Effect of adding amino acids residues in N- and C-terminus of Vip3Aa16 (L121I) toxin
Abstract
To study the importance of N- and C-terminus of Bacillus thuringiensis Vip3Aa16 (L121I) toxin (88 kDa), a number of mutants were generated. The addition of two (2R: RS) or eleven (11R: RSRPGHHHHHH) amino acid residues at the Vip3Aa16 (L121I) C-terminus allowed to an unappropriated folding illustrated by the abundant presence of the 62 kDa proteolytic form. The produced Vip3Aa16 (L121I) full length form was less detected when increasing the number of amino acids residues in the C-terminus. Bioassays demonstrated that the growth of the lepidopteran Ephestia kuehniella was slightly affected by Vip3Aa16 (L121I)-2R and not affected by Vip3Aa16 (L121I)-11R. Additionally, the fusion at the Vip3Aa16 (L121I) N-terminus of 39 amino acids harboring the E. coli OmpA leader peptide and the His-tag sequence allowed to the increase of protease sensitivity of Vip3Aa16 (L121I) full length form, as only the 62 kDa proteolysis form was detected. Remarkably, this fused protein produced in Escherichia coli (E. coli) was biologically inactive toward Ephestia kuehniella larvae. Thus, the N-terminus of the protein is required to the accomplishment of the insecticidal activity of Vip3 proteins. This report serves as guideline for the study of Vip3Aa16 (L121I) protein stability and activity.
Introduction
Vegetative insecticidal proteins (Vips) are an insecticidal toxins isolated from B. thuringiensis (Bacillus thuringiensis) and produced during the vegetative growth stage starting at mid-log phase until the beginning of sporulation 1-3. Vip proteins have been classified into four groups according to their sequence homology: Vip1, Vip2, Vip3, and Vip4 4. Vip1 and Vip2 function as a binary toxin toxic to coleopteran insect larvae such as Diabrotica virgifera virgifera 5-7. However, the insecticidal proteins Vip3 target the lepidopteran insect larvae including Agrotis ipsilon, Spodoptera exigua, Chilo partellus, Helicoverpa punctigera, and Diatraea saccharalis 1, 2, 8-12. Vip3 proteins are highly conserved and have been identified from different B. thuringiensis strains 4, 13-15. It was postulated that their C-terminus are divergent contrarily to their N-terminus which are more conserved 16. Upon ingestion by susceptible insect larvae, Vip3A protoxin is processed by midgut proteases into the active toxin of about 62 kDa molecular weight. This toxin binds to specific receptors in the midgut membrane, allowing to the disruption of the epithelial cells by the formation of pores in the apical membrane and then to the larvae death 17-19. The Vip3 protein sequences do not exhibit any similarity with the Cry proteins considered as the first generation of insecticidal proteins and some differences were found when comparing their action modes. Hence, toxicity symptoms provoked by ingestion of Vip3 were delayed by 36–48 h compared with those of Cry proteins toward Agrotis ipsilon 20 and Vip3 has at least 260-fold higher toxicity against Agrotis ipsilon than some Cry1A proteins 21. Additionally, the Vip3 toxin bound to different receptors in brush border membrane vesicles (BBMV) in comparison with Cry1A protein 22. All these factors emphasized the possible benefit of Vip3 use in resistance management strategies instead Cry proteins, since Vip3 could be useful as a biological control agent against resistant or insensitive pests 23.
Few studies were reported on the assessment of effects due to the adding of amino acids residues in Vip3 protein extremities. During this study, some mutants of vip3Aa16 (L121I) gene were constructed and the stability of the heterologously produced proteins was analysed. Bioassays against the second instar larvae of Ephestia kuehniella were performed to evaluate the impact of the protein modifications on the Vip3Aa16 (L121I) toxicity.
Materials and methods
Bacterial strains, growth conditions, and DNA manipulations
E. coli strain Top10 (Invitrogen, USA) was used as the cloning host. It was grown at 37 °C in Luria-Bertani (LB) medium 24. B. thuringiensis strains S1/4 3 and BUPM95 25 were cultivated in LB medium at 30 °C and their plasmid DNAs were extracted by alkaline lyses in order to PCR amplify the vip3Aa16 (L121I) (Genbank accession no.: JQ768411) and vip3Aa16 (Genbank accession n°: AY739665) genes, respectively. PCR assays were carried out in a reaction mixture including 50 mM KCl, 10 mM Tris-HCl, 2.5 mM MgCl2, 200 μM of each dNTP, 1 μM of each primer, DNA, 1.25 U high-fidelity Pfu DNA polymerase (Amersham). Thermal cycler conditions consisted of a denaturing step at 94 °C for 2 min, followed by 28 cycles constituted of denaturing at 94 °C for 50 s, annealing at 48 °C for 45 s and extension at 72 °C for 90 s. The pGEM-T easy (Promega) and pKJ3 26 plasmids were used for DNA cloning. E. coli plasmid DNA was extracted by the alkaline lyses procedure 24.
Cloning of vip3Aa16 (L121I) and vip3Aa16 genes
The sequence of the open reading frame corresponding to the vip3Aa16 (L121I) differs from vip3Aa16 by two substitutions (143: C->A) (codon CGG: Arginine in the place of AGG: Arginine) and (361: A->C) (codon ATT: Isoleucin 121 in the place of Leucin 121 CTT). In order to study the effect of I121L substitution, vip3Aa16 and vip3Aa16 (L121I) open reading frame sequences were cloned in pGEMT-easy vector with PS12 and vip13 primers (Table 1). The obtained plasmids were called pG-vip3Aa16 (L121I) and pG-vip3Aa16.
| Vip3Aa16(L121I) primers | Sequences (5′->3′) |
|---|---|
| PS12 | CTAGTATCGATTAGCTGAAAAGGAAGGTCGACATGAACAAGAATAATACTAAATT |
| vip13 | ATTTGGAAAGATATAAAACTTATAA |
| PS14 | ATACTGAATTCCCCGGGAACTTAAGATCTCTTAATAGAGACATCGTAAAAATG |
| PJ3R | ACTGTCGCATGCGAATTCTTAGTGATGATGATGATGATGCCCGGGTCTAGATCTC TTAATAGAGACATCGTAAAAATG |
| PJSiD1 | CCATCATGGATCCGGTACCCTCGAGGAGCTCATGAACAAGAATAATACTAAATTAAG |
| PJSiD2 | ACCGTAGCGCAGGCCATGCCATGGATGCAT CAC CAT CACCATCATGGATCC GGTACC |
| PJSiD3 | AGCTATCGCGATTGCAGTGGCACTGGCTGGTTTCGCTACCGTAGCGCAGGCCATG |
| PJSiD4 | GAATTCATTAAAGAGGAGAAAAGATCTATGAAAAAGACAGCTATCGCGATTGCAGTG |
| PJ3Ro | ACCCGGGGTTACTTAATAGAGACATCGTAAA |
Construction of vip3Aa16 (L121I) mutant genes in C-terminus
Two amino acid residues were added to the C-terminus of vip3Aa16 (L121I) gene via PCR using DNA from the B. thuringiensis S1/4 strain as template and primers PS12 and PS14, where PS14 adds the R and S residues (Table 1). The resulted plasmid was named pG- vip3Aa16 (L121I)-2R (Table 2). On the other hand, a sequence of 11 amino acids containing the 6 His-tag was also added in the C-terminus extremity via PCR using (PS12, PJ3R) primers. The new plasmid was called pG-vip3Aa16 (L121I)-11R (Table 2).
| Plasmids | Size (kDa) | Added Aa residues | References |
|---|---|---|---|
| pGEM-T | 3 | Promega | |
| pG-vip3Aa16 (L121I) | 5.4 | This study | |
| pG-vip3Aa16 | 5.4 | This study | |
| pG vip3Aa16 (L121I)-2R | 5.4 | RS | This study |
| pG-vip3Aa16 (L121I)-11R | 5.43 | RSRPGHHHHHH | This study |
| pG-6His-tag-vip3Aa16 (L121I) | 5.4 | MRGSHHHHHHGSGTLEELMNK NNTKLA | This study |
| pG-vip3Aa16(L121I)-39R | 5.5 | MKKTAIAIAVALAGFATVAQAMPW MHHHHHHGSGTLEELMNKNNTKLA | This study |
| pKJ3 | 4.1 | 26 | |
| pK-6His-tag-vip3Aa16 (L121I) | 6.5 | MRGSHHHHHHGSGTLEELMNKNNTKLA | This study |
| pK-vip3Aa16(L121I)-39R | 6.6 | MKKTAIAIAVALAGFATVAQAMPW MHHHHHHGSGTLEELMNKNNTKLA | This study |
Construction of vip3Aa16 (L121I) mutant genes in N-terminus
A number of 39 amino acids residues were added in N-terminus comprising the 6 His-tag sequence and the OmpA leader peptide. Firstly, the vip3Aa16 (L121I) gene was amplified using the primers (PJSiD1, PJ3Ro) followed with a second PCR with the primers (PJSiD2, PJ3Ro) to add a sequence of 27 amino acids residues containing the 6 His-tag (Table 1). This fragment was further cloned in pGEMT-easy and PKJ3 plasmids to obtain pG-6His-tag- vip3Aa16(L121I) and pK-6His-tag-vip3Aa16(L121I) plasmids, respectively (Table 2). The pK-6His-tag-vip3Aa16(L121I) was used to verify the expression of the new fused protein, however, the pG-6His-tag-vip3Aa16(L121I) plasmid was subjected to two more PCR amplifications with (PJSiD3, PJ3Ro) followed by (PJSiD4, PJ3Ro) to completely introducing the sequence which includes the OmpA leader peptide of 21 amino acids residues corresponding to the signal sequence of E.coli 27 (Table 1). The resulted fused sequence was cloned in pGEMT-easy vector to obtain pG-vip3Aa16(L121I)-39R plasmid and next to pKJ3 vector via a double digestion with EcoRI and XmaI restriction enzymes to obtain the pK-vip3Aa16(L121I)-39R plasmid (Table 2).
Protein analysis
Protein extracts were suspended in Laemmli sample buffer and boiled for 5 min. They were analyzed by SDS–PAGE on a 9% polyacrylamide separating gel as described by Laemmli 28 and stained using Coomassie blue or transferred to nitrocellulose membranes, and finally probed with rabbit polyclonal antibody raised against Vip3A proteins. Horseradish peroxidase-labelled goat anti-rabbit was used as secondary antibody to visualize Vip3A proteins by enhanced chemiluminescence using ECL+ kit (Amersham Biosciences, France).
Insecticidal bioassays
Oral toxicity assays against E. kuehniella were carried out using 700 μl of the sonicated recombinant E. coli cells mixed with 1 g of wheat semolina, placed in a Petri dish, dried at room temperature and then ground. Ten second-instar larvae were added to each dish and were incubated at 25 °C. The experiment was replicated four times. For these tests, the larval weights were recorded periodically by using a suitable microbalance. Statistical analyses were carried out using Excel software to calculate the mean values of four experiments and their standard deviations.
Results
Impact of I121L substitution on the expression of Vip3Aa16 (L121I) and Vip3Aa16 proteins
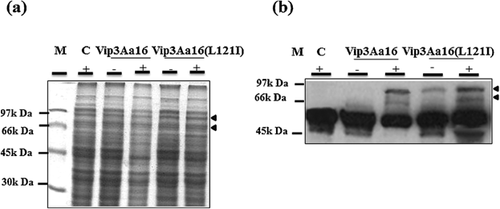
The pG-vip3Aa16 (L121I) and pG-vip3Aa16 plasmids were expressed in E. coli. Western blot analysis revealed the presence of Vip3Aa16 (L121I) and Vip3Aa16 proteins displaying a molecular weight of about 88 kDa. The two proteins showed the same level of expression, suggesting that the I121L substitution did not affect their expression (Fig. 1).
Effects of R, S amino acids and 6His-tag sequence additions in the C-terminus on the expression of Vip3Aa16 (L121I)
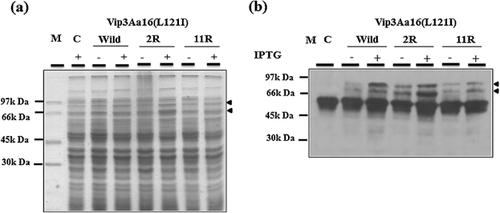
Synthesis of Vip3Aa16 (L121I)-2R and Vip3Aa16 (L121I)-11R proteins in E. coli was done for the purpose of studying the impact of the additions on their production and stability. The recombinant strains Top10(pG-vip3Aa16 (L121I)-2R) and Top10(pG-vip3Aa16 (L121I)-11R), together with the positive control Top10(pG-vip3Aa16 (L121I) and the negative control Top10(pGEM-T) were grown in liquid LB medium, induced with IPTG, and analyzed by SDS–PAGE and Western blot using rabbit antibodies raised against Vip3A protein. Addition of an Arginine and Serine amino acids in the C-terminus of Vip3Aa16 (L121I) leads to a clear unappropriated folding illustrated by the abundant presence of the proteolytic form of Vip3Aa16 (L121I) (62 kDa) and the decrease of the 88 kDa form comparing with the wild type protein (Fig. 2). Moreover, adding of (11R) amino acids including an arginine, serine, arginine, proline, Gglycine, and 6His-tag caused a reduction of Vip3Aa16 (L121I)-11R full length form production as well as the proteolytic form comparing with Vip3Aa16 (L121I)-2R and the wild type Vip3Aa16 (L121I) proteins (Fig. 2). Production of the full length form of Vip3Aa16 (L121I) decrease by the addition of increase number of amino acids in the C-terminus.
Impact of the fusion of Vip3Aa16 (L121I) protein with the 6 His-tag and the E. coli OmpA leader peptide in N-terminus
For the purpose of redirecting the synthesis of Vip3Aa16 (L121I) protein into the periplasmic space of E. coli followed by its purification via the 6 His-tag, we fused its open reading frame (ORF) with the OmpA leader peptide corresponding to the signal sequence of E. coli and the 6His-tag sequence. Initially, impact of the 6His-tag addition in N-terminus was studied. SDS–PAGE analysis showed that the full length form of the 6His-tag-Vip3Aa16 (L121I) protein was detected from pK-6His-tag-Vip3Aa16 (L121I) comparing with the negative control, concluding that this addition did not affect the expression of the protein (Fig. 3).
Secondly, cultures and the IPTG inductions of Top10(pG-vip3Aa16(L121I)-39R) and Top10(pGEM-T) were done followed by analysis of the pellets. Interestingly, Vip3Aa16 (L121I)-39R full length form was not detected with SDS–PAGE and Western blot (Fig. 4A and B). However, a 62 kDa proteolysis form was clearly perceived. On the other hand, in order to try the improvement of the production level of this fusion, the corresponding ORF was cloned in the pKJ3 plasmid containing a more efficient expression system. After culturing Top10(pKJ3), and Top10(pK-vip3Aa16 (L121I)-39R) recombinant strains, pellets were recuperated and analyzed by SDS–PAGE and Western blot. Figure 4C and D revealed only the presence of the 62 kDa proteolysis form in the two tested recombinant clones comparing with the negative form. Besides, examination of supernatants confirmed the absence of the full length and proteolysis forms of Vip3Aa16 (L121I)-39R.
Oral toxicity of the modified vip3Aa16 (L121I) genes against E. kuehniella
Toxicity of Vip3Aa16 (L121I) protein produced from all constructions was evaluated against the second-instar larvae of E. kuehniella. Growth curves revealed that the sonicated Top10(pGEM-T) and Top10(pKJ3) strains used as negative controls, were non-toxic toward E. kuehniella since the growth of the larvae was not affected. A remarkable loss of weight was noted using the sonicated Top10(pG-vip3Aa16 (L121I)) and Top10(pG-vip3Aa16). There was no difference between Vip3Aa16 (L121I) and Vip3Aa16 proteins toxicities (Fig. 5A). Furthermore, it was demonstrated that E. kuehniella growth was little affected by the sonicated Top10(pG-vip3Aa16 (L121I)-11R) and not affected by Top10(pG-vip3Aa16 (L121I)-2R) comparing with the control (Fig. 5B). On the other hand, when testing the Top10(pG-vip3Aa16(L121I)-39R), the same gain of weight was found as the control Top10(pGEM-T) showing the total loss of activity of Vip3Aa16 (L121I)-39R (Fig. 5C). Same results were found when testing Top10(pKJ3) and Top10(pK-vip3Aa16 (L121I)-39R) recombinant strains (Fig. 5D).
Discussion
Protein engineering has been successfully used to elucidate the role of some amino acids residues in the structure stability of Cry toxins and to generate improved B. thuringiensis δ-endotoxins with higher and broader insecticidal activity 29-31. Here, we reported on Vip3 protein stability and toxicity by creating mutant proteins.
This study suggests that the Vip3 C-terminus is critical to its stability and insecticidal activity, since the addition of the residus (R, S) and (RSRPGHHHHHH) provoked the abundant presence of the proteolytic form traduced by the decrease of toxicity toward E. kuehniella. This might be explained by the vulnerability of the C-terminus modified proteins to the proteases. In fact, the alignment of 16 Vip3 sequences showed that their C-terminus sequences were less conservative than their N-terminus sequences, suggesting an accumulation of many mutations during their evolution 16. In this report, we suggest that the occurrence of C-terminus mutations might be the result of the spectrum diversification but not a randomly accumulation owing to the tolerance of this terminus, since the addition of 2R and 11R amino acid residues in C-terminus resulted the substantially decrease of toxicity. Besides, we concluded that the stability of Vip3 decreased when adding more amino acids in its C-terminus. Likewise, Li et al. 32 demonstrated that the deletion of the last three amino acid residues (SIK) or the addition of eight residues (LQHHHHHH) at the C-terminus of the fused protein Vip3AcAa abolished totally the insecticidal activity. They also showed that the substitution of the last two residues from IK to LG or LR conducted to the total loss or to the increase of its activity against the lepidopteran Helicoverpa armigera and Spodoptera exigua, respectively.
The Vip3Aa16 (L121I) N-terminus was also investigated. Since Vip3 protein was secreted in culture medium by B. thuringiensis, many manipulations were made in order to produce higher quantities of the protein and to facilitate its purification. So, we tried to heterologously produce a chimeric Vip3Aa16 (L121I) protein containing a supplementary 39 amino acids, harbouring the OmpA leader peptide and a 6His-tag at its N-terminus. In fact, OmpA leader peptide was used in many reports to redirect expression into periplasmic space. In fact, to solve the problems of insolubility and the weakness amounts of the deglycosidase enzyme production, Loo et al. 33 expressed the corresponding ORF as a fusion with the OmpA leader sequence, and a hexahistidine-tag sequence. The fused protein was efficiently processed, exported to the E. coli periplasm, and easily purified. Surprisingly, addition of OmpA leader sequence in our case allowed to the total proteolysis of Vip3Aa16 (L121I) full length form protein and only to the production of the 62 kDa form. Toxicity bioassays demonstrated that this 62 kDa proteolysis form was inactive toward E. kuehniella, although that it was admitted that inside the midgut epithelium of susceptible larvae, Vip3 protein is proteolyzed into the active form of about 62 kDa, binds to specific receptors, and forms pores leading to the larvae death 18. This suggests that the protein was not correctly folded into a functional insecticidal protein, proposing that the N-terminus is required for the correct folding of the Vip3 protein even though that it was removed and not required for Vip3 toxicity in the midgut of susceptible larvae.
In this context, many reports described the impacts of N-terminus modifications on larvae toxicities. For example, Chen et al. 34 showed that the activity toward first 27 amino acids at the N- terminus of Vip3A-S184 corresponding to its signal peptide were essential for the insecticidal activity toward Spodoptera exigua, Helicoverpa armigera, and S. litura. Contrarily, the truncated Vip3Aa14 protein (devoid of its signal peptide) did not lose its Spodoptera litura and Plutella xylostella 14. The deletion of 39 amino acids from the N- terminus of Vip3 protein did not alter its toxicity against Chilo partellus larvae, however, a remarkable reduction in its toxicity was observed against Spodoptera litura larvae 8. On the other hand, the I121L mutation existing in Vip3Aa16 (L121I) seems to be without effect on the protein stability comparing to Vip3Aa16. This could be explained by the localization of this modification in the first 200 amino acids of N-terminus which will be discarded after the protein activation, even though that it was demonstrated that this terminus was necessary to the right folding of Vip3 proteins.
The correctly folded Vip3 proteins should form a strongly structured protease resistant core, while modifications at the C- or N-terminus caused the disruption of their three dimensional structures and making them susceptible for proteases and less toxic to larvae.
Acknowledgments
This work was supported by grants from the Ministry of Higher Education, Scientific Research and Information Technology, and Communication, Tunisia. We wish to thank Mr. Zouhair Essafi, an English professor for having proofread this paper.
Conflict of interest
There is no conflict of interest in this paper.




