Optical spectroscopic determination of human meniscus composition
ABSTRACT
This study investigates the correlation between the composition of human meniscus and its absorption spectrum in the visible (VIS) and near infrared (NIR) spectral range. Meniscus samples (n = 24) were obtained from nonarthritic knees of human cadavers with no history of joint diseases. Specimens (n = 72) were obtained from three distinct sections of the meniscus, namely; anterior, center, posterior. Absorption spectra were acquired from each specimen in the VIS and NIR spectral range (400–1,100 nm). Following spectroscopic probing, the specimens were subjected to biochemical analyses to determine the matrix composition, that is water, hydroxyproline, and uronic acid contents. Multivariate analytical techniques, including principal component analysis (PCA) and partial least squares (PLS) regression, were then used to investigate the correlation between the matrix composition and it spectral response. Our results indicate that the optical absorption of meniscus matrix is related to its composition, and this relationship is optimal in the NIR spectral range (750–1,100 nm). High correlations (R2uronic = 86.9%, R2water = 83.8%, R2hydroxyproline = 81.7%, p < 0.0001) were obtained between the spectral predicted and measured meniscus composition, thus suggesting that spectral data in the NIR range can be utilized for estimating the matrix composition of human meniscus. In conclusion, optical spectroscopy, particularly in the NIR spectral range, is a potential method for evaluating the composition of human meniscus. This presents a promising technique for rapid and nondestructive evaluation of meniscus integrity in real-time during arthroscopic surgery. © 2015 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 34:270–278, 2016.
The menisci are C-shaped complex cartilaginous tissues positioned between the tibia and femur in the medial and lateral compartments. They function as secondary stabilizers1, 2 in the knee joint and provide load distribution,3 and they have been postulated to facilitate joint lubrication during movement,4 nutrition of the articular cartilage5 and proprioception.6 Meniscus is highly hydrated (water: 72%), with the remaining 28% comprised of organic matter, mostly extracellular matrix (ECM), and fibrochondrocytes.7, 8 In general, collagens make up majority (75%) of this organic matter, followed by proteoglycans (PGs) (17%), DNA (2%), adhesion glycoproteins (<1%), and elastin (<1%).8, 9 Structurally, the menisci are primarily composed of an interlacing network of collagen fibers (predominantly type I collagen) interposed with cells, an ECM of PGs, and glycoproteins.10 The composition and structure may vary depending on age, injuries, and other pathological conditions.11, 12 Meniscus consists of three different regions: The vascularized outer periphery “red-red zone”, the transitional region which has properties from both adjacent regions “red-white zone”, and the avascular inner region, often termed the “white-white zone”.13, 14 The fibrocartilaginous red–red zone, consists mainly of collagen type I, at approximately 80% composition by dry weight. In the hyaline-like white–white zone, collagen makes up 70% of the tissue by dry weight, of which 60% is collagen type II, and 40% is collagen type I.15
Meniscal injuries often occur from age-dependent degenerative changes in the tissue composition or mechanical trauma. Degenerative changes in meniscus composition can affect functional competence of the tissue and lead to more fragile tissue that is liable to tears, either spontaneously, or with minimal trauma. Degenerative tears have been suggested to account for about 50% of meniscal tears16 and are described as “lesions of middle age”, and this pattern of tear was most frequently seen in the posterior horn of the medial meniscus.17 These injuries tend to impair the load bearing capacity of meniscus and decreases the biomechanical integrity of the tissue, resulting in overloading of cartilage, and ultimately to osteoarthritis (OA).18-20 Hence, early detection of changes in meniscal composition is essential for prevention, or management of meniscal pathologies and control of OA.
Like most biological tissues, and connective tissues such as articular cartilage, the inherent structure-function coupling, that is the relationship between the tissue structure (constituents) and function, suggests that alteration of the tissue composition will also alter its function. During degeneration, the water content of human meniscus has been shown to increase consistently (from about 70 to 85% of wet weight) with the level of degeneration. The matrix collagen and total PG contents were also observed to decrease with the level of degeneration. These observed alterations in meniscus water, collagen, and PG contents at various stages of degeneration were not due to age-dependent changes.8 Thus, monitoring the changes in meniscus composition can be used as a marker to track the tissue's health and degenerative state.
Clinical diagnosis and evaluation of joint defects, including meniscal injuries, and degeneration is mostly restricted to magnetic resonance imaging (MRI), X-ray imaging, and visual evaluation via conventional arthroscopy.21 Although current diagnostic imaging techniques are noninvasive in nature, some drawbacks limit their application for accurate diagnosis of meniscal injuries, and degeneration. X-ray imaging is often used in diagnostics of joint problems, however the contrast between soft tissues is poor in radiographic images, and hence it cannot detect changes in the internal structure of meniscus.21 Current clinical MRI lacks the resolution to evaluate meniscal degeneration or provide information on the tissue composition, and its availability poses further limitations. While visual evaluation via conventional arthroscopy is the most commonly used technique for diagnosis, and repair of meniscal pathologies, it is limited to the evaluation of meniscus superficial structures, and the outcomes tend to differ between the observers.22 More so, arthroscopy has been reported to suffer from large variation in inter-observer reliability, and depends on the grading system, and surgeon experience.23, 24 Due to these limitations of existing diagnostic methodologies, there is a need for techniques capable of rapid, and nondestructive assessment of meniscus integrity.
In recent years, optics, and spectroscopy-based techniques have emerged as promising modalities for characterizing connective tissues, particularly articular cartilage. One of these prominent techniques is near infrared spectroscopy (NIR). When light interacts with meniscus, as with other biological tissues, two main phenomena are observed: scattering and absorption, and these are related to the composition of the tissue. Therefore, the visible (VIS), and NIR spectra gives an indication of the tissue's composition and structure. Thus, characterization of the optical properties of the tissue can be used to evaluate its health and integrity. The capacity of light in the visible region to evaluate biological tissues, such as musculoskeletal tissues,25, 26 skin,27-29 and blood,30, 31 has been studied. Our choice of including the VIS region in our investigation was motivated by recent studies by Johansson et al.,25 who suggested that light in the VIS region can be used to predict tissue thickness. More so, the studies of Kinnunen et al.26 showed that this region is capable of distinguishing between normal and degenerated articular cartilage.
In earlier studies, the capacity of spectroscopy-based method to characterize the integrity and health of articular cartilage has been demonstrated.32-40 Adult human knee menisci possess proteoglycan macromolecules of similar size and glycosaminoglycan (GAG) content to those present in articular cartilage, although at lower tissue concentrations.8 Meniscus collagen is predominantly type I, which has chemical, and structural similarities to type II that is abundant in articular cartilage. Therefore, the fundamental molecular structure of meniscus is similar to that of articular cartilage, which has been characterized using spectroscopic techniques in the previous studies referenced above. Thus, we hypothesized that the optical spectral response of meniscus can be used to determine its matrix composition. To test this hypothesis, we investigated the relationship between the tissue's spectral data and its composition using principal component analysis (PCA) and partial least squares (PLS) regression multivariate statistical techniques.
METHODOLOGY
Sample Preparation
Meniscus samples were obtained from nonarthritic knees of human cadavers with no history of joint diseases (n = 24, 11 males, 1 female; 24–80 years old, mean age = 51.9). The human cadaver knees were obtained from Jyväskylä Central Hospital, Jyväskylä, Finland under ethical approval by the National Authority for Medicolegal Affairs, Helsinki, Finland (Permission 1781/32/200/01). After extraction, the samples were carefully wrapped in towels soaked in phosphate-buffered saline (PBS; Euroclone, Paignton-Devon, UK) containing inhibitors of proteolytic enzymes (ethylenediaminetetraacetic acid dehydrate [EDTA]; Merck, Darmstadt, Germany) and benzamidine HCl (Sigma, St. Louis, MO), and sealed in zip-lock bags prior to storage at −20°C to minimize freeze drying, and thus keeping the meniscus water content relatively stable. Prior to experiments, the samples were thawed and immersed in PBS supplemented with inhibitors of proteolytic enzymes and meniscus specimens (n = 72) were prepared from the samples. The specimens were extracted at predefined locations on the meniscus samples (Fig. 1a).
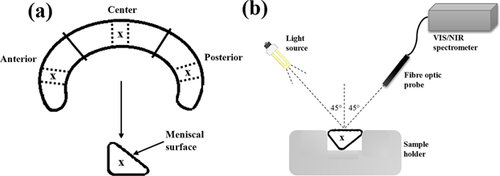
Optical Spectroscopy of Meniscus Samples
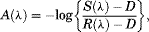 (1)
(1)Spectral Pre-Processing and Multivariate Analyses
Since spectral data are highly multi-collinear, de-correlation, and feature extraction techniques are often required to analyze these multi-dimensional data. PCA, a multivariate feature extraction technique, was used to investigate and understand the underlying structure in the spectral data. PCA constructs a combination of the original variables (spectral data) in a new feature plane (principal components) to create a new set of variables that maximizes the variance in the original data.
 (2)
(2)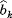 = regression coefficient, k = 1, 2, …, K (number of predictor data points, K = 1234 when the full spectral data is used as predictor variable). PLS regression is of particular interest because, unlike multiple linear regression (MLR), it can analyze data with strongly collinear (correlated), noisy, and numerous variables (e.g., spectroscopic data), and also simultaneously model several response variables, that is properties/parameters of the samples.43 Optimal use of this technique requires correct selection of the amount of latent variables (number of components) to include during predictive model development, too much components would lead to over-fitting and too little to under-fitting.
= regression coefficient, k = 1, 2, …, K (number of predictor data points, K = 1234 when the full spectral data is used as predictor variable). PLS regression is of particular interest because, unlike multiple linear regression (MLR), it can analyze data with strongly collinear (correlated), noisy, and numerous variables (e.g., spectroscopic data), and also simultaneously model several response variables, that is properties/parameters of the samples.43 Optimal use of this technique requires correct selection of the amount of latent variables (number of components) to include during predictive model development, too much components would lead to over-fitting and too little to under-fitting.To optimize multivariate correlation between the predictor and response variables, spectral nonlinearities, and baseline offsets44 were corrected using pre-processing methods prior to analyses. A third order Savitzky–Golay moving average filter was used for spectral smoothing, then derivative pre-treatment was used for baseline correction and spectral transformation. Multivariate analyses was then performed separately with data from the VIS range (400–750 nm), NIR range (750–1100 nm), and total spectrum (400–1100 nm). Since light in the NIR range penetrates deeper into biological tissues, this spectral range was further sub-divided into three distinct regions (Table 1) and used for analyses in order to investigate whether or not there is a relationship between specific regions of the NIR spectrum and the different constituents of meniscus composition. This is based on an approach adopted in an earlier study.33
| Uronic acid | Hydroxyproline | Water | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wavelength region (nm) | R2(%) | RMSECV | Error (%) | R2(%) | RMSECV | Error (%) | R2(%) | RMSECV | Error (%) |
| Combined (400–1100) | 56.8 | 0.64 | 23.2 | 59.2 | 4.61 | 21.5 | 43.6 | 3.76 | 18.3 |
| Visible (400–750) | 48.3 | 0.61 | 21.9 | 52.3 | 4.77 | 21.2 | 35.8 | 3.48 | 16.9 |
| Near infrared (750–1100) | 86.9 | 0.58 | 19.5 | 81.7 | 5.1 | 23.8 | 83.8 | 4.11 | 19.9 |
| NIR1 (750–850) | 70.4 | 0.7 | 27.3 | 65 | 5.85 | 27.3 | 62.4 | 4.16 | 20.2 |
| NIR2 (850–950) | 46.4 | 0.77 | 27.9 | 63.8 | 5.23 | 24.4 | 54.7 | 4.01 | 19.5 |
| NIR3 (950–1100) | 80.2 | 0.55 | 20.0 | 69.1 | 5.41 | 25.2 | 65.9 | 4.06 | 19.7 |
- Spectral Pre-Processing (Smoothing + 2nd Derivative) was Performed Prior to Analyses and 5 PLS Components Were Used for all Analyses [RMSECV: Root Mean Square of Cross Validation].
PCA was performed on the smoothed and derivatized (1st) spectra, and PCA scores, and loadings were obtained for inspection. PCA scores describe the new variables, obtained as a combination of the original spectral data in a new feature plane (the principal components), while the loadings define the weight of the new variables in the principal component axes. These often have relationship with properties of the system under investigation (human meniscus in this case). For multivariate prediction analysis, single y-variable PLS algorithm (PLS1) was used to relate the spectral data with the composition of the tissues. (Table 1).
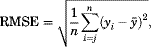 (3)
(3)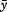 = mean of measured responses. Spectral pre-processing and PLS analyses were performed using custom-written program in MATLAB (ver. R2014a, Mathworks, Natick, MA).
= mean of measured responses. Spectral pre-processing and PLS analyses were performed using custom-written program in MATLAB (ver. R2014a, Mathworks, Natick, MA).Reference Measurements
For analyses of meniscus composition, three sections (thickness 1 mm) were cut laterally through the full thickness of each sample, one per measurement location. The meniscus tissue wet weight was measured. Subsequently, the specimens were freeze-dried to determine the dry weight of the tissue, and the water content was calculated from this information.45 Following water content estimation, hydroxyproline, and uronic acid contents, which correspond to the tissue collagen and PG contents, respectively, were then determined biochemically. This was undertaken by digesting the sections in papain (1 mg/ml) and 150 mM sodium acetate including 50 mM Cys–HCl and 5 mM EDTA at 60°C and pH of 6.5 for 3 h to digest the PGs. The papain digested sections were freeze dried and hydrolyzed, and the hydroxyproline content was determined spectrophotometrically.46 The uronic acid content was quantified from the ethanol-precipitated residue of the digested sections. For each sample, the uronic acid and hydroxyproline contents were determined three times and averaged. The contents were then normalized to the wet weight of the section to compensate for the variation in section sizes.
For histology, 3 μm thick sections were obtained from the samples, and stained with Safranin-O, a cationic dye that binds stoichiometrically to negatively charged GAGs, and is indicative of the PG content of the tissue. The spatial PG distribution of the stained sections were obtained using a light microscope (AxioImager M2, Carl Zeiss, Oberkochen, Germany).
RESULTS
Spectral variations in the NIR region were consistent with differences in meniscus composition, which is indicative of the tissue's morphology and health, as can be observed in the representative plot (Fig. 2). Hence, changes in meniscus composition influences the tissue's spectral response. Since the differences in baseline between samples may be due to multiplicative light scatter, derivative pre-processing, which eliminates constant offset, was employed in order to closely inspect spectral variations (inset of Fig. 2). This variation was observed to be consistent in the NIR region because of the full-depth tissue penetration of light in this region. The reverse is the case in the VIS region of the spectrum, which is restricted to the superficial layer of the meniscus.
Comparison of the absorbance value at the water peak (about 950 nm) of the 1st derivative spectra (Fig. 2) showed significant difference (p = 0.022) between the spectra of samples obtained from the three different sections of the meniscus. The water content in these regions depend upon whether the medial or lateral meniscus is being examined, with higher water content observed in the medial meniscus. Samples extracted from the central meniscus had the highest amount of water content, and the posterior the least amount. These were consistent with the literature.41
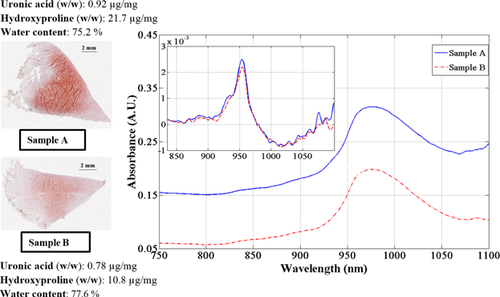
PCA of the 1st derivative pre-processed spectra reveals certain relationships that are indicative of the tissue composition. Only the first three principal components were considered, and they show strong relationships with the original data. The first principal component (PC1) explains about 65% of the variations in the original spectral data and the loadings plot (Fig. 3a) suggests that this is mostly indicative of meniscus water content, which is represented by the 3rd overtone water (OH) peak observed in the derivatized spectra around 953 nm. PC1 also bears some relationship with the solid matrix components (collagen and PGs), particularly indicated by the 3rd overtone CH peak around 830 nm. The second and third PC, which represent the remaining variations (along with higher components), are mostly related to the solid matrix.
This is supported by the direct relationship between the second PC scores and the tissue matrix content (collagen in this case, quantified by hydroxyproline content) observed in the scatter plot of Figure 3(b). Although, other minor matrix components such as glycolipids and glycoproteins could also be responsible for variances in the higher PCs. Peaks observed in the regions between 1000–1100 nm (10000–9091 cm−1) of the second and third PC loadings are indicative of 3rd overtone absorptions due to the matrix constituents of cartilage.32
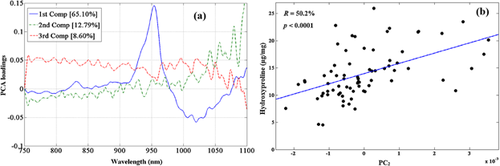
From preliminary experiments, spectral smoothing, and 2nd derivative pre-processing with 5 PLS components were determined to be optimal for correlating the predictor (spectra) to the response (reference) data. PLS regression of the pre-processed spectra and the response (tissue composition) variables yielded strong correlations (p < 0.001) between the spectra predicted and measured tissue composition (Table 1). The correlations were best with data from the NIR region of the spectrum and poorest in the VIS region (Table 1).
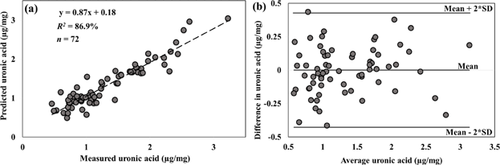
The high correlations (p < 0.001) obtained with spectral data from the NIR region is demonstrated in the plots of predicted vs. measured reference data of the samples (Figs. 4-6a). The corresponding Bland–Altman plots (Figs. 4-6b) show agreement between the predicted and measured tissue composition of the samples measured. The bias between the predicted and reference measurement, estimated in the Bland–Altman plots as the mean of the difference between both measurements, were negligibly small (<0.01%) for all the parameters.
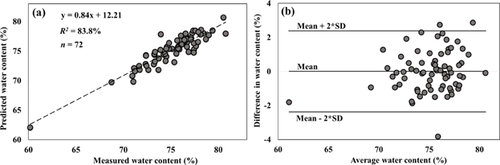
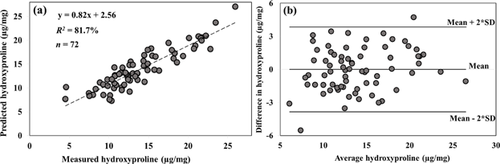
The mean values (given as mean ± SD) and range for the reference measurements are: Water, 75.2 ± 3.0 (60.2–80.8) %, hydroxyproline, 13.96 ± 4.57 (4.53–25.96) µg/mg, and uronic acid, 1.33 ± 0.60 (0.46–3.23) µg/mg contents. The model prediction errors (RMSECV) in the NIR region, relative to the range of samples used in this study were 19.9% for water content, 23.8% for hydroxyproline content and 19.5% for uronic acid content. While these percentage error values seem relatively high, we believe this would decrease as the number of samples, and range of parameters increase.
DISCUSSION
In this study, we investigated the relationship between the optical absorption response of human meniscus and its matrix composition. We demonstrated the potential of optical spectroscopy in the NIR spectral range to estimate the biochemical composition of human meniscus samples (Figs. 2, 4-6a). The agreement between the spectral predicted and reference measurements, revealed by the Bland–Altman plots (Figs. 4-6b), demonstrates the reliability of the present method. The relative errors observed in the models are partly due to the limited number of samples used in present study. Thus, before this technique can be fully adapted for clinical applications, the models would need to be optimized using a large number of samples with different levels of tissue degeneration (i.e., larger sample variance). Differences in the water content (associated with the spectral water peak) observed in the three regions of meniscus were consistent with the literature,41 with higher water content in the medial meniscus. Samples extracted from the central meniscus had the highest water content, and the posterior the lowest.
The findings in this study are supported by results from earlier studies on articular cartilage,32-36, 47 which consists of similar matrix components as meniscus, although the collagen types differ. The NIR region where correlation was optimized is also consistent with earlier studies on cartilage.34, 35, 48 It is worth noting at this stage that the present optical method is not proposed as a replacement for the reference techniques, but merely as a means of estimating the composition of meniscus during arthroscopy. Although the depth of penetration of light into meniscus was not investigated in this study, a previous study49 suggests that NIR radiation penetrates 4–5 mm into articular cartilage, which has similar constituents as meniscus. Thus, the acquired spectra can be attributed to the meniscus matrix, which had average thickness of 4.05 mm in this study. Meniscus structural organization is different from that of articular cartilage and further study is required on how the structural organization of meniscus collagen affects the amount of scatter.
The proposed optical technique takes advantage of light interaction with meniscus matrix on a molecular level, and is sensitive to micro- and macroscopic changes in the tissue structure and composition.32, 34, 48 Thus, it could be useful for rapid tracking of changes in meniscus composition, resulting from either matrix degeneration or tissue recovery/regeneration in follow up procedures after repair surgeries. It would contribute to the quest for modern quantitative arthroscopy techniques and could be used to augment and eliminate subjectivity in traditional arthroscopy. For example, the tissue composition of an individual patient could possibly be predicted from spectra obtained from the patient using optimized predictive models relating the spectra to the tissue composition. Since the predicted composition are absolute values, the tissue health can be evaluated either relative to benchmark values from a population of patients, or to the composition of healthy tissue from the same patients.
The poor correlations (35.8 ≤ R2 ≤ 52.3) between spectral data in the VIS range and meniscus composition suggest poor penetration depth of light in this wavelength range. This is in line with reported attenuation of visible light to about one third of the incident light intensity at approximately 0.6 mm in articular cartilage.50 Although visible light is expected to penetrate deep into the cartilage (> 5 mm, depth of penetration increases with decreasing wavelength),49, 51 it experiences strong attenuation in cartilage, which possesses similar constituents as meniscus, although with different matrix architecture. Thus the amount of light reaching the mid or deep matrix would have been diminished significantly. In addition, in terms of the optical windows for characterization of biological tissues, Sordillo et al.52 showed that light in this region experiences stronger attenuation compared to regions within the optical windows. Hence, spectral data from this region would arguably be limited to characterization of meniscal superficial layer. This explains the limited capacity of spectral data within this range to predict meniscus composition (Table 1), thus limiting its use for probing full-thickness human meniscus. Nevertheless, this region may be sensitive to changes in meniscus properties associated with superficial matrix tear or crack, alteration and degeneration. On the molecular level, spectral absorptions encountered in this region are associated with higher overtone (e.g., fourth and fifth overtones) molecular vibrations that tend to be very weak and are of limited analytical significance.
Light in the NIR range penetrates deeper into biological tissues,33, 53, 54 particularly in the wavelength range used in this study (750–1100 nm),49 thus permitting full-depth probing of meniscus matrix. This is evident from the significant improvement in correlation obtained with data from this spectral range relative to the VIS region (Table 1). Since spectral absorption of biological materials in the NIR range arise predominantly from CH, NH, OH, and SH bonds,55, 56 the fundamental constituents of meniscus matrix components, data in this region is indicative of both micro- and macroscopic properties of the tissue.32 Thus, the spectral response of meniscus incorporates latent information on the composition and structure32, 34 of its matrix as demonstrated by the PCA results (Fig. 3); this explains the improved correlation with the reference measurements (Figs. 4-6).
The spectral absorptions encountered in the NIR region are associated with third overtone molecular vibrations that may be weak, yet of useful analytical significance. The pronounced and dominant peak at approximately 977 nm is the 3rd overtone OH peak due to the abundant water content of meniscus, while the 3rd overtone CH stretch bands which are dominant between 845–878 nm are indicative of the collagen (hydroxyproline) and PG (uronic acid) content of the matrix. Although the CH peak seems to be weak and slightly masked by the prominent and ubiquitous OH peak due to the tissue's water content, spectral pre-processing (Fig. 2) proved to be effective in extracting and amplifying the effects of these peaks. This improved the outcome of the multivariate correlation analyses.
The statistically significant difference in spectral response of samples obtained from different sections of meniscus, that is mid-meniscus, anterior, and posterior horns, is possibly due to the differences in matrix organization in these anatomical sections of the tissue. Due to the limited number of cadaver joints used in this study, further analyses with larger number of joints, and samples would need to be performed in order to fully validate the models developed and extend the technique to clinical arthroscopic application. Although the meniscus is known to be partially vascularized, the presence of blood was not accounted for due to the preparation of the samples used in this study, that is, they were stored in PBS after extraction, thus flushing any remaining blood in the tissue. Hence, the results reported here would need to be optimized in vivo in order to account for the spectral signature of blood during the analyses. Nevertheless, this does not distort the underlying hypothesis of this study.
Another limitation of the current study is that extension of the experimental protocol (set-up) to in vivo conditions may not be feasible. However, the system can easily be optimized, for example integration of both light source and spectrometer (detector) into one unit applied via a contact optical fiber probe, prior to adaptation of this optical technique for arthroscopic application. The relative errors observed in the predictive models developed in the current study were higher than those obtained by Hanifi et al40 using an infrared fiber optic probe (IFOP). However, it is worth noting that the mid-IR spectral range used by Hanifi et al is more sensitive, but, it suffers from limited depth of penetration (maximum of 10 μm in cartilage).57
CONCLUSION
We have evaluated the potential of optical spectroscopy in the NIR spectral region for estimating tissue composition of human meniscus. The uronic acid (indicative of PGs), hydroxyproline (indicative of collagen), and water content, measured via conventional biochemical analyses, were found to correlate significantly with the spectral response in the NIR spectral range. This presents potential for nondestructive characterization of human meniscus in real-time during arthroscopic evaluation of joint conditions.
AUTHORS' CONTRIBUTIONS
Ala-Myllymäki J. was involved in data acquisition and analyses, and was also involved in the preparation of manuscript drafts. Honkanen J.T.J. contributed in reference measurements and interpretation of data. Töyräs J. contributed in the study conception and interpretation of data. Afara I.O. contributed in the study conception, experimental design, data analyses and interpretation. All authors contributed in the preparation and approval of the final submitted manuscript.
ACKNOWLEDGMENTS
This study was supported by funding from the Academy of Finland (project 267551, University of Eastern Finland) and Kuopio University Hospital (VTR projects 5041750 and 5041744, PY210 Clinical Neurophysiology).




