications observed with the non-degradable synthetic materials such as foreign body reactions or synovitis. While many polymers have the potential for use in ligament reconstruction, we chose PCL because it is biologically inert, non-toxic, degrades slowly in vivo, and is easily manufactured into a desired conformation.4 PCL is also mechanically robust and shows little plastic deformation under mechanical stress.30 Its use has been established in the bone tissue engineering literature as a reliable reservoir for mineralization and type I collagen deposition due to its aligned nanofiber structure when electrospun.31 Also, it has been shown that the high surface to volume and short diffusion length scale of the small diameter fibers in electrospun PCL mats are favorable for controlled drug delivery and used in tissue engineering.32
In this study, we examined two PCL grafts with different molecular weights, UHMWPCL (500 kDa) versus PCL (80 kDa), for ACL reconstruction. As expected, the electrospun UHMWPCL scaffolds degraded at a slower rate in vitro as compared to the PCL scaffolds. In vivo, the degradation rate of both scaffolds would likely be more rapid due to the presence of cells and enzymes that could help accelerate the degradation process. By 16 post-implantation, both scaffolds had been resorbed and replaced by neo-ligament with minimal inflammatory response. Histologically, both of the electrospun polymer scaffolds facilitated cell infiltration and collagen alignment in the regenerated ACL. In addition, both grafts showed successful bony integration. It does not appear that UHMWPCL was in any way inferior to the PCL scaffolds in terms of biological suitability for ACL tissue engineering. There was no difference in cellularity of the two graft types after 16 weeks.
The implantation of both PCL and UHMWPCL grafts resulted in the formation of neo-ligament with type I collagen elaboration. UHMWPCL had more aligned fibers than PCL grafts, and unlike PCL grafts, they were similar to allografts in this metric. It should also be noted that previously, the birefringence of collagen-coated PCL grafts stained with picrosirius red were quantified prior to implantation and found to be 0.7%.15 This ensured that the increased birefringence of 37.5 in the UHMWPCL group and 26.7 in the PCL group was due to elaborated collagen in the neo-ligament rather than to the very thin layer of collagen coating which was applied to the scaffolds prior to implantation.
As expected, the UHMWPCL grafts had a significantly higher peak load to failure than the PCL grafts at the time of implantation. Interestingly, by 16 weeks post-operatively, when all the polymer in both groups had already degraded and been replaced by neo-ligament, the UHMWPCL grafts still had significantly higher peak load to failure than the PCL grafts. A possible explanation for this is that the UHMWPCL polymer degrades more slowly, allowing the aligned electrospun fibers to guide the newly elaborated collagen fibers longer, resulting in improved collagen fiber alignment at 16 weeks. The better collagen fiber alignment of the UHMWPCL grafts would explain its increased peak load to failure as compared to the PCL grafts. The superior peak load to failure may also be attributed to improved neo-ligament formation in the presence of an initially more stable knee. It should be noted that caution should be applied in comparing mechanical testing values of the PCL grafts in the present study and our previous study15 because of the use of different rat species. Because the present study did not employ the implantation of xenogeneic cells, the decision was made to use immunocompetent rats, providing the benefit of allowing us to observe any inflammatory response to the UHMWPCL. Likewise, differences in animal species must be recognized when comparing the values found in this study to previously published reports. Due to obvious challenges in obtaining human reconstructed ACL specimens, there is insufficient data on strength of human allografts over time. Based on animal models and knowledge of scar healing, it is generally accepted that allografts are weaker than native ACL after reconstruction.4 Hence, the allograft control group is valuable in allowing us to assess the efficacy of the UHMWPCL graft. We demonstrated in this study that 16 weeks after implantation, the UHMWPCL grafts were not significantly different from the FDL allografts in terms of cellularity, peak load to failure, stiffness, and collagen fiber alignment. Continued work may be warranted to further improve mechanical properties of the engineered ACL replacement. An area of possible future investigation would be to use a composite material or to alter graft geometry in an attempt to improve mechanical properties.
CONCLUSIONS
This study demonstrates the potential of a tissue engineered approach to treatment of ACL rupture. By 16 weeks after implantation, UHMWPCL grafts were not significantly different than FDL allografts in terms of cellularity, peak load to failure, stiffness, and collagen fiber alignment. This in vivo study establishes the superiority of the higher molecular weight version of polycaprolactone over PCL as a scaffold material for ACL reconstruction.
AUTHORS' CONTRIBUTIONS
NL designed experiment, performed animal surgeries, and drafted manuscript. NK fabricated scaffolds, performed mechanical testing, and analyzed mechanical testing data. AA performed mechanical testing and edited manuscript. AN performed histological and immunohistochemical staining and data analysis. JJ helped with study design, scaffold staining, and manuscript preparation. FP, BW, and DM helped with study design and manuscript preparation. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This project was funded by the OREF Clinician Scientist Training Grant (NL), H H Lee Surgical Research Grant (NL), Veteran's Administration BLR&D Merit Review 1 I01 BX00012601 (DRM) and Musculoskeletal Transplantation Foundation Young Investigator Award (FP).




