Interactions of environmental conditions and mechanical loads have influence on matrix turnover by nucleus pulposus cells
Abstract
Disc degeneration is associated with several changes in the physicochemical environment of intervertebral disc cells. Nucleus pulposus (NP) cells in the center of degenerated discs are exposed to decreased glucose supply, osmolarity, pH, and oxygen levels. To understand the complexity of these interactions on a cellular level, we designed standardized experiments in which we compared responses to these environmental factors under normal levels with those seen under two different degrees of disc degeneration. We hypothesized that these changes in environmental stimuli influence gene expression of matrix proteins and matrix degrading enzymes and alter their responses to cyclic hydrostatic pressure (HP). Our results suggest that a simulation of degenerative conditions influences the degradation of disc matrix through impairing matrix formation and accelerating matrix resorption via up- or down-regulation of the respective target genes. The greatest effects were seen for decreases in glucose concentration and pH. Low oxygen had little influence. HP had little direct effect but appeared to counteract matrix degradation by reducing or inverting some of the adverse effects of other stimuli. For ongoing in vitro studies, interactions between mechanical stimuli and factors in the physicochemical environment should not be ignored as these could markedly influence results. © 2011 Orthopaedic Research Society Published by Wiley Periodicals, Inc. J Orthop Res 30:112–121, 2012
Intervertebral disc (IVD) degeneration is strongly associated with back pain, a disorder which affects a large proportion of the population and is a burden for the affected patients, and, because of its high health-care and social costs, for society.1 Disc degeneration is a complex problem with multiple factors contributing to this phenomenon and despite an increasing number of studies the pathogenic pathway is not fully understood.
Although genetic predisposition appears overriding,2, 3 degenerative pathways are assumed to be influenced by factors such as mechanical loads4, 5 and alterations to the physicochemical environment6 of the disc cells. These signals do not act independently, however, and there is little information on how these different factors interact and influence each other to affect alterations of structure, function, and composition of the disc matrix.
Of the environmental factors thought to influence degenerative changes in the disc, decreased nutrition is assumed to be a key contributor.7 Normal healthy discs are avascular and nutrient supply and removal of metabolic degradation products occurs predominantly via diffusion from the blood vessels at the cartilaginous endplate. Disc cells metabolize primarily by glycolysis even in the presence of oxygen; they thus require glucose and produce lactic acid at a high rate.8, 9 A reduction in supply of essential nutrients or failure of routes for lactic acid removal is assumed to be one—if not the major—reason for disc degeneration. Indeed calcification of the cartilaginous endplates leading to a decreased permeability for nutrients and metabolites increases with increase in degree of disc degeneration. Measurements with microelectrodes or by biochemical assays have shown low levels of nutrients such as glucose and also acidic pH levels (arising from lactic acid accumulation) in many degenerated discs.10
Disc cells are very sensitive to alterations of nutritional substrates and accumulation of metabolites. In vitro experiments have shown that disc cells need critical concentrations of glucose, a suitable pH and oxygen supply to stay viable and metabolically active.8, 11 Disc cells die if glucose levels fall below around 0.5 mM. Moreover, disc cells are particularly sensitive to the fall in pH arising from accumulation of lactic acid which reduces rates of proteoglycan biosynthesis but does not decrease the activity of matrix-degrading enzymes and thus contributes to degenerative changes of IVDs.12
Mechanical loads are another factor, which could potentially affect disc cell function adversely. The principal role of the discs is biomechanical; they act as joints of the spine providing mobility and flexibility. Discs are thus exposed to complex mechanical loads and their responses are governed by the properties of the extracellular matrix and hence will alter when the disc tissue changes its structure because of tissue degradation. In particular, swelling pressure, which is regulated by the osmotic contribution of the polyanionic glycosaminoglycans, falls as a consequence of aggrecan loss in degenerated discs. Thus, during degeneration the cells in the disc are exposed to a variety of alterations in their nutritional, osmotic, and mechanical microenvironments.
In several separate studies it has been shown that these factors all influence disc cell function; variations in glucose, oxygen, and pH levels each altered disc cell gene expression and viability.13-15 Hydrostatic pressure (HP) application has also been shown to affect cell function but results vary with loading regime and possibly between species.16, 17 Different experimental protocols make the comparison of the observed effects from different studies difficult. Moreover, there is very little information on interaction between environmental signals; for instance does glucose or oxygen level or osmolarity influence responses of disc cells to different mechanical stresses?
In order to gain some understanding of the effects of such interactions, we have used a standardized experimental protocol to investigate how changes in levels of some factors in the physicochemical environment (glucose, oxygen, pH, and osmolarity) influence responses to a mechanical load (intermittent HP) when applied to bovine nucleus pulposus (NP) cells.
In the present study, we hypothesized that alterations of the glucose, osmolarity, oxygen, and pH environment within ranges that are known to occur in degenerated disc tissue, have influence on NP cell viability and gene expression of proteins of matrix formation and degradation. Our second hypothesis was that these environmental variations alter the cells' responses to their respective predominant physiological load, which is HP for NP cells.
METHODS
As there is only limited availability of normal non-degenerated human disc tissue, the present study was performed with NP cells isolated from bovine caudal discs of young animals as bovine disc cells are a well-accepted model system for normal, healthy lumbar discs15 with the advantage of unlimited availability.
Isolation and Culture of Intervertebral Disc (IVD) Cells
Bovine IVD cells were isolated from caudal discs of cattle from a local abattoir; tails from 10 animals were used for results reported here. All animals were younger than 24 months. Within few hours after slaughter, up to six bovine discs from each specimen were removed from the cattle tails, separated into annulus fibrosus and NP and cells isolated from the pooled tissue regions. Cells from the NP region were used for the present study.
Cell isolation was performed by enzymatic digestion with collagenase as described previously.18, 19 The composition of the digestion media for NP tissue was collagenase-1 (0.8 mg/ml), and DNAse (2.6 units/ml) in Dulbecco's modified Eagle's medium (DMEM—Cat. No. 22320-022, Gibco, Karlsruhe, Germany) supplemented with 5% penicillin/streptomycin (Biochrom, Berlin, Germany), 5% fungizone (Gibco) and osmolarity adapted to 400 mOsm by addition of 1.5% of a 5 M NaCl/0.4 M KCl solution. After digestion and centrifugation, cell pellets were washed twice with PBS and expanded in monolayer culture to increase the cell number. All experiments were performed on passage II–III cells. NP cells were cultured with standard DMEM (with 5 mM glucose) supplemented with 1% penicillin/streptomycin, 5% fetal bovine serum, 1% non-essential amino acids, 1% L-glutamine (all from Biochrom) 0.5% fungizone, and 1.5% of 5 M NaCl/0.4 M KCl solution at reduced oxygen atmosphere (37°C, 100% humidity, 5% CO2, and 6% O2) during the entire cell expansion period. The medium was changed twice a week. Primary cultures were sub-cultured using trypsin/EDTA (0.05%/0.02%) (Biochrom).
Alginate Bead Cultures
After in vitro expansion, NP cells were harvested and seeded into alginate beads as previously described.14 Briefly, NP cells were seeded into 3D-alginate beads at a density of 4 × 106 cells/ml (40,000 cells/bead) using a 1.2% low-viscosity alginic acid sodium salt (A2158-250G, Sigma-Aldrich, Steinheim, Germany) solution and a syringe with a 22-gauge needle. After polymerization in a 102 mM CaCl2 solution and washing in phosphate-buffered saline (PBS) and standard medium, the cell-seeded alginate beads were cultured under standard conditions for 3 days. For alteration of the medium environment, a complete medium change was performed for the different conditions as described below.
Variation of Environmental Conditions
In three different experimental parts each of the following environmental conditions was varied according to the scheme shown in Figure 1.
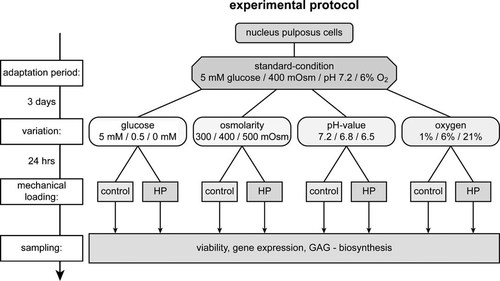
Scheme of the standardized protocol used for the different environment variation experiments.
In all experimental parts, one condition represented the estimated normal average value of the respective factor for the disc center. The other conditions represented deviations of this normal average value that can occur during disc degeneration. Each experiment was repeated with NP cells from six to eight animals. The total number of repetitions for each experiment is shown in the respective figure legend.
Variation of the Glucose Environment
Culture medium was replaced by serum-free DMEM (Biochrom) with different glucose concentrations (0 mM glucose, 0.5 mM glucose, 5 mM glucose) for 24 h until load application, and harvesting of the cells. The different glucose media were prepared as recently described by using serum-free DMEM (Biochrom) with different glucose concentrations (F0405: glucose-free = 0 mM, F0415: 1 g glucose/L = 5 mM, and 90% F0405 plus 10% F0415 = 0.5 mM glucose).14 The 5 mM glucose condition was considered as standard for the glucose supply and the other two variations represented reduced glucose conditions.
Variation of the Osmotic Environment
Culture medium was replaced by serum-free DMEM (Biochrom) with 5 mM glucose and adjusted to different medium osmolarities by adding variable volumes of a high salt solution (5 M NaCl/0.4 M KCl) to achieve the average-physiological osmolarity for the NP of a normal non-degenerated disc (400 mOsm), hypo-osmolarity (300 mOsm—representing a condition in degenerated discs), or hyper-osmolarity (500 mOsm—a condition that may occur if a normal disc looses fluid during diurnal loading) as described in a previous study.20
Variation of the pH Environment
Variations of pH level were achieved by addition of appropriate volumes of 1 N HCl to the culture medium. For study of the effect of pH, the standard culture medium DMEM (pH 7.2) was replaced by medium adjusted to pH 7.2 (representing the normal condition) or to the pH-reduced conditions pH 6.8 or 6.5 (representing degenerative conditions). These media were equilibrated in filter tissue flasks in the CO2-incubator that was used for the experiments for 24 h prior to addition of this medium to the cell cultures. At the end of the 24 h incubation period loaded samples were exposed to HP and unloaded samples were maintained without any load application as described below.
Variation of the Oxygen Environment
Variations of the oxygen level were performed by using an incubator that allowed an automatically controlled adjustment of the oxygen environment via addition of nitrogen gas (HeraCell 240, Heraeus, Hanau, Germany). All oxygen experiments were performed under standard medium conditions (DMEM with 5 mM glucose, pH 7.2, 400 mOsm). After 3 days of pre-culturing at average disc normoxic conditions for the NP (6% oxygen) both loaded samples and the respective unloaded controls of each experimental group were maintained for further 24 h at the required oxygen levels (1%, 6%, or 21% oxygen).
At the end of the 24 h period, equal numbers of beads were transferred into syringes filled with standard medium and transferred into the cylindric tube of our HP apparatus (see below, HP experiments). Parallel cultures that were maintained at unloaded conditions were incubated under the same conditions but without application of HP.
Cell Viability
At the time of sampling each two beads from each experimental group were dissolved in dissolution buffer (5 min in 150 mM NaCl, 28 mM EDTA, pH 8.0) and viability was determined by microscopic observation of cells after staining with trypan blue and the percentage of living cells was calculated.
Mechanical Stimulation by Application of Hydrostatic Pressure
NP cells were pre-cultured at the different environmental conditions as described above. Directly before application of HP, alginate beads containing NP cells were transferred into syringes filled with the respective culture medium (adjusted to the different glucose, osmolarity, or pH conditions as described above); residual air was removed. These syringes were exposed to mechanical stimulation in a custom-made pressure chamber filled with fluid maintained at 37°C, as recently described.14 Intermittent HP was applied for 30 min at a pressure magnitude of 2.5 MPa and a frequency of 0.1 Hz (180 cycles). This pressure regime simulates the upper range of HP that can occur in the disc center during daily activities as shown previously.18, 19 Controls cultured at similar conditions (transfer into syringes with the respective culture medium) were placed in an incubator (37°C) with no additional pressure application. Following mechanical stimulation, alginate beads of stimulated samples and unloaded controls were dissolved in dissolution buffer (5–10 min in 150 mM NaCl, 28 mM EDTA, pH 8.0). The cell suspension was centrifuged and the NP cell pellet was resuspended in RLT-buffer (Qiagen, Hilden, Germany) and frozen at −80°C until RNA isolation and determination of gene expression.
RNA Isolation and Quantitative Real-Time RT-PCR
Cellular responses were examined as previously described.14 Directly after cessation of mechanical stimulation, gene expression of matrix proteins and matrix degrading enzymes was analyzed in relation to the respective unstimulated control cultures by real-time RT-PCR. Briefly, total RNA was prepared, measured, and cDNA synthesis was performed, as described previously.18 Specific primers were designed for the different target genes (see Table 1) based on published gene sequences (NCBI Nucleotide database). Gene expression was determined by real-time RT-PCR using the iCycler analysis system (Biorad, Munich, Germany) with cloned standards for quantification as described.14 Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was chosen as the housekeeping gene after verifying that its expression in stimulated samples and unstimulated control samples differed by only 0.1 in regard to the CT-value (Δct). As we found higher variations in GAPDH expression at different oxygen levels, samples of the oxygen experiments were normalized to the bovine ribosomal housekeeping gene RPL30 to exclude oxygen-influenced variations of GAPDH expression. The amount of the respective amplification product was determined in duplicate measurement of each sample normalized to GAPDH or RPL30 (oxygen experiments). Normalized values of mechanically stimulated cells were compared to the respective unloaded controls.
| Gene | Species | Sequence (forward and reverse primer) | PubMed sequence accession no. |
|---|---|---|---|
| GAPDH | Bovine | 5′-ACC CAG AAG ACT GTG GAT GG-3′ | XM_001252511 |
| 5′-CAA CAG ACA CGT TGG GAG TG-3′ | |||
| RPL30 | Bovine | 5′-AGG AAG GCT CAA CGA GAA CA-3′ | BC102445 |
| 5′-CGA GGA GCA GAA ACC TTC AC-3′ | |||
| Aggrecan | Bovine | 5′-ACA GCG CCT ACC AAG ACA AG-3′ | NM_173981 |
| 5′-ACG ATG CCT TTT ACC ACG AC-3′ | |||
| Collagen-I | Bovine | 5′-TGA GAG AGG GGT TGT TGG AC-3′ | NM_174520 |
| 5′-AGG TTC ACC CTT CAC ACC TG-3′ | |||
| Collagen-II | Bovine | 5′-CCT GTA GGA CCT TTG GGT CA-3′ | X02420 |
| 5′-ATA GCG CCG TTG TGT AGG AC-3′ | |||
| MMP-2 | Bovine | 5′-ACC AGA GCA CCA TTG AGA CC-3′ | NM_174745 |
| 5′-AAC CGT AGC GGA GTC ACA TC-3′ | |||
| MMP-3 | Bovine | 5′-AAT CAG TTC TGG GCC ATC AG-3′ | AF069642 |
| 5′-CTC TGA TTC AAC CCC TGG AA-3′ |
Glycosaminoglycan Assay
In samples that showed a stronger alteration of aggrecan expression, the effect found on gene expression level was confirmed on protein level by using the Blyscan glycosaminoglycan assay (Biocolor, Carrickfergus, UK). Briefly, medium supernatants of alginate beads from parallel cultures that were assayed 48 h after variation of the respective environment condition were analyzed as described by the manufacturer. The standards were diluted with the respective medium to obtain the given concentrations in 100 µl following the manufacturer's protocol. By centrifugation (7,500g, 15 min, 4°C) of the conditioned media via Amicon Ultra centrifugation filter devices (Millipore, Carrigtwohill, Ireland), a 40-fold concentration of the samples was obtained. To each 100 µl sample, 1 ml of Blyscan Dye Reagent was added (final volume: 1.1 ml) and all samples were thoroughly mixed for 30 min. Samples were centrifuged (13,000g, 10 min) and the supernatants removed. The pellets were dissolved in 1 ml of Blyscan Dissociation Reagent and mixed for further 10 min. All steps were performed at room temperature. Two hundred microliters of each sample were added into each well of a 96-well microtiter plate and measured in duplicate by a spectrophotometer at a wavelength of 656 nm. The sample absorption was correlated to the absorption of the defined standard concentrations. The calculated mean glycosaminoglycan (=GAG) concentration of each sample was normalized to live cell number (trypan blue).
Data Evaluation and Statistics
Statistical evaluations were performed on mean values of gene expression, normalized to the housekeeping genes (GAPDH for glucose, osmolarity, and pH experiments, the bovine ribosomal housekeeping gene RPL30 for oxygen experiments). The normalized gene expression values at each altered condition (variation of glucose, osmolarity, oxygen, or pH) were related to the respective estimated standard condition for the NP (Figs. 2a, 3a, 4a, and 5a), which is represented in these graphs by the reference line (“1”). For each condition, the mean ± standard deviations of these ratios from the six to eight repetitions of each experiment with cells from different bovine specimens are shown in the graphs.
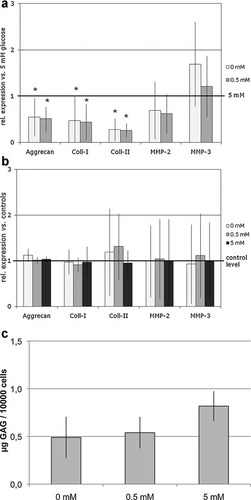
(a) Variation of the glucose environment: Effects on gene expression of bovine NP cells (n = 8) at glucose-reduced conditions (0/0.5 mM glucose) relative to their respective parallel cultures maintained at normal glucose supply (5 mM) represented here by the reference line (“1” = gene expression at 5 mM glucose; *p < 0.05). The graph shows mean ± standard deviations of experiments with NP cells from eight different bovine tail specimens. (b) Influence of hydrostatic pressure (HP) under alteration of the glucose environment: Effects of HP on gene expression of bovine NP cells (n = 6) at three variations of the glucose environment (0/0.5/5 mM glucose) relative to their respective unloaded parallel cultures at each glucose condition represented here by the reference line (“1”). The graph shows mean ± standard deviations of experiments with NP cells from six different bovine tail specimens. (c) Influence of the glucose environment on glycosaminoglycan production by NP cells: GAG content (normalized to cell number) in the concentrated medium samples of NP cells cultured in alginate beads after 48 h exposure to variations of the glucose environment (0/0.5/5 mM glucose). The graph shows mean and standard deviations of three different experiments.
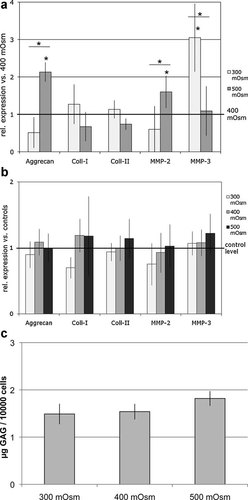
(a) Variation of the osmotic environment: Effects on gene expression of bovine NP cells (n = 8) at hypo-osmotic (300 mOsm) and hyper-osmotic (500 mOsm) conditions relative to their respective parallel cultures maintained at an average normal osmolarity value for the disc center (400 mOsm) represented here by the reference line (“1” = gene expression at 400 mOsm; *p < 0.05). The graph shows mean ± standard deviations of experiments with NP cells from eight different bovine tail specimens. (b) Influence of hydrostatic pressure (HP) under alteration of the osmotic environment: Effects of HP on gene expression of bovine NP cells (n = 6) at three variations of the osmotic environment (300/400/500 mOsm) relative to the respective unloaded parallel cultures at each osmotic condition represented here by the reference line (“1”). The graph shows mean ± standard deviations of experiments with NP cells from six different bovine tail specimens. (c) Influence of the osmotic environment on glycosaminoglycan production by NP cells: GAG content (normalized to cell number) in the concentrated medium samples of unloaded NP cells cultured in alginate beads after 48 h exposure to variations of the osmotic environment (300/400/500). The graph shows mean and standard deviations of three different experiments.
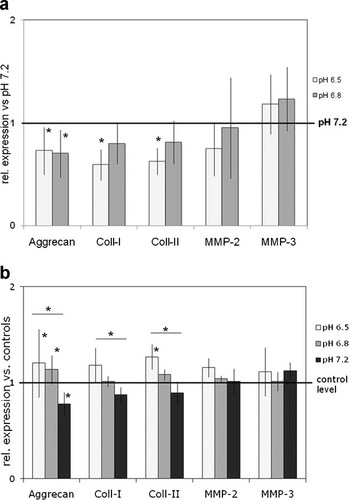
(a) Variation of the pH environment: Effects on gene expression of bovine NP cells (n = 6) at pH-reduced conditions (pH 6.5/6.8) relative to their respective parallel cultures maintained at normal pH 7.2 represented here by the reference line (“1” = gene expression at pH 7.2; *p < 0.05). The graph shows mean ± standard deviations of experiments with NP cells from six different bovine tail specimens. (b) Influence of hydrostatic pressure (HP) under alteration of the pH environment: Effects of HP on gene expression of bovine NP cells (n = 6) at three variations of the pH environment (pH 6.5, 6.8, 7.2) relative to the respective unloaded parallel cultures at each pH condition represented here by the reference line (“1”). The graph shows mean ± standard deviations of experiments with NP cells from six different bovine tail specimens.
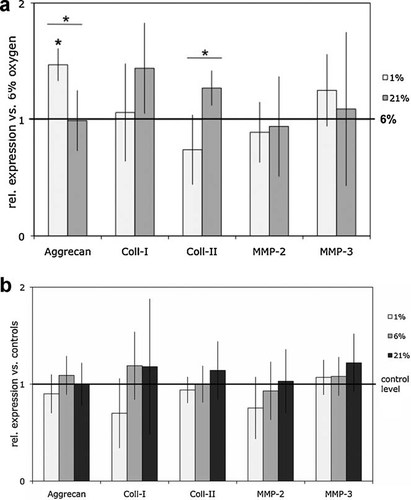
(a) Variation of the oxygen environment: Effect on gene expression of bovine NP cells (n = 6) at hypoxia (1% oxygen) or atmospheric oxygen (21% oxygen environment) relative to their respective parallel cultures maintained at average disc-normoxic conditions (6% oxygen) represented here by the reference line (1 = gene expression at 6% oxygen; *p < 0.05). The graph shows mean ± standard deviations of experiments with NP cells from six different bovine tail specimens. (b) Influence of hydrostatic pressure (HP) under alteration of the oxygen environment: Effects of HP on gene expression of bovine NP cells (n = 6) at three variations of the oxygen environment (1%, 6%, 21% oxygen) relative to the respective unloaded parallel cultures at each oxygen condition represented here by the reference line (“1”). The graph shows mean ± standard deviations of experiments with NP cells from six different bovine tail specimens.
The effects of HP are shown in separate graphs (Figs. 2b, 3b, 4b, and 5b) for each of the three different conditions per test group (e.g., glucose, osmolarity, oxygen, and pH) by determination of the ratio of each loaded sample and the respective unloaded control for each condition (e.g., for osmolarity: (HP-loaded sample at 300 mOsm)/(control sample at 300 mOsm)). The normalized values of the mechanically stimulated cultures were referred to the associated unloaded control samples at the same environmental condition. All figures showing load-induced effects of HP show the means and standard deviations of all replicate experiments with the NP cells from the different specimens; the reference line (“1”) represents the level of the unloaded controls at each experimental condition. A non-parametric Wilcoxon signed-rank test was performed to detect differences between the variables of each test condition and the respective standard condition, and between the HP-stimulated group and associated unloaded control group (null hypothesis = 1 in case of no difference between groups, significance level: p < 0.05). Multiple testing was not considered.
RESULTS
Cell Viability
A microscopic evaluation of cell viability by trypan blue staining showed no effect on viability of NP cells during the 24 h exposure to different environmental conditions (oxygen, osmolarity, pH). There were 85–95% of living cells in all samples with no significant differences between the samples from the different experimental groups. A reduction of glucose supply had some effect on NP cell viability during the 24 h incubation period of these experiments with highest viability at 5 mM glucose and a slight fall in cell viability with fall in glucose concentration. Even in the glucose-free group, cell viability did not fall below about 80% of living cells over the 24 h glucose-free-incubation period. Differences in viability between groups were not significant.
Variation of Glucose
In unloaded control cultures, gene expression results at glucose-reduced conditions (0/0.5 mM) were compared to values of parallel cultures that were maintained at normal glucose supply (5 mM). The variation of glucose supply significantly altered bovine NP cell responses for most target genes. Expression of the anabolic matrix molecules aggrecan (0.55/0.51), collagen-I (0.47/0.44), and collagen-II (0.28/0.26) was significantly down-regulated under reduced glucose supply (p < 0.05, Fig. 2a). This effect was also confirmed at protein level with a decreased GAG concentration in medium samples of NP beads exposed to the glucose-reduced conditions compared to those cultured in 5 mM glucose (Fig. 2c). Furthermore, MMP-2 expression was not significantly altered, whereas gene expression levels of MMP-3 tended to be increased (1.69-fold) at glucose-free conditions. Effects on MMP expression were less uniform with high variations between NP cells from different specimens.
Compared to the effects at unloaded conditions, application of HP did not significantly alter the observed effects with high variability for MMP-2 and MMP-3 expression: in some experiments a decrease, in others an up-regulation of MMP expression by HP could be observed (Fig. 2b). Due to high variability between NP cells from different specimens, no consistent response pattern for these target genes was observed.
Variation of Osmolarity
Alteration of the osmotic environment changed gene expression of aggrecan, MMP-2 and MMP-3 expression with significant differences compared to the average normal osmolarity value (400 mOsm) (Fig. 3a). Aggrecan (2.13-fold) and MMP-2 (1.6-fold) expression was significantly up-regulated at hyper-osmolarity (500 mOsm), MMP-3 expression showed a significant increase at hypo-osmolarity (3-fold). Comparison between groups showed a significant difference between the lowest and highest osmotic value for aggrecan and for MMP expression (p = 0.03). This gene expression result for aggrecan was also confirmed at protein level by a 1.5-fold increase of GAG concentration measured in medium samples from the highest compared to the lowest osmotic condition (Fig. 3c). Compared to the influence of osmotic changes, the effects of mechanical loading were less powerful with only small and non-uniform alterations of gene expression seen for most target genes.
Variation of pH
A reduction of the pH value to the more acidic conditions which might occur during disc degeneration (pH 6.5/6.8) significantly (p = 0.031) decreased gene expression of all matrix proteins in the unloaded control samples of bovine NP cells (Fig. 4a). Aggrecan expression was equally decreased under both pH conditions by (0.73/0.70), respectively. For collagen-I (0.59/0.8) and collagen-II expression (0.63/0.81) the decrease was more pronounced in the unloaded samples at the more acidic pH value (Fig. 4a). MMP expression was not significantly changed by alterations of the pH environment.
Application of mechanical loads altered this gene expression pattern. The down-regulation effect of matrix protein expression was diminished or even inverted by application of HP to pH-reduced cultures compared to the effects found at normal pH value (Fig. 4b) with significant differences between samples maintained at pH 7.2 and 6.5 for aggrecan (p = 0.005), collagen-I (p = 0.01), and collagen-II expression (p = 0.001). MMP-2 and MMP-3 expression was not significantly altered by mechanical loading.
Variation of Oxygen
The effect of oxygen variation on expression of target genes under unloaded conditions was minor and varied from experiment to experiment. Compared to the average normoxic conditions in IVDs (6% oxygen), a reduction of oxygen to 1% pO2 slightly up-regulated aggrecan expression (1.47-fold, p = 0.039) and tended to down-regulate collagen-II expression (Fig. 5a). A significant difference could be observed with regard to both these target genes between cultures maintained at hypoxia and atmospheric oxygen with opposite effects: while aggrecan was increased under hypoxia, collagen-II expression was higher at atmospheric oxygen. Mechanical stimulation by HP failed to alter these responses under any of the different oxygen conditions (Fig. 5b).
DISCUSSION
During disc degeneration, a number of changes that alter the microenvironment of IVD cells occur and interact with each other; these result in part from impaired transport of nutrients and metabolites and hence a reduced glucose supply,21, 22 alterations of oxygen supply and a more acidic pH8, 11, 20, 23, 24 as well as degradation of disc proteoglycans resulting in a reduced tissue osmolarity.25, 26 These changes have been recently reviewed.6, 27-29 In order to determine responses of disc cells to such environmental changes, we followed a standardized experimental protocol to study how these influence matrix gene expression of bovine disc nucleus cells and examined interactions between these signals and HP (Fig. 1). We found in agreement with others, that changes in the physicochemical environment influenced the gene expression pattern of NP cells; a reduced glucose environment, alteration of osmolarity and a more acidic pH had the strongest impact. Expression of aggrecan, collagen-I, and collagen-II was significantly down-regulated after culture at low glucose or acidic pH; whereas MMP-3 was up-regulated though not at levels of significance (Figs. 2a and 4a). Low osmolarity also had adverse effects, significantly reducing expression of aggrecan while stimulating that of MMP-3; by contrast high osmolarity stimulated aggrecan expression and also expression of MMP-2 (Fig. 3a). Oxygen levels also had opposite effects on matrix gene expression as low oxygen stimulated aggrecan expression significantly but collagen-II expression was stimulated in atmospheric oxygen (Fig. 5a). Pressure application appeared to have little effect but, when interactions with other environmental signals were examined, we found that pressure altered or even reversed some responses seen in its absence. In particular, pressure application partially reversed the down-regulation of aggrecan and collagen expression seen at acidic pH (Fig. 4b).
Our results are in agreement with others who have found that hyperosmolarity20, 30, 31 and low oxygen15 each up-regulate aggrecan expression or proteoglycan production by NP cells but have little effect on collagen-I and collagen-II expression.32 However, in a previous study, an up-regulation of collagen-II by hyperosmolarity was reported20 and while we found here that collagen-II expression was greatest in atmospheric oxygen (Fig. 5a) others report that it is greatest at low oxygen.33 Differences in findings might result from differences in culture conditions and time of exposure to the environmental signals but may also result from differences in cell type; in regard to effects of oxygen, our findings come from experiments on mature NP cells cultured in 3-dimensional matrices whereas in other reports oxygen stimulated collagen expression in notochordal NP cells cultured in monolayer.33 Our results also support other reports that low pH16 and glucose8, 11, 24, 34 both suppress expression of all matrix proteins. Thus in general our results and those of others find that conditions seen in more degenerate discs, viz. low glucose, acidic pH and low osmolarity tended to inhibit expression of aggrecan and collagen genes but may stimulate expression of MMP-3, able to degrade matrix proteins and activate other matrix metalloproteinases.35 The decreased matrix expression together with an increased expression of MMPs could contribute to an imbalance in matrix turnover at conditions seen in degenerate discs thus possibly promoting accelerated matrix degradation.
It should be noted, as seen also by others on studies on bovine articular cartilage3, 36 that we found high animal–animal variability in responses to changes in environmental condition. This heterogeneity might be influenced by animal–animal genetic differences and by variations in the cell cultures due to different in vitro expansion times. Although cattle all fell into a relatively narrow age range (18–24 months old) skeletal maturation might not be complete in all specimens. As shown by other authors, maturation influences disc cell responses to externally applied stimulation37 and might be responsible for the observed variations in cell responses of different animals. In addition, we also examined the effects of some of these environmental conditions on disc cells from human donors. We found in general that the pattern of response to these stimuli was similar to that shown here for bovine discs; however, the variability was considerably higher than seen for bovine discs; results suggested that some donors are “high responders” and others “low responders” to the same stimulus (not shown). As availability of normal healthy human disc tissue is very limited for research purposes and disc cell responses in herniated or degenerated disc tissue might also be influenced by the disease, we have focused on bovine disc cells in the present study.
Compared to the effects caused by changes in the physicochemical environment discussed above, application of HP, the main mechanical signal seen by NP cells, had little influence on disc cell gene expression (Figs. 2-5b). Similar levels of HP are, however, reported to stimulate sulfate incorporation (as a measure of proteoglycan synthesis) by NP explants and rabbit NP cells in alginate.38-40 Differences between these reports and our studies may reflect differences in culture systems, cell phenotypes, animal age, and pressure protocol. However, even though in general changes in gene expression were not markedly influenced by HP, pressure inhibited expression of collagen-II at low osmolarity and low oxygen even though not reaching level of significance (Fig. 5b), whereas the fall in matrix gene expression in acidic conditions was significantly diminished or even reversed if the cell cultures were exposed to HP (Fig. 4b).
Little is known about some of the signaling pathways that lead from changes in the physicochemical and mechanical environment to changes in gene expression in disc cells. Most work has been carried out on effects of changes in osmolarity on aggrecan expression. One pathway arises through osmotic stimulation of the osmoregulatory transcription factor TonEPB41 involved in regulation of aggrecan expression. Changes in osmolarity also induce intracellular calcium transients in disc and cartilage cells,42-45 TonEPB activation by intracellular calcium controls transcription of the gene for (beta)1,3-glucuronosyltransferase-I, an enzyme involved in glycosaminoglycan synthesis. Signaling pathways induced by application of HP have also been explored in disc to a limited extent where its effect on matrix synthesis appears to act in part through stimulation of nitric oxide production.46 More is known about effects of HP on articular chondrocytes where it affects activity of membrane transporters involved in maintaining a constant intracellular ionic milieu.47 Of particular relevance to this study is the effect of pressure on the sodium–hydrogen exchanger NHE, the main transporter involved in removing intracellular protons and preventing intracellular acidification.48 Accumulation of protons and consequent intracellular acidification leads to a fall in matrix synthesis.49 NHE is ordinarily only able to maintain a neutral intracellular pH if extracellular pH remains above pH 6.8,49 with further fall in extracellular pH, intracellular pH acidifies leading to a reduction in matrix synthesis and gene expression (Fig. 2a). HP, however, stimulates NHE activity in chondrocytes,50 preventing intracellular acidification even in a relatively acid environment; our results suggest that a similar stimulation of NHE activity by pressure occurs in the disc and is responsible reversing the acid-induced fall in matrix gene expression (Fig. 2b) seen in unpressurized NP cells (Fig. 2a).
Other pathways may involve factors which reduce intracellular ATP as a fall in matrix synthesis by cells of other cartilages correlates with a fall in ATP levels.47 Our results are in line with this finding; low glucose levels both reduced matrix gene expression in results reported here (Fig. 2a) and in other studies14 and would also be expected to reduce intracellular ATP level as disc cells like chondrocytes rely on an adequate supply of glucose to obtain energy via the glycolytic pathway.8, 9 Intracellular ATP also falls with fall in environmental pH and hence reduction on ATP levels may be one of the factors leading to the pH-dependent fall in matrix synthesis rates seen in the disc and in other cartilages13, 49 as well as the fall in matrix gene expression seen here (Fig. 4a). Oxygen is not involved in ATP production by NP cells8, 9 and the factors leading to the increase in aggrecan gene expression seen at low oxygen in the present study (Fig. 5a) and by others15 are unknown. However, as aggrecan inhibits endothelial cell invasion,51 the increase in aggrecan expression under hypoxia would enable the disc to protect itself from blood vessel ingrowth under low oxygen supply via up-regulation of this anti-angiogenic matrix molecule.
In summary, we found that simulation of environmental conditions thought to occur during degeneration appeared to impair matrix formation and support matrix degradation. As environmental conditions not only altered the gene expression pattern of disc cells but also their responses to mechanical load, it is important for future in vitro studies with IVD cells to take the influence of these environmental factors into consideration.
Acknowledgements
We thank Mrs. Daniela Kuschel for excellent technical assistance. This work was supported in part by the German Research Foundation DFG (NE 549/3-1) and the European Community's Seventh Framework Programme (FP7, 2007-2013 under grant agreement no. HEALTH-F2-2008-201626).




