Nanotechnological approaches as a promising way for heavy metal mitigation in an aqueous system
Funding information:
None.
Abstract
The ever-rising environmental problems because of heavy metals emerging from anthropogenic activities pose an impending threat to all biota globally. Considering their persistence and possibility in biomagnification, they are prominent among pollutants. There has been an apparent shift of research interest in advancing cost-effective and competent technologies to mitigate environmental contaminants, specifically heavy metals. In the recent two decades, tailored nanomaterials (NMs), nanoparticles, and NM-based adsorbents have been emerging for removing heavy metal pollution on a sustainable scale, especially the green synthesis of these nanoproducts effective and nonhazardous means. Hence, this review explores the various avenues in nanotechnology, an attempt to gauge nanotechnological approaches to mitigate heavy metals in the aqueous system, especially emphasizing the recent trends and advancements. Inputs on remediating heavy metal in sustainable and environmentally benign aspects recommended future directions to compensate for the voids in this domain have been addressed.
Abbreviations
-
- Ag-NPs
-
- Silver Nanoparticles
-
- Al-NPs
-
- Aluminum Nanoparticles
-
- Al2O3/silica NPs
-
- Aluminum oxide/Silica nanoparticles
-
- CMC
-
- carboxymethyl cellulose
-
- CS
-
- blended chitosan
-
- Fe-Pd-NPs
-
- Iron-palladium-nanoparticles
-
- HAP
-
- Hydroxyapatite
-
- HAP-ALG
-
- HAP-Alginate
-
- HAP-CMC
-
- HAP-carboxymethyl cellulose
-
- HAP-CTS
-
- HAP-nanocomposites with chitosan
-
- HAP-GEL
-
- HAP-Gelatin
-
- kDa
-
- kilo Dalton
-
- MCM
-
- magnetic composite microspheres
-
- MnO2
-
- NPs-Manganese Oxide Nanoparticles
-
- MOF
-
- Metal-organic framework
-
- MWCNT
-
- Multi-walled carbon nanotubes
-
- NMs
-
- nanomaterials
-
- NPs
-
- nanoparticles
-
- OMCs
-
- Organic matter constituents
-
- oMWCNT/PPy
-
- Polypyrrole coated oxidized MWCNTs
-
- PAAs
-
- poly(acrylic acid)
-
- PEI
-
- polyethyleneimine
-
- PMMA
-
- Poly (methyl methacrylate)
-
- PNCs
-
- Polymeric nanocomposite
-
- RO
-
- Reverse osmosis
-
- ROS
-
- Reactive oxygen species
-
- TiO2-NPs
-
- Titanium oxide nanoparticles
-
- UV
-
- Ultra violet
1 INTRODUCTION
Any materials with nanosized dimensions can be considered nanomaterials (NMs), and nowadays, it gains attention to scientists due to their more comprehensive applications. For example, biological NMs consist of interdisciplinary science, including biotechnology, physics, quantum technology, chemistry, material science, and engineering sectors [1, 2]. Similarly, nanobiotechnology gives space to take advantage of the quantum and surface phenomenon that facilitated the industrial processes. Moreover, nanobiotechnology, associated with data science and cognitive skills, has been projected to give new avenues in the upcoming decades in innumerable arenas such as agriculture, environmental, biomedical, military, and various commercial sectors [3].
In recent decades, nanotechnology has gained attention for an all-pervading research scientist in the field of environmental xenobiotics mitigation [4]. Very notably, nanoparticles (NPs) and their application in heavy metal removal. Typically, NMs with sizes ranging from 1–100 nm include nanosized metal-based particles such as zero-valent metal, metal oxides, metal-containing NPs, clay minerals, graphene, activated carbon, and carbon nanotubes [5]. Nanotechnology has gained greater attention in contaminant removal from wastewater, especially heavy metals in the past two decades. In recent years, nanotechnology gives various ideas to identify and generate possible nano adsorbents for environmental applications. For environmental applications, it is essential to identify novel NMs with superior performance. In recent years, emerging science and technology rely on the application of NMs [6]. Notably, environmental appliances of nanosourced constituents have established much interest in the public and gain attention, and it's time to explore sustainability in the remedial process [7]. Few of the characteristics features of NMs, such as enormous specific areas, minor adjustment of temperature, tenable pore size, a shorter space of interparticle diffusion, a large number of associated adsorption sites, and divergent surface chemistry, NMs are widely used as absorbents and catalysts for contaminant removal [8-11].
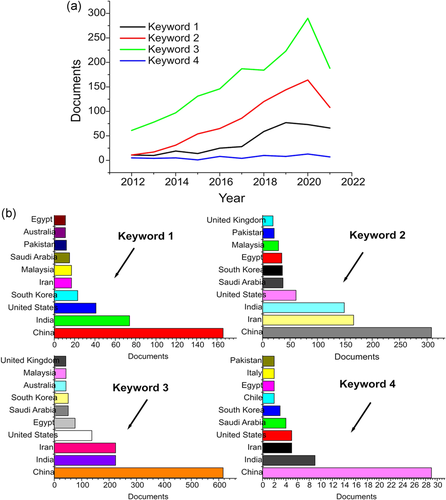
Nanotechnology applications in heavy metal removal have received much attention in recent years worldwide due to their associated environmental problems. Numerous scientific publications have described the impacts of various NMs with diverse remediation techniques. Scopus database analysis for the research output in the keyword “Emerging contaminants” for 2012–2021 is illustrated in Figure 1. The four different keywords are provided as follows “Keyword 1- {nanomaterials} AND {heavy metal} AND {removal}, Keyword 2- {nanocomposite} AND {heavy metal} AND {removal}, Keyword 3- {nanoparticles} AND {heavy metal} AND {removal}, and Keyword 4- {nanostructure} AND {heavy metal} AND {removal}.” The database showed 382, 800, 1585, and 65 documents for the keywords 1–4, respectively. Their year-wise publication is depicted in Figure 1a, and the top 10 countries which hold a significant number of publications are illustrated in Figure 2b. Due to the growing interest and demand for various NMs to expel heavy metal, a systemic review is most required to highlight the NP-metal interactions and issues.
2 NEED FOR HEAVY METAL ELIMINATION
The primary source of heavy metals is mining, electroplating, pesticides, paint, batteries, and ceramics discharge [12]. Due to industrialization and urbanization, a dramatic increase in the widespread of heavy metals. Heavy metals such as chromium (Cr), arsenic (As), cadmium (Cd), copper (Cu), mercury (Hg), lead (Pb), selenium (Se), zinc (Zn), and nickel (Ni) concentration increased over the period. Around 10 million major contaminated sites were identified globally, of which 50% of sites were heavy metals and metalloid contaminated sites [13]. These heavy metals are nonbiodegradable and can amass inside the human body as a toxic or carcinogenic agent, causing severe health issues that are incurable. Researchers have found that metals are the primary cause of diseases in human beings and increased attention is given to identifying and removing the toxic metals from the wastewater. The least the quantity of metals in food, water, and other constituents associated with the human lifestyle, if reduced over a period of time, the more substantial the health improvement on the future generation; hence, the need for mitigatory measures to regulate the presence of heavy metals in the environment.
Heavy metals ions can be removed from wastewater by either physical or chemical methods. On the basis of the concentration of the pollutants, different methods are combined. Traditional methods like the ion exchange method, precipitation method are used to expel heavy metals at the least magnitude, but having disadvantages like high cost, low efficiency, and can cause secondary pollution [14]. Many other methods like sorption, organic systems, chemical precipitation, reverse osmosis, membrane procedures, ultrafiltration, electrochemical procedures, and so forth, are in use. Recently, new technologies have been developed using natural adsorbents for heavy metal removal as they are economical, cost-effective, more efficient, and feasible.
A variety of sorbents are used for metal ion adsorption from the wastewater, and a few of them are categorized as bio sorbents [15], activated carbon, polymeric fibers [16], oxide minerals [17], and resins. Owing to the upsurge in industrialization, many heavy metals are released into the environment and the need to identify cheap, environmentally friendly, and sustainable pollutant removal strategies without impacting the economics [18].
3 FABRICATION OF NPS
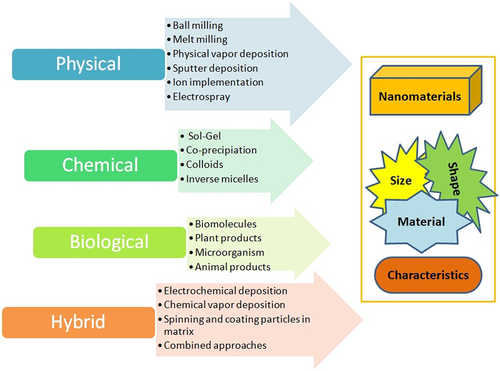
Fabrication of NPs and NMs is much demanded and emerging in areas in nano-based research. The NMs are synthesized via various physical, chemical, biological, hybrid, or combined mechanisms (Figure 2). Among those, biologically mediated synthesis of NPs receives much attention in the recent decade. Green synthesis procedures have successfully fabricated many green NPs. For example, for the fabrication of NPs, bacteria, fungi, yeast, and algae were engaged [19]. Apart from microorganisms, studies revealed that green materials (olive oil, gum acacia, waste coconut coir dust, wood-derived sugar carbon, eggshells, etc.) and support materials (Perlite, cellulose acetate, halloysite nanotubes, polydopamine functionalized graphene, etc.) showed better efficiency when we fabricated with NPs [20]. Microbial products, namely, lactic acid from Lactobacillus and extracellular protein (21 and 24 kDa) from fungus, act as reducing agents and facilitate the synthesis of titanium dioxide (TiO2) and Ag NPs [19, 20].
The selected list of NMs employed for the heavy metal removal and their fabrication process are summarized in Table 1. When contrasting with orthodox NPs, green harnessed NPs are highly susceptible to amalgamating into aggregates attributed to the extended surface energy, relatively strong van der Waals forces than conventional NPs, and magnetic interactions, which always act as hindering factors for mobility and reactivity with target pollutants. Green catalysts and support constituents have been established and extensively used for environmental applications to avoid this scenario. Various studies revealed that green support materials act as a stabilizing agent in NPs synthesis. He and Zhao [21] proposed synthesizing palletized iron (Fe-Pd) NPs by a greener approach. Similarly, Liang and Zhao [22] demonstrated the practice of starch as a stabilizing mediator in magnetite NPs synthesis and their in-situ immobilization of As (V) heavy metal removal in loamy sand soil.
| Sl. no. | Method/process involved | Fabricated NPs/NMs | Heavy metals studied | References |
|---|---|---|---|---|
| 1 | Co-precipitation, reduction, and adsorption | Fe-NPs | Cr (VI) | [23] |
| 2 | Reduction of bioaccumulation study | Citrate-coated silver NPs | As (V) and Cu | [24] |
| 3 | Adsorption | Magnetic inverse spinal iron oxide NPs | Pb (II) and Cr (III) | [25] |
| 4 | Adsorption | Iron-oxide NPs | Cd (II) | [26] |
| 5 | Stabilisation and transformation | Green iron oxide NPs | Arsenate—As (V) and Arsenite—As (III) | [27] |
| 6 | pH-dependent | Iron NPs | Ni | [28] |
| 7 | Adsorption and reduction | Iron NPs | Cr (VI), Cu (II), Pb (II), and Zn (II) | [29] |
| 8 | Reduction and adsorption efficiency decreased by the presence of coexisting anions | FeS- sodium alginate NPs | Se (IV) | [30] |
| 9 | Role of cell surface binding site of yeast, subsequently reduction via FeO/Fe3O4 | FeO/Fe3O4 nanocomposites | Cr (VI) | [31] |
4 NANOMEMBRANES FOR HEAVY METAL REMOVAL
The membrane's process is one of the highly efficient methodologies compared to other physical wastewater treatment methods or water purification. Mainly, membrane technology removes organic and organic pollutants and bioproducts from wastewater [32]. The construction of ultrafine fiber or nanofiber resulting in a membrane for wastewater treatment and contamination removal is one of the numerous types of advancements that have resulted from it. Micro, ultra, and nanofiltration (NF) are now being incorporated. Electrospinning has led to the development of electrospun nanofibers membranes, which have resulted in a cost-effective, highly efficient, and easier approach, and it has now emerged as a newer method of wastewater treatment. Another factor to consider is a higher surface-to-volume ratio, as well as religious nature. It is extremely desirable and seen as something that has the potential to entirely alter a situation because of its ease of manipulation in all areas, including diameter orientation, structural alignment, and composition, by adjusting parameters such as distance, voltage, and concentration [33].
Tight NF membranes with molecular weight cut-offs of about 150–350 Da are typically used for water treatment and recycle; as they have better organic retention and moderate inorganic ion removal than reverse osmosis (RO) membranes and are more energy-efficient [34]. The advancement and alteration of membrane surface chemistry and structure is a productive strategy for improving NF performance for specific feed circumstances by optimizing the chemistry on the surface of membranes [35]. Functionalization via nanocomposite is one way to tune the nanomembrane response and acquire desired physical and chemical properties [36]. Figure 3 shows the four primary ways of functionalizing nanomembranes. In any application, nanomembrane efficiency always relies on molecular structure and weight, pore size, hydrophobicity and hydrophilicity, and polarity. Both nanofiltration and nanomembrane are widely used to treat drinking water and groundwater [37, 38]. Generally, nanomembrane is divided into organic (organic polymer-based membranes) and inorganic nanomembrane [39]. Zeolite, silicon dioxide, two-dimensional graphene-sourced membranes are the best example of inorganic nanomembrane. The organic polymeric membranes are widely made up of natural polymers, such as cellulose acetate [40] and chitosan (CS), whereas synthetic polymeric membranes are made up of polyacrylonitrile, polyamidoamine, polyurethane, polyethersulfone, polysulfone, and poly(vinyl alcohol)/polyamide. As of now, numerous studies revealed that integrating NMs to the organic polymeric or inorganic membrane, namely, hydrophilic metal oxide Al2O3/silica NPs, zeolites, and antimicrobial Ag NPs [40-47].
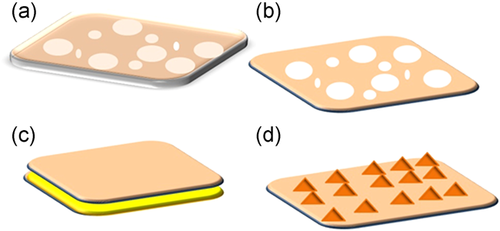
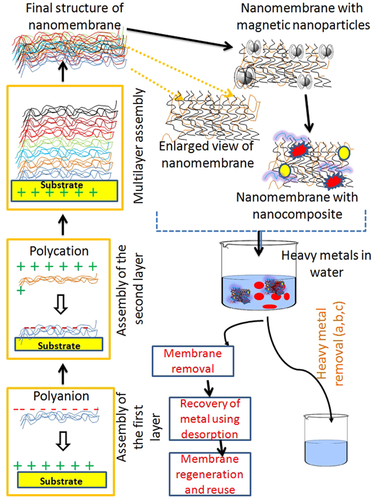
Soyekwo et al. [35] constructed the NF membrane with a selective layer made of an aminophosphonate produced by the KabachnikFields reaction, which aided metal cation integration and resulted in metal–aminophosphonate networks that were firmly fixed on the membrane. The Fe3+ added to the membranes improved electropositivity, resulting in good heavy metal ion removal (over 98%). According to Zeng et al. [48], the NF membrane modified with halloysite nanotubes functionalized with 3-aminopropyltriethoxysilane had removal efficiencies (%) of 47.9, 44.2, and 52.3 for Cu (II), Cd (II), and Cr (VI), respectively. For the elimination of heavy metals, a unique nanocomposite membrane was designed, and its achievement was equated to that of polymeric membranes. While polymeric membranes may reject heavy metals to a degree of 77% to 99%, nanocomposite membranes can reject heavy metals altogether (up to 100%) [49]. A schematic illustration of the layer-by-layer nanomembrane assembly technique and heavy metal expulsion from an aqueous setup is depicted in Figure 4. Integration of organic polymeric nano/membranes with any inorganic NPs always leads to a massive impact on the efficacy of NMs for water purification with functionality was improvised.
5 BIOPOLYMER-NPS COMPOSITES FOR METAL REMEDIATION
In recent years, nanoengineering focus on tailoring the surface structure of NPs or green NPs, with the conjunction with biopolymers such as starch, cellulose, guar gum, and other polysaccharides from a plant source; alginates, galactans, and carrageenan from algal source; and collagen, gelatin, albumin, and proteins from animal-sourced; and polymers such as dextran, gellan gum, pullulan, and polyhydroxybutyrate from microbial origin were studied. Biopolymers are obtained from eco-friendly agro wastes, namely, corn starch, pea starch, vegetable oil, and so forth [50, 51]. These biopolymers are classified based on monomeric units. Namely, (1) polysaccharides, (2) polynucleotides, and (3) polypeptides. These kinds of biopolymeric material are always obtained from renewable, eco-friendly materials, so it always shows great attention to the development of various composites; moreover, it exhibits economically and environmentally friendly ways to get sustainable technology by conjugated polymers. In general, this group of polymers is renewable, nontoxic, economical, and eco-friendly, with these scope and broad advantages, these biopolymers are widely used in various applications which include environmental, biomedical, packaging, pharmaceutical, energy, catalysis, and food industries, and so forth [52-55].
In nanotechnology, nanocomposites and NMs are widely used to minimize the risk associated with environmental pollution, especially wastewater treatment. One of the well know and well-exploited biopolymer, cellulose, used to synthesis cellulose-based NMs such as cellulose nanofibers, nanofibrils, nanocrystals, or nanowires for environmental pollution mitigation, in particular, to remove both organic and inorganic pollutants in aqueous solution by adsorption principle [56, 57]. After cellulose, chitin is considered as another second naturally available polymer. It is a primary polysaccharide, mostly present in the shells of crabs. The difference of chitin with cellulose is the presence of acetamide group rather than hydroxyl group.
Similarly, Lignin, another second most biopolymer next to cellulose and chitin that abundantly available in nature. Recently, it has been contemplated as a substitute for fossil fuel resources, and it showed unique attributes such as antimicrobial action, antioxidative features, with characteristics features namely, low density, good stiffness, elevated carbon quotient, and ultraviolet (UV) radiation fortification [58]. For the synthesis of composite-biomaterials from lignin, Kai et al. [59] harnessed a variety of composite biomaterials by packing lignin in PLA via polymerization of lactide on top of collectively alkylated lignin molecule; similarly, this copolymer system was initially blended with poly(l-lactide) and electrospun and they finally fabricated nanofibers with uniform diameters was achieved. Like cellulose, starch is another most commonly available biodegradable polymer and it is one of the widely available natural polymers next to cellulose [60]. Several literature studies revealed that evidence of various forms of starch, namely, starch crystallites, starch nanocrystals, microcrystalline starch, and hydrolyzed starch, were synthesized from starch [61, 62].
6 REMOVAL MECHANISMS OF HEAVY METAL
6.1 Adsorption of heavy metals
Refinement of wastewater with a metal load of more than 1000 mg/L is done by means of lime precipitation. Precipitation reagents like lime and limestone are mainly used due to their least cost and ease of obtainability in many countries [63]. However, it has a drawback that chemicals are required to decrease the load of the metal in chemical precipitation. This can be overcome by using the adsorption technique which requires no/or much less chemicals for the remove heavy metals. Adsorption is considered one of the alternative treatment methods for the removal of heavy metals. Recently many cheap adsorbents from industrial by-products, agricultural waste, natural materials, or tailored-biopolymers have been deliberate for the elimination of heavy metals from metal-saturated wastewater [64, 65]. Critical factors for selecting the most suitable adsorbents are low cost, and technical applicability and the other advantages are cheap equipment, safe handling, and simplicity. In most studies, adsorption efficacy is always affected by pH, temperature, and original adsorbate dosage under in-vitro studies [66]. The physicochemical properties of NMs have an impact on the heavy metal removal process, and a few of those are given in Table 2.
| Sl. no. | NPs/nanomaterials (NMs) | Physicochemical properties | References |
|---|---|---|---|
| 1 | Nano-composite adsorbent | Highly porous structure and with a high surface area | [67] |
| 2 | TiO2 NPs | Globular shape with particle size approximately 4 nm | [68] |
| 3 | Silica-based nano-conjugate NMs | Mesoporous structure | [69] |
| 4 | Nano zero-valent iron NPs | Tiny particle size with powder state | [3] |
| 5 | Carbon nanotubes | Carbon nanotubes are graphene sheets, and they can be rolled up in cylinders with a diameter of 1 nm, hexagonal shape | [70] |
| 6 | Al2O3 NPs | Mostly rod-shaped, approximately 40 nm in length | [71] |
| 7 | Nano hydroxyapatite | Crystalline structure, hexagonal shape | [72] |
6.2 Reduction of heavy metals by nano-photocatalyst
Many different types of wastewater cleanup procedures were in practice, but photocatalysis remains one of the finest as it may eliminate or diminish the pollutant rather than only moderate, trap, or isolate it. The use of several types of semiconductor materials, such as zinc oxide (ZnO), TiO2, and as a light-responsive material to treat organic-laden wastewater has piqued the interest of researchers. When light is irradiated, these semiconductors collect photons and excite electrons into a higher energy state, forming electron pair holes that are transferred on the semiconductor's surface, resulting in reactive oxygen species (ROS) such as OH● and O2●− [73]. However, the success rate of employing NMs for the photocatalytic removal of heavy metal is not a highly appropriate suggestion due to an inability to degrade metallic ions. Hence, the use of photocatalyst in the removal of heavy metal is rarely researched. However, the NMs as photocatalysts, on the contrary, have shown potential in lowering the adverse effects of heavy metals by converting metal ions into less hazardous by-products. When bombarded with light, these semiconductors can release powerful oxidative free radicals capable of eliminating a wide spectrum of organic pollutants and reducing heavy metal ions. In this aspect, the NMs-assisted photocatalytic approach is suggested to reduce heavy metal contamination and its associated adverse effects [74]. Crystal phase, lattice impurities, crystallinity, surface area, and other factors influence the photocatalytic activity of NMs. The performance of NMs in photocatalytic reduction of heavy metal can be accelerated by implementing some countermeasures such as doping of metals, doping of non-metals, co-doping, and other surface modifications [75]. In most cases, the photocatalytic process is been used as co-reaction along with adsorption of heavy metals [76-79].
7 KEY NPS FOR HEAVY METAL MITIGATION
Metallic salts are employed as catalysts to speed up the oxidation generation process [80]. Among the several metal oxide NPs, TiO2 is widely used due it has a strong ability to break down inorganic pollutants, is photo corrosion resistant, has high thermal stability, and is inexpensive [81, 82]. TiO2 NPs are effective and robust sorbents for the elimination of metallic pollutants. TiO2 NPs are a naturally safe substance with a lot of photocatalytic activity in UV light but very little in visible light. Due to their unique chemical and physical qualities, such as strong oxidizing and reducing ability, high permeability, and exceptional optical/electrical properties, TiO2 NPs have received a lot of attention. It is an excellent semiconductor that is used in several applications in light science. TiO2 NPs may interact with pollutants by altering bioavailability and toxicity. Hence, TiO2 NPs for the sorption of hazardous heavy metals from wastewater have a lot of potential in the future [83]. Various materials were studied to prepare the TiO2 NPs-based adsorbents for heavy metals removal. In addition, TiO2 nanocomposites were synthesized using biological compounds are also been used for heavy metal removal. For example, biocomposite of cyclodextrin-polycaprolactone TiO2 yielded 98% removal of Pb (II) (10 ppm), and its been found nanocomposite surface area was improved by adding TiO2 1.3-fold compared to unmodified composite [81].
Another well-known NPs with high binding energy (60 meV), with unique characteristics, is the ZnO NPs, which serve wide applications. The removal of Cd2+, Cu2+, Ni2+, and Pb2+ from aqueous solutions using ZnO by adsorption in single and multiple component model is assessed by Mahdavi et al. [84] and reported that ZnO NPs are the most promising sorbent due to their high adsorption competence. Wang et al. [85] reported the ZnO nanosheets as better nano adsorbents compared to simple nanopowders due to their special features such as high porosity, huge surface areas, and simple separation from solution as forms micro/nanostructured self-aggregates, and so forth. Various ZnO-based NMs such as pristine ZnO NPs, ZnO nanocomposites, doped ZnO nanostructures, and surface-modified ZnO NPs for the removal of different heavy metal ions (Cd2+, Hg2+, As3+, Pb2+, Cr6+, Ni2+, Co2+, and Cu2+) are extensively assessed by Dhiman and Kondal [86]. The mechanism for adsorptive removal of heavy metal ions (Ag+, Cu2+, Cr6+, Pb2+, Cd2+, Ni2+, and Mn2+) using ZnO NPs, the efficiency of adsorption comparison under irradiation to visible and UV light sources and the metal loaded ZnO characterization after adsorption are reported by Le et al. [87]. They found that the adsorption of metal ions like Mn2+, Cu2+, Ni2+, and Cd2+ was not responsive, while Pb2+, Cr6+, and Ag+ were responsive to the type of light sources with 1 h exposure time. According to Le et al. [81], the ZnO NPs can pursue two possible mechanisms for heavy metal ions removal: (1) Physical adsorption and (2) Reduction/oxidation by photo-generated e−/h+ pairs.
Iron oxide (FeO) NPs received considerable traction amidst metal oxide NPs for their maximal inherent reactivity of the surface domains [88]. Magnetic FeO has recently piqued the interest of scientists and environmentalists owing to their extended range of uses in sectors such as catalysis, pigments, biosensors, magnetic resonance imaging, data storage, photocatalysis, and environmental applications. Because of their usefulness in environmental research, several magnetic iron oxides of various sizes and morphologies have been manufactured in this regard [89, 90]. Among the eight known phases of iron oxides, the most common and prospective magnetic particles for water treatment are Fe3O4 (magnetite), FeO (wustite), α-Fe2O3 (hematite), and γ-Fe2O3 (maghemite). Hematite iron (III) oxide is divided into four phases: α-Fe2O3, β-Fe2O3, γ-Fe2O3, and ε-Fe2O3 being the most stable iron (III) oxides [91, 92]. As nanosized sorbents, iron oxides-based systems are of particular interest because, in addition to having a large surface area, significant adsorption capabilities, and a low cost, they also have magnetic characteristics that make separation and recovery easier. FeO-NPs has employed for removal heavy metal such as Cr6+, As5+, Cd2+, Hg2+, Pb2+, Cu2+, and Cd2+, and so forth, in various studies [93].
In functional NMs, layered graphite-based nanostructures (carbon nanotubes, graphene, and graphene oxide sheets) have been employed as solid support for iron-oxide NPs. The findings in hybrid nanostructures suggested that a synergetic adsorptive effect between carbon-based and iron oxide may be developed that outperforms both of its individual building blocks for water cleanup [94]. Some iron oxides behave like semiconductors, but their employment has some limitations due to quick electron-hole pair recombination and limited separation efficiency. However, it can be altered by tailoring Fe metal-organic framework to enhance photocatalytic degradation of hazardous organic pollutants in water [95].
Silica NPs contain wealthy –Si–OH functional groups and show enhanced catalytic activity based on catalysts (Pt, Pd, Cu, etc.) embedded with the nanostructures [96, 97]. Even this group of NPs can be used as an adsorbent to remove some colored pollutants in wastewater [98]. The NPs possesses many advantages, including it prevents iron oxide aggregation, Improves chemical stability in acid medium, and protects against toxicity, and is negatively charged at mild acid to alkaline pH. The procedure for the modification of the exterior of silica-gels by glycine, diglycine, and triglycine in the company of copper ions is to fashion a novel form of copper-engraved-sorbent, having an elevated adsorptive capability and discernment for Cu-ions, even when other metal ions compete with their load several times elevated than the dosage of Cu.
Recently, Kaur et al. [99] have experienced the CaFe2O4–NGO nanocomposite for the superior elimination of heavy metal ions and photocatalytic degeneration of organic xenobiotics by the combined mechanism of adsorption and photocatalysis. To some extent, photocatalysis is most suited for the photodegradation and photoreduction of effluent infused with heavy metal ions and organic contaminants, hence amplifying its resourcefulness [99]. For example, the electrons excite towards the particle surface during light irradiation of photocatalyst are consumed by the Cr (VI) ions, allowing for a single photoreduction. As a result of the constant existence of an electron-hole pair, the catalyst becomes reactive. To take advantage of this mechanism, researchers are combining Cr (VI) metal ion photoreduction with organic contaminants. Here, the organic pollutant serves as an electron source for the photocatalyst (electron deprived), resulting in a sequential reaction of Cr (VI) photoreduction and organic pollutant degradation [75, 100]. Selected nano and nanocomposite adsorbents tested for heavy metal removal studies are given in Table 3.
| Sl. no. | Nano adsorbents | Heavy metal adsorbed | Removal efficiency (mg/g) | References |
|---|---|---|---|---|
| 1 | Fe/Mn oxy-hydroxide | As (III) | 0.0067 | [101] |
| 2 | Iron oxide/activated carbon | Cr (VI), Cu (II), and Cd (II) | 8.06 for Cr (VI), 3.2 for Cu (II), and 2.15 for Cd (II) | [102] |
| 3 | Magnesium ferrite nanocrystallites | As (V) | 83.20 | [103] |
| 4 | l-Cysteine Functionalized magnetite | Cr (VI) and Pb (II) | 34.5 for Cr (VI) and 18.8 for Pb (II) | [104] |
| 5 | Anatase nanoadsorbent | As (III) | 16.98 | [105] |
| 6. | Magnetite kaolinite nanocomposite | Pb (II), Cu (II), Cr (VI), Cd (II), and Ni (II) | 106 for Pb (II), 98 for Cu (II), 100 for Cr (VI), 97 for Cd (II), and 95.2 for Ni (II) | [106] |
| 7 | Mercapto-functionalized nano-Fe3O4 magnetic polymers | Hg (II) | 140.0 | [107] |
| 8 | ZVI decorated on graphene nanosheets | Cr (VI) | 21.72 | [108] |
| 9 | Ferrite-coated apatite magnetic NMs | Eu (III) | 157.14 | [109] |
| 10 | Graphene oxide (GO)/chitosan aerogel | Pb (II), Cu (II), and Cr (VI) | 747 for Pb (II), 457 for Cu (II), and 292 for Cr (VI) | [110] |
| 11 | Carboxymethyl-b-cyclodextrin Fe3O4 NPs | Cu (II) | 47.20 | [111] |
| 12 | Chitosan/sulfydryl functionalized GO | Cd (II), Pb (II), and Cu (II) | 177 for Cd (II), 447 for Pb (II), and 425 for Cu (II) | [112] |
| 13 | Gum tragacanth/GO | As (I), Cd (II), and Pb (II) | 132 for As (I), 112 for Cd (II), and 142 for Pb (II) | [113] |
| 14 | EDTA-functionalized magnetic chitosan/GO | Cu (II), Pb (II), and As (II) | 207 for Cu (II), 312 for Pb (II), and 45 for As (II) | [114] |
| 15 | Phytic acid-induced graphene | Hg (II) | 361 | [115] |
| 16 | Methyl-β-cyclodextrin modified GO | Pb (II) | 312 | [116] |
| 17 | Magnetic GO grafted polymaleicamide dendrimers | Pb (II) | 181 | [117] |
8 OTHER NOTABLE NMS FOR HEAVY METAL REMOVAL
In the past two decades of NMs and their application in the remediation of heavy metals, many technological methods/processes were developed and implemented to remove carcinogenic metals from various contaminated sites by chemical precipitation [118], ion exchange [119], adsorption [120], membrane filtration [121], electrochemical treatment [122], and so forth. The most common type of NMs employed and the approaches for removing heavy metals from wastewater are summarized in Figure 5.
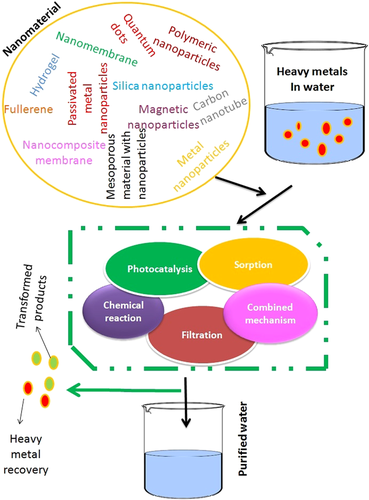
8.1 Carbon nanotubes
Primarily, Carbon nanotubes are divided into two types, (a) single-walled carbon nanotubes and (b) multiwalled carbon nanotubes (MWCNT) [123]. Generally, these carbon nanotubes exhibit high superiority in the removal of heavy metals in wastewater, mainly because of large specific surface area, high adsorption capacity, and fast adsorption kinetics [67]. Several studies revealed that it exhibits excellent adsorption effects towards heavy metals such as Mn (VII), TI (I), Cu (II), Pb (II), and Cr (VI) [124, 125].
8.2 Hydroxyapatite (HAP) NMs
HAP generated from synthetic or biogenic waste resources has shown acceptable physical and chemical properties, allowing it to attach to various contaminants faster and more efficiently. HAP is a biomaterial that is biocompatible and nontoxic in nature, with calcium and phosphorus as its main constituents. HAP is an affordable but effective adsorbent that is widely used for decontaminating wastewater and soils damaged by heavy metals. The nano-HAP is a suitable material for the disposal of contaminants because of its high adsorption capacity for heavy metals [126]. The features of HAP as an advanced adsorbent material were discussed by Nayak et al. [127] in the context of its efficacy in removing metal ion pollutants from not only a single metal ion suspension but also a multimetal ion solution. HAP's great specificity is further shown when it's used as an adsorbent. Fernando et al. [128] used a simple wet chemical in situ precipitation approach to make HAP nanocomposites with CS (HAP-CTS), alginate (HAP-ALG), carboxymethyl cellulose (HAP-CMC), and gelatin (HAP-GEL). The powder form of HAP-CTS is the best sorbent, according to gravity filtration research, with breakthrough capacities of 3000, 3000, 2600, and 2000 ml g−1 for Pb (II), Cd (II), As (V), and F–, respectively. HAP is widely used to remove nickel and lead [129], cadmium [130], copper [131], zinc [132], and arsenic [133], and so forth.
8.3 Other metallic NPs
Silver (Ag) NPs possess catalytic activity and a relatively high adsorption capacity and surface area at their nanoscale [134]. Recently, Verma and Bharadvaja [135] reported the maximum Cr and Cd removal as 47.84% and 5.68%, respectively, in the case of biogenic Ag-NPs. Manganese oxides (MnOs) exhibit high deposition efficiency at their nanoszie than microsize due to the polymorphic assemblies and utmost active sites. Previously, MnO2 has been discouraged for adsorption of heavy metal ions from wastewater, but later growth in nanotechnology has made some improvements in surface modification to create MnO2–NPs as vibrant tools for heavy metal removal [136]. Aluminum (Al)-based NPs are also investigated for heavy metal removal by various studies is reported by Tahir et al. [136]. In their review, it reported that around 99.9% removal of Hg, Ar, and Ti (III) by Al-NPs. A critical review performed by Manyangadze et al. [137], reported the enhancing adsorption capacity of nanoadsorbents via surface modification. Performance was assessed by comparisons on adsorption capacities of differently modified NPs (Si, FeO, and Al2) on different heavy metal ions (As, Cu, Pb, Co, Cr, Zn, Mn, Cd, Ni, and Hg) and found that the modified materials gave superior adsorption capacities.
8.4 Polymeric nanocomposites (PNCs)
PNCs are a type of adsorbent that has a lot of potential for heavy metal extraction because of their unique properties. Because adsorption governs the majority of the removal process, certain parameters in the chemical structure of PNCs, as well as metal solutions, can have a substantial impact on the adsorption process [138]. The outcome of Zhao et al. [139] critically analyzed the applicability of various PNCs for heavy metal removal. Their report includes several PNCs such as polypyrrole (PPy)/polyacrylonitrile core-shell structures for Cr (VI) removal, copolymer composites such as amphiphilic NPs with PMMA cores and PEI shells for the removal of Cu (II) ions, magnetic composite microspheres, consisting of Fe3O4 NPs and poly(acrylic acid)-blended CS for Cu (II) removal, CMC/CS-grafted MWCNTs for uranium [U (VI)] removal, and PPy-coated oxidized MWCNTs were applied to remove Pb (II) and Cu (II). Recently, Azad and Mohsennia [140] prepared the free-standing polyvinyl butyral-polyacrylonitrile/ZnAl-layered double hydroxide nanocomposite membrane for the removal of heavy metals from wastewater.
9 NPS FATE AND TRANSPORT IN AQUATIC SETUPS
It is imperative to deliberate the dynamics for envisaging the fate and transportation conduct of NPs with the impact of the physiochemical progressions in the aquatic setup and its influence on NPs [141]. The NPs-fate-model includes the dynamics of developments, counting homoaggregation, heteroaggregation, dissolution, oxidation, and sulfidation. The precision of estimates of NPs' release and transport relays on the mechanisms that influence the interactions between the heavy metal ions (ligands) and NPs usually culminates as complexation reactions, impacting their amenability to conventional wastewater treatment strategies; the nature of complexes also affects NPs attenuation, ultimately their fate and toxicity. The NPs and their transformed residues could impede microbial growth via cell membranes disruptions, protein-oxidation, disruption of energy transduction, generation of ROS. Colloidal constancy stimulates NPs performance in the aqueous setups, owing to NPs inherent attribute to counterattack aggregation for an elevated time. Analogous NPs interact and homoaggregate, while the presence of elevated levels of organic molecules endorses heteroaggregation; these processes also continue during the transport of NPs, exerting its impact on the NPs fate. The transportation of NPs encompasses collision amidst them, a step accelerating the accumulation [142].
Brownian-motion, fluid-motion, and differential sedimentation can stimulate NPs fate and transport. As NPs transpor1table in aqueous setups via Brownian movement, solid substratum capture NPs and disturb their travels, triggering collision and sedimentation. The exteriors of charged particles networking can culminate in charge neutralization or reversal. Capping agents like polymers get committed to NPs surfaces decelerating aggregation. Changes in pH, ionic forte, and magnitudes can alter the attributes impacting the NPs complete surface charge. Organic matter constituents (OMCs) in the aqueous settings can disturb the charge as the exchanges with OMCs induce steric or electrostatic stabilization. As the NPs interrelate with xenobiotics in the ecosystem, the attributes of the NPs linger to transform. The prime physicochemical feature of NPs that influence their conduct in aquatic setups encompasses composition, mass, particle quantum and dosage, surface area volume, size dispersal, specific surface area, zeta potential, and dissolution. The environmental circumstances of aqueous setups also impact the performance of NPs. The ionic strength, pH, the concentration of OMCs occurring in the aquatic environment, ambient temperature, flow-velocity, and other constraints are all integral that regulate the fate and transport of NPs. Even though an array of attributes impacts the fate and conduct of NPs in the aqueous ecosystem, NPs are chiefly influenced by their size; owing to their minuscule size, the NPs are more widespread and reactive; enormously impeding to detect and quantify NPs. Hence, both the surface attributes of NPs and aqueous environmental constraints will regulate their fate and transport [143-148]. Table 4 elucidated the fate and conduct of NPs in aquatic setup [141].
| Mode of alteration progression | Consequences of transformation | |
|---|---|---|
| Chemical and photochemical processes | Photochemical responses | NPs surface coatings and surface assets changes vary based on the photochemical reactions; impact the aggregation, agglomeration of NPs. |
| Redox responses | NPs endure oxidation or reduction; Ecological parameters play a crucial part in determining the extent of the outcome. | |
| Dissolution/speciation | NPs dissolve; dissolution be contingent on particular NP and the water parameters. | |
| Physical processes | Aggregation/agglomeration | Aggregation happens with strong chemical bonds, or electrostatic interactions trigger clustering (permanent). |
| Agglomeration happens under weak van der Waals forces triggering clustering (alterable) and reliant on water parameters. | ||
| Sedimentation/deposition | NPs agglomerate settle is impacted by NPs attributes and limited by reaction rate. | |
| Interactions with surfaces/substances | Organic matter constituents adsorption | Fixes to the surface of NPs, shifting the attributes and behavior of the NP in aqueous setups. |
| Other substratum sorption | NPs attach to another substratum (a rare and extreme type of hetero-aggregation). | |
It is advisable to choose appropriate NPs synthesis process by weighing pros and cons, altogether with above-discussed features of specific types of NPs-sourced NMs for superior expulsion of a specific or broad range of heavy metals in the aqueous setup to limit operational cost and also for scaled-up expulsions of metals from the aqueous environment (Table 5).
| Preparation approaches | Merits | Demerits |
|---|---|---|
| Solvothermal/hydrothermal combination | [i] NPs with superior purity and minimal flaws | [i] Extensive time consumption |
| [ii] Effortlessly amendable assembly | [ii] Elevated energy requirements | |
| [iii] Simplest maneuver | [iii] Possibility of solvent explosion | |
| Sono-chemical approach | [i] Homogeneous crystal nucleation | [i] Unbalanced reactions |
| [ii] Rapid process | [ii] Higher instrumentation requirements | |
| [iii] Least appropriate for large-scale making | ||
| Mechanochemical approach | [i] Suitable for large-scale making | [i] Distorted or malformed crystals |
| [ii] Least consumption of consumables | [ii] uneven NP size | |
| [ii] Energy conservative and competent to work with | [iii] Maximal impure outputs | |
| Microfluidic approach | [i] Process reproducibility | [i] Highest operational cost among methods |
| [ii] Unremitting preparation | [ii] heterogeneously irregular size of NPs | |
| [iii] Maximal productivity | ||
| [iv] Scaled-up processing | ||
| Dry-gel conversion approach | [i] least precursors generated | [i] Least output |
| [ii] Compact reactors | [ii] Maximal process period | |
| [iii] Continuous production | [iii] Maximal energy consumption | |
| Electrochemical approach | [i] NPs with maximal purity with least byproducts | [i] Requirement for complex apparatus |
| [ii] Conducive experimental settings | [ii] Uneven NPs | |
| [iii] Lest costly instruments | [iii] Difficult to extract NPs | |
| Microwave approach | [i] NPs with utmost dispersibility and uniformity | [i] Difficult to extract NPs |
| [ii] Rapid process | [ii] Unbalanced setup | |
| [iii] Maximal harvest | [iii] unsuitable for large-scale making | |
| [iv] Poor reproducibility |
10 CLUSTER ANALYSIS OF LITERATURE FOR THEORETICAL MAP
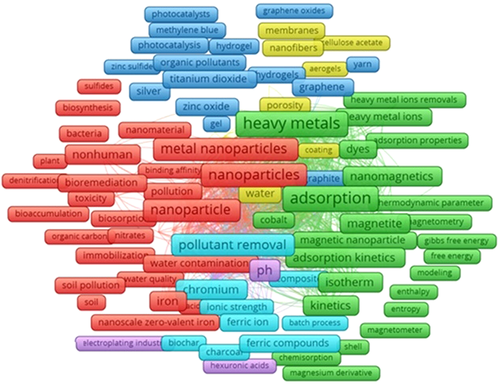
The mechanism of NMs remediation of heavy metals in an aqueous system, especially in actual wastewater, is more intricate. Heavy metal pollution in water is a global problem, hence, strengthening water remediation theory and technology research is vital to protecting the water streams. Therefore, based on the relevant literature on NMs-assisted removal of heavy metal research in the Scopus database, the scientific knowledge map can identify the research topics that match the keywords. The map can better help researchers quickly understand the topic structure and research topics from large-scale scientific literature in the field of a specific keyword. The thematic map (Figure 6) was generated by cluster analysis using VOSviewer version 16.16 for the document information obtained from 382 articles found in the Scopus database to identify the most interacted keywords and technologies (Keywords provided as “TITLE-ABS-KEY {nanocomposites} AND {heavy metal} AND {removal}”). The thematic map indicates the incidence of terms such as and NPs, nanomagnetic, adsorption, isotherms, thermodynamic parameter, membranes, polymers, and so forth. The generated knowledge map highlights several factors, and its network association would substantially impact the efficiency of NPs-mediated removal of heavy metal for environmental restoration. However, several key factors have been reported, and the heavy metal removal process is mainly associated with the four major groups such as type of NMs employed and their characteristics, removal mechanism, heavy metal type and characteristics, and operational conditions (pH, temperature, salinity, acidity, etc.).
11 SUMMARY
Nanotechnology has advanced significantly in recent years, and this advancement facilitates novel research, especially in the mitigation of toxic heavy metals from the contaminated sites or form wastewaters are remediated or eradicated via suitable NPs or NMs. Biological routes are considered environmentally benign for the synthesis of NMs and play a prominent role in the remediation of these heavy metals. Transformation of the attributes and functional optimization in nanotechnology has been fruitful for the nanoremediation of heavy metals. The effective mode of harnessing NMs requires specific consideration, especially to advance innovative NMs to handle explicit heavy metal. In this review, the use of nanotechnology for the mitigation of xenobiotics, especially heavy metals been summarized, contrasted, and explored. NMs mediated expulsion of heavy metals from water and wastewater has been extensively explored but primarily at the bench-scale level. Considering the economics and health risk issues remain critical bottleneck problems impeding large-scale NPs application. However, specific and futuristic research should consider and evaluate simulation models for more extensive-scale operations. The assimilation of multicomponent NPs and NMs, integrated with agricultural wastes-residue incorporated as tailored NM composites, would be the future, especially, in the domain of mitigation of heavy metals.
ACKNOWLEDGMENT
Authors would like to acknowledge their affiliated institutions for the support.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




