Vascular endothelial growth factor polymorphisms in patients with steroid-induced femoral head osteonecrosis
Abstract
To investigate an association between steroid-induced femoral head osteonecrosis (FHON) and functional vascular endothelial growth factor (VEGF) gene (−2578A/C, −1154A/G, −634C/G, and +405C/G) polymorphisms polymerase chain reaction-restriction fragment length polymorphism genotyping was performed in 160 patients (86 idiopathic FHON and 74 steroid-induced FHON) and 160 gender- and age-matched controls. The steroid-induced subgroup had a significantly lower prevalence of −1154A allele (7.4% vs. 18.1%, odds ratio (OR) = 0.363) and genotype carrying −1154A (14.9% vs. 32.5%, OR = 0.333 in a recessive model) than controls. In a dominant model, the frequency of genotype carrying +405G (74.3% vs. 84.4%, OR = 0.492) was significantly lower in steroid-induced FHON than in controls. The distribution of haplotypes was significantly different between controls and FHON patients (p = 0.00011). Especially, when haplotypes were classified into high (CGCG and AAGG) or low (CGGC and AGGC) VEGF inducing haplotypes, patients with steroid-induced FHON had a significantly lower prevalence of high inducing haplotypes (7.4% vs. 15.9%, OR = 0.424) and a significantly higher prevalence of low inducing haplotypes (4.7% vs. 0.6%, OR = 7.894) than controls. Low inducing VEGF haplotypes may confer an increased risk and high inducing haplotypes have a protective effect for the development of steroid-induced FHON in Korea. © 2011 Orthopaedic Research Society Published by Wiley Periodicals, Inc. J Orthop Res 30:21–27, 2012
Glucocorticoids are commonly prescribed for the treatment of patients with autoimmune inflammatory diseases, neoplastic diseases, or organ transplantation. However, they have been a leading cause of non-traumatic femoral head osteonecrosis (FHON).1, 2 Since not all patients who are treated with steroids develop FHON, the presence of additional risk factors or individual variation of glucocorticoids sensitivity has been suggested. However, no specific risk factor has been confirmed to date.
Although the pathogenesis of steroid-induced FHON is poorly understood, an impairment of angiogenesis has been proposed as a mechanism of FHON and pathogenic mechanisms include dysregulation of endothelial cell activating factors and angiogenetic factors.3 Vascular endothelial growth factor (VEGF), which is induced by hypoxia, is a strong angiogenic protein and also plays a role in the formation of cartilage and bone. Increased VEGF expression has been observed in the edematous area of the reparative zone around the necrotic portion of the femoral head from patients with FHON or Legg–Calve–Perthes disease.4-6 Gene transfection studies of FHON using animal models have shown that VEGF enhances revascularization of necrotic bone.7, 8 Early incremental VEGF expression can stimulate vascular invasion and resorption of necrotic tissue and seems to initiate the repair process or limit the extent of infracted bone damage.4, 9 Collectively, VEGF production is a major mechanism by which angiogenesis and osteogenesis are coupled during the bone repair process.10, 11
Both direct and indirect pathomechanisms of steroid-induced FHON including apoptosis, suppressed precursor production of osteoblast and osteocytes, endothelial dysfunction, enhanced coagulability, increased production of reactive oxygen species, inhibition of angiogenesis, and increased adipogenesis have been proposed.2 Suppression of VEGF production by glucocorticoids can inhibit angiogenesis and shorten osteoblast survival.
Therefore, we hypothesized that the genetic polymorphisms of VEGF are associated with the development of steroid-induced FHON. To test this hypothesis, we investigated the polymorphisms in the promoter and 5′-untranslated region (UTR) of the VEGF gene in patients with steroid-induced FHON because polymorphisms within these regions have lead to differences in VEGF expression.12-14
MATERIALS AND METHODS
Study Subjects
Among patients with non-traumatic FHON, who were diagnosed by evidence of osteonecrosis on magnetic resonance imaging in stage 1 of Association Research Circulation Osseous (ARCO) classification system15 and plain radiographs in stages 2, 3, and 4 at two university hospitals, we enrolled 160 patients with idiopathic and steroid-induced FHON (108 males and 52 females; mean age, 39.2 years; range 20–67 years). Steroid-induced FHON was defined by a history of a mean daily dose of ≥16.6 mg or highest daily dose of 80 mg of predinosolone equivalent within 1 year prior to the development of symptoms or radiological diagnosis in asymptomatic cases.16, 17 Idiopathic FHON was defined by exclusion of cases with steroid-induced FHON, alcohol-induced FHON (pure ethanol consumption ≥800 mg/week18) or possible combined causes. The study population consisted of 86 idiopathic FHON patients (57 males and 29 females; mean age, 38.6 years; range 20–64 years) and 74 steroid-induced FHON patients (51 males and 23 females; mean age, 39.9 years; range 20–67 years). The DNA samples partially overlapped with those analyzed in the previous study;19 29 (33.7%) in idiopathic and 47 (63. 5%) in steroid-induced FHON. Healthy control subjects were matched with patients for gender and age (3-year range) and enrolled from subjects who had no significant medical problems on routine medical checkups (mean age, 39.3 years; range 20–68 years). Underlying diseases in steroid-induced FHON were allergic respiratory or cutaneous diseases (n = 21 patients), glomerulonephritis or kidney transplantation (n = 14), systemic lupus erythematosus (n = 9), idiopathic thrombocytopenia purpura (n = 5), systemic vasculitis (n = 5), psoriasis (n = 3), inflammatory bowel disease (n = 3), optic neuritis or uveitis (n = 3), sudden sensorineural hearing loss (n = 2), tuberculous meningitis (n = 2), intracranial hemorrhage (n = 2), systemic sclerosis (n = 2), and others (n = 3). The proportion of ever-smoker and total exposure of smoking (pack-year) were compatible between healthy controls and total FHON patients. However, patients with steroid-induced FHON had a significantly higher proportion of ever-smoker (age- and gender-adjusted odds ratio (OR) was 2.27 [95% confidence interval 1.11–4.64]) and higher exposure of smoking (p = 0.014 by one-way ANOVA) when compared with healthy controls and idiopathic FHON patients. This study was approved by the Institutional Review Board at our hospitals after reviewing the ethical issues (IRB protocol #B-0412/015-010).
DNA Isolation and Genotyping
DNA samples were extracted from peripheral mononuclear cells using the QIAamp Blood kit (Qiagen, Valencia, CA). Polymerase chain reaction (PCR) genotyping for VEGF promoter and 5′-UTR polymorphisms (−2578A/C (reference single nucleotide polymorphism (SNP) ID rs699947), −1154A/G (rs1570360), −634C/G (rs2010963), and +405C/G (rs2010963)) was performed by PCR-restriction fragment length polymorphism (RFLP) analysis. PCR primers were synthesized according to previous reports with some modifications (Bioneer, Daejeon, South Korea) (Supplementary Material). The −2578A allele, −1154G allele, and −634G and 405G alleles result in the gain of sites for BglII (Takara Bio, Inc., Shiga, Japan), MnlI (New England Biolabs, Ipswich, MA), and BsmFI (New England Biolabs), respectively.14, 20, 21 The PCR products were digested by an appropriate restriction enzyme described above and then separated by 2–3% agarose gel electrophoresis. DNA fragments were stained with GelRed™ fluorescent nucleic acid dye (Komabiotech, Seoul, Korea). To confirm the accuracy of the method employed, 20 samples were randomly selected and analyzed by direct sequencing; all results were in accordance with the PCR-RFLP results.
Statistical Analyses
Chi-square goodness-of-fit testing was used to determine the fit between observed genotype frequencies and those expected under Hardy–Weinberg equilibrium (HWE). The allele frequencies of healthy controls and FHON groups were compared using the chi-square test or Fisher's exact test. In case of multiple comparisons, Bonferroni's correction was applied. An association between the genotypes and FHON was analyzed by logistic regression analysis adjusted with sex, age, and smoking status under codominant, dominant, or recessive model, and the homozygote for allele with a higher promoter activity (i.e., −2578CC, −1154GG, −634CC, and +405GG) was used as a reference.12-14 Degrees of linkage disequilibrium between the genotype pairs were analyzed using the JLIN program (version 1.6.0).22 Haplotype frequencies were computed using the PHASE program (version 2.1) and differences in the haplotype frequencies of the FHON patient and control groups were analyzed by permutation testing (100,000 random permutations).23 To calculate the OR adjusted with sex, age, and smoking status, logistic regression analyses were performed in analysis for haplotypes with a frequency >1% assigned by the PHASE program. We determined the effect size estimation for chi-square or Fisher's exact test with the phi coefficient (Cramer's Variance (V); V = 0.1 is a small effect size, 0.3 is a medium effect size, and 0.5 is a large effect size) if p or corrected p (pc) < 0.05. All statistical analyses were performed using PASW Statistics (version 17.0.2, SPSS, Inc., Chicago, IL) and the statistical power was calculated using G*Power (version 3.0).24
RESULTS
Distributions did not deviate from HWE in both groups, with the exception of −1154A/G (p = 0.017) in FHON patients. Moreover, these polymorphic alleles were found to be in a state of significant linkage-disequilibrium with each other (p < 0.00001 by chi-square test,  = 0.46–0.97). There was no significant difference in the distributions of allele frequencies between healthy controls and FHON patients (Fig. 1). However, steroid-induced FHON subgroup had a significantly lower prevalence of −1154A allele than controls (7.4% vs. 18.1%, p = 0.00024, OR = 0.36 [0.19–0.71], V = 0.14). In addition, there was no skewed distribution of genotypes in whole FHON patients when compared with those in healthy controls (Fig. 2). In subgroup analyses, the idiopathic FHON subgroup had a significantly higher prevalence of the −1154GA genotype in a co-dominant model (46.5% vs. 28.7%, p = 0.013, OR = 2.01 [1.16–3.49], V = 0.18) and the genotype carrying −1154A in a recessive model (46.5% vs. 32.5%, p = 0.034, OR = 1.80 [1.05–3.11], V = 0.18) than controls. On the contrary, the frequency of −1154GA in a co-dominant model (14.9% vs. 28.7%, p = 0.010, OR = 0.37 [0.18–0.79], V = 0.15) and that of genotype carrying −1154A in a recessive model (14.9% vs. 32.5%, p = 0.004, OR = 0.33 [0.16–0.70], V = 0.19) was significantly lower in the steroid-induced FHON than in controls. Furthermore, steroid-induced FHON significantly decreased the frequency of genotype carrying +405G (74.3% vs. 84.4%, p = 0.045, OR = 0.49 [0.25–0.98] in a dominant model, V = 0.12) when compared with controls.
= 0.46–0.97). There was no significant difference in the distributions of allele frequencies between healthy controls and FHON patients (Fig. 1). However, steroid-induced FHON subgroup had a significantly lower prevalence of −1154A allele than controls (7.4% vs. 18.1%, p = 0.00024, OR = 0.36 [0.19–0.71], V = 0.14). In addition, there was no skewed distribution of genotypes in whole FHON patients when compared with those in healthy controls (Fig. 2). In subgroup analyses, the idiopathic FHON subgroup had a significantly higher prevalence of the −1154GA genotype in a co-dominant model (46.5% vs. 28.7%, p = 0.013, OR = 2.01 [1.16–3.49], V = 0.18) and the genotype carrying −1154A in a recessive model (46.5% vs. 32.5%, p = 0.034, OR = 1.80 [1.05–3.11], V = 0.18) than controls. On the contrary, the frequency of −1154GA in a co-dominant model (14.9% vs. 28.7%, p = 0.010, OR = 0.37 [0.18–0.79], V = 0.15) and that of genotype carrying −1154A in a recessive model (14.9% vs. 32.5%, p = 0.004, OR = 0.33 [0.16–0.70], V = 0.19) was significantly lower in the steroid-induced FHON than in controls. Furthermore, steroid-induced FHON significantly decreased the frequency of genotype carrying +405G (74.3% vs. 84.4%, p = 0.045, OR = 0.49 [0.25–0.98] in a dominant model, V = 0.12) when compared with controls.
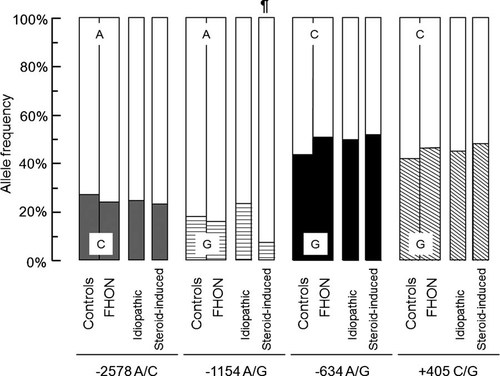
Allele distributions of VEGF promoter and 5′-UTR polymorphisms in controls and FHON patients. The prevalence of −1154A allele was significantly lower in the steroid-induced FHON than in controls (¶ 7.4% vs. 18.1%, p = 0.00024 by chi-square test, OR = 0.36 [0.19–0.71]).
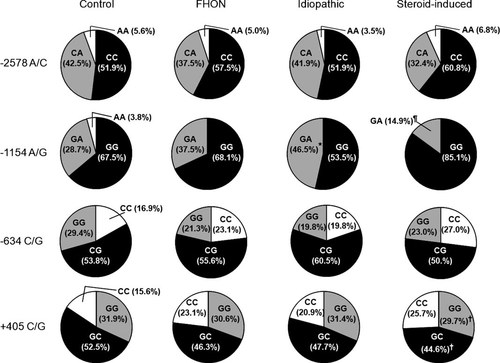
Genotype distributions of VEGF promoter and 5′-UTR polymorphisms in controls and FHON patients The prevalence of −1154GA genotype was significantly higher in the idiopathic FHON, when compared with controls (*p = 0.013, OR = 2.01 [1.16–3.49]). Conversely, the frequency of −1154GA (¶p = 0.010, OR = 0.37 [0.18–0.79]) was significantly lower in the steroid-induced FHON than in controls. The genotype frequency carrying −1154A allele was significantly higher in the idiopathic FHON (* 46.5% vs. 32.5%, p = 0.034 in a recessive model, OR = 1.80 [1.05–3.11]) and lower in the steroid-induced FHON (¶ 14.9% vs. 32.5%, p = 0.004 in a recessive model, OR = 0.33 [0.16–0.70]) compared with that of controls. Moreover, the frequency of genotype carrying +405G was significantly decreased in the steroid-induced FHON († 74.3% vs. 84.4%, p = 0.045, OR = 0.49 [0.25–0.98] in a dominant model) when compared with controls. All p values were calculated by logistic regression adjusted with sex, age, and smoking status.
A possible 15 haplotypes were assigned by the PHASE program and nine had a frequency >1.0% (Fig. 3). The distribution of haplotypes was significantly different between controls and FHON patients (p = 0.00011). FHON group had a significantly lower frequency of AAGG haplotype (5.6% vs. 12.4%, pc = 0.021, OR = 0.44 [0.26–0.75], V = 0.12) and lower prevalence of AAGG haplotype carriers (13.8% vs. 27.5%, p = 0.002, OR = 0.41 [0.23–0.72], V = 0.17) than controls. In subgroup analyses, AAGG haplotype frequency (3.4% vs. 12.4%, pc = 0.004, OR = 0.21 [0.08–0.52], V = 0.16) or its carriers prevalence (6.8% vs. 27.5%, p = 4.72 × 10−4, OR = 0.170 [0.06–0.46], V = 0.24) was significantly lower in steroid-induced subgroup than in controls. In addition, steroid-induced FHON showed a higher prevalence of CGGC carriers than controls (8.1% vs. 1.3%, p = 0.018, OR = 7.30 [1.40–37.94], V = 0.18).
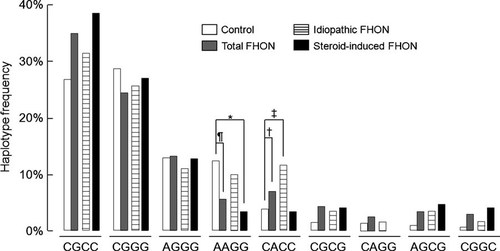
Haplotype distributions of VEGF promoter and 5′-UTR polymorphisms in controls and FHON patients. The distribution of haplotypes was significantly different between controls and FHON patients (p = 0.00011 by permutation test). The frequency of AAGG haplotype was significantly lower in the total (¶ 5.6% vs. 12.4%, pc = 0.021 by chi-square test with Bonferroni's correction, OR = 0.44 [0.26–0.75]) and steroid-induced FHON (* 3.4% vs. 12.4%, pc = 0.004, OR = 0.21 [0.08–0.52]) than controls. On the contrary, CACC haplotype frequency was significantly higher in total († 7.0% vs. 3.9%, pc = 0.048, OR = 2.93 [1.37–6.27]) and idiopathic FHON (‡ 11.6% vs. 3.9%, pc = 0.001, OR = 4.55 [2.06–10.04]) than in controls.
We classified haplotypes into high inducing (CGCG and AAGG) and low inducing (CGGC and AGGC) haplotypes13, 25 (Table 1). Total patients with FHON had a significantly lower frequency of high inducing haplotypes (10.6% vs. 15.9%, p = 0.048, OR = 0.637 [0.40–1.00], V = 0.08) and tended to have a higher frequency of low inducing haplotypes (2.8% vs. 0.6%, p = 0.0632, OR = 4.60 [1.11–19.02]). Furthermore, the carriers with high inducing haplotypes was significantly less prevalent (18.8% vs. 30.0%, p = 0.016, OR = 0.52 [0.31–0.89], V = 0.13) and carriers with low inducing haplotypes was significantly more prevalent (6.3% vs. 1.3%, p = 0.032, OR = 5.42 [1.16–25.35], V = 0.13) in whole FHON patients than in controls. Especially, the steroid-induced subgroup had significantly decreased frequency of high inducing (7.4% vs. 15.9%, p = 0.012, OR = 0.42 [0.22–0.83], V = 0.12; statistical power 73.9%) and increased frequency of low inducing haplotypes (4.7% vs. 0.6%, p = 0.0055, OR = 7.89 [1.84–33.80], V = 0.14; statistical power 79%). Furthermore, the proportion of subjects carrying high inducing haplotypes was significantly lower (13.5% vs. 30.0%, p = 0.006, OR = 0.33 [0.15–0.71], V = 0.18; statistical power 80.4%) and that of subjects carrying lower inducing haplotypes was significantly higher (9.5% vs. 1.3%, p = 0.008, OR = 9.23 [1.80–47.27], V = 0.20; statistical power 79.2%) in the steroid-induced subgroup than in controls. However, the distributions of high or low inducing haplotypes were not significantly different between idiopathic FHON subgroup and controls.
| Control, Frequency | FHON Patients | ||||||
|---|---|---|---|---|---|---|---|
| Total | Idiopathic | Steroid-Induced | |||||
| Frequency | OR [95% CI]* | Frequency | OR [95% CI]* | Frequency | OR [95% CI]* | ||
| Haplotypes | n = 320 | n = 320 | n = 172 | n = 148 | |||
| High inducing haplotypes# | 15.9% | 10.6% | 0.63 [0.395–1.00]¶ | 13.4% | 0.81 [0.48–1.38] | 7.4% | 0.42 [0.22–0.83]† |
| Low inducing haplotypes# | 0.6% | 3.1% | 4.60 [1.11–19.02] | 1.7% | 2.82 [0.56–14.25] | 4.7% | 7.89 [1.84–33.80]‡ |
| Carriers | n = 160 | n = 160 | n = 86 | n = 74 | |||
| High inducing haplotypes | 30.0% | 18.8% | 0.52 [0.31–0.89]** | 23.3% | 0.69 [0.37–1.26] | 13.5% | 0.33 [0.15–0.71]¶¶ |
| Low inducing haplotypes | 1.3% | 6.3% | 5.42 [1.16–25.35]†† | 3.5% | 2.93 [0.48–18.01] | 9.5% | 9.23 [1.80–47.27]‡‡ |
- *Odds ratio [95% confidence interval] when compared with controls; ¶p = 0.048 by chi-square or Fisher's exact test; †p = 0.012; ‡p = 0.0055; **p = 0.016 by logistic regression adjusted with sex, age, and smoking status; ¶¶p = 0.004; ††p = 0.032; ‡‡p = 0.008; #high inducing haplotypes were CGCG and AAGG, and low inducing haplotypes were CGGC and AGGC according to the Refs.11,22
DISCUSSION
A pathophysiological model of glucocorticoid-induced FHON is a multiple hit theory and intravascular thrombosis, aberrant lipid metabolism, and steroid sensitivity have been mainly explored as a major pathogenic event. In this regard, candidate genes such as plasminogen activator inhibitor-1 (PAI-1), apolipoprotein B (ApoB), and cytochrome P450 (CYP3A4) have been investigated.26-29 A recent genome-wide association study reported genetic variants of acid phosphatase 1 (ACP1) were associated with FHON in children with acute lymphoblastic leukemia.30 However, until now, there has been no study concerning a gene related with angiogenesis.
VEGF production by stimulated mononuclear cells from subjects with the −2578CC, −1154GG, or +405GG genotypes is significantly higher than those with the −2578AA, −1154AA, or +405CC, respectively.12, 13 In addition, the −634CC genotype has been reported to be associated with higher serum VEGF levels when compared with others.14 Kim et al.19 recently analyzed VEGF −2578C/A, −634G/C, and +936C/T polymorphisms and reported that the VEGF −634C carrier state is associated with non-traumatic FHON. They enrolled patients having non-traumatic FHON regardless of etiology and most of them were those with ethanol-induced FHON. But, we studied functional VEGF promoter polymorphisms such as −2578C/A, −1154A/G, −634C/G, and +405C/G in idiopathic and steroid-induced FHON patients. Because chronic ethanol exposure increases VEGF expression,31, 32 we excluded ethanol-induced FHON subgroup to make clear the extent to which functional VEGF promoter polymorphisms contribute on the development of FHON.
Presently, steroid-induced FHON displayed significantly different frequencies of genotype carrying −1154A in a recessive model (OR = 0.33) or +405G in a dominant model (OR = 0.49) when compared with those in controls. But, these genotype results did not indicate a functional implication because the frequencies of both genotype with low promoter activity (−1154A carrier) and one with high promoter activity (+405G carrier) were less common in steroid-induced FHON than in controls. This may have been a result of the highly polymorphic nature of VEGF gene. Therefore, VEGF haplotypes are implicated more strongly with VEGF expression than a SNP.
The AAG and CGC haplotypes of −2578A, −1154, and −634 sites show significantly higher hypoxia-stimulated VEGF expression than AGG and CGG in human myoblasts.25 Although Lambrechts et al. reported that AAG and AGG haplotypes had lower promoter activity in hypoxia than CGC using a luciferase reporter assay in GI-1 glioma cells,33 transcriptional VEGF regulation is affected by the genetic makeup of tumor cells11 and the region from −2468 to −1177 was absent in the reporter construct designed by Lambrechts et al. The +405G/C polymorphism has been reported to be strongly in linkage disequilibrium with the −2578A/C polymorphism and haplotype containing +405G was found to be associated with the high production of VEGF.34, 35 According to the results of the aforementioned studies, we classified hapotypes for −2578, −1154, −634, and +405 into high inducing (CGCG and AAGG) and low inducing haplotypes (CGGC and AGGC). In our study, steroid-induced FHON was positively associated with low inducing VEGF haplotypes and negatively associated with high inducing haplotypes. Unfortunately, a clear gene dosage effect of these haplotypes was not found, perhaps due to the small number of subjects carrying low or high inducing diplotypes (low inducing diplotypes, 0.0% in all subjects; high inducing diplotypes, 1.9% in controls and 1.4% in steroid-induced FHON). But, these functional VEGF haplotypes were found not to be associated with idiopathic FHON.
Glucocorticoids directly decrease VEGF production in bone marrow stromal cells, osteoblasts, and chondrocytes as well as endothelial cells.36-38 Furthermore, their inhibitory activity on VEGF production is potentiated in hypoxic condition.39 Moreover, Wang et al.40 reported that the levels of VEGF were significantly suppressed in the early stage of steroid-induced FHON in a rabbit model. In this context, we are tempted to speculate that a synergic effect between glucocorticoids induced VEGF suppression and the carriage state of low inducing VEGF haplotypes by hypoxia increases the risk of development of steroid-induced FHON. Thus, idiopathic, ethanol-induced, and steroid-induced FHON belong to a clinical syndrome that would have a different pathogenesis or genetic basis.
There are several limitations in this study. The sample size was not large enough to generalize our results, as like the previously reported studies (n ≤ 69 patients).26-30, 41, 42 We enrolled 74 patients with steroid-induced FHON; to the best of our knowledge, the sample size is the largest in genetic polymorphism studies concerning steroid-induced FHON conducted so far. In our study, the calculated effect sizes were relatively small for all comparisons, which may be secondary to a small sample size. But, this limitation should not be mistakenly translated into clinically insignificant results because FHON is considered a pathophysiologically heterogeneous disease and the effect size of single gene variants could not be large. Furthermore, even though steroid is a well-defined risk factor of non-traumatic FHON, the clinical definition of steroid-induced FHON has not been established. In a view of dosage, we referred to a large epidemiologic study in Japan.16 Also, to clarify the temporal relationship, the gap between glucocorticoids use and FHON development was restricted to within 1 year.17 Moreover, steroid-induced FHON subgroup had diverse underlying diseases in our study and it could affect the distributions of VEGF polymorphisms. Thus, it is ideal to compare the polymorphisms between patients having and not having steroid-induced FHON, who were diagnosed as a single inflammatory disease. However, this heterogeneity is a real issue in clinical practice when we are confronted with steroid-induced FHON.
Despite these limitations, the results suggest that VEGF is a novel susceptibility gene in steroid-induced FHON and low inducing VEGF haplotypes confer an increased risk for the development of steroid-induced FHON in Korea. To confirm our results, further studies are warranted on larger patients from other ethnic groups.
Acknowledgements
This study was supported by a grant no 02-207-006 from the SNUBH Research Fund.




