Actinophage R4 integrase-based site-specific chromosomal integration of non-replicative closed circular DNA
Abstract
The actinophage R4 integrase (Sre)-based molecular genetic engineering system was developed for the chromosomal integration of multiple genes in Escherichia coli. A cloned DNA fragment containing two attP sites, green fluorescent protein (gfp) as a first transgene, and an antibiotic resistance gene as a selection marker was self-ligated to generate non-replicative closed circular DNA (nrccDNA) for integration. nrccDNA was introduced into attB-inserted E. coli cells harboring the plasmid expressing Sre by electroporation. The expressed Sre catalyzed site-specific integration between one of the two attP sites on nrccDNA and the attB site on the E. coli chromosome. The integration frequency was affected by the chromosomal location of the target site. A second nrccDNA containing two attB sites, lacZα encoding the alpha fragment of β-galactosidase as a transgene, and another antibiotic resistance gene was integrated into the residual attP site on the gfp-integrated E. coli chromosome via one of the two attB sites according to reiterating site-specific recombination. The integrants clearly exhibited β-galactosidase activity and green fluorescence, suggesting the simultaneous expression of multiple recombinant proteins in E. coli. The results of the present study showed that a step-by-step integration procedure using nrccDNA achieved the chromosomal integration of multiple genes.
Introduction
In gene-function studies, integrative gene transfer methods are limited by variable transgene expression. Site-specific recombinases are attractive valuable tools for basic research and molecular genetic engineering 1, 2. The temperate phage encodes the integrase that catalyzes site-specific recombination between two attachment sites on the bacterial host chromosome (attB) and phage chromosome (attP). This reaction generates the integrated prophage sandwiched by the recombinant attL and attR sites 3, 4. The integration step, which occurs in a site-specific manner, is a uni-directional reaction. The temperate phage R4 isolated from Streptomyces albus J1074 has a wide host range for Streptomyces species 5. To date, 19 actinophage genomes have been sequenced completely, and comparative genomic analyses have revealed that the R4 phage genome is dominantly clustered with six Streptomyces phage genomes and also that R4-cluster phages have sequence similarities to mycobacteriophage subclusters A1 and A2, suggesting the conservation of an ancestral genome architecture 6. The actinophage R4 encoded integrase (Sre) 7 belonging to the large serine recombinase family 8, which catalyzes site-specific recombination between attP and attB on the R4 phage and Streptomyces parvulus 2297 chromosome 9, 10. Serine-type site-specific recombination events are applied to the gene integration system for hetero hosts. In vivo recombination analyses revealed that Sre catalyzed site-specific recombination without requiring any accessory proteins 11. The Sre-based site-specific integration system functions in a wide range of other species, including E. coli, cyanobacteria, human cells, and insect cells 11-15. In our previous study, a step-wise multiple gene integration system was provided for photosynthetic cyanobacteria, leading to the controlled expression of intergenes by different promoters 12. This step-wise integration system allows fragments larger than 10-kb and containing fundamental DNA elements of the vector to be inserted into the desired site-specific positions in the chromosome. However, other DNA elements, excluding assembled attachment sites and antibiotic-resistant genes as a selection marker, are redundant in this site-specific integration system.
The genetic engineering tool is generally used to analyze the host-vector system in E. coli. Although generally considered to be very useful, difficulties associated with incompatibility, antibiotic selection, and the expression stability of the gene introduced have been reported 16-19. Its efficient accessibility and high-efficiency transformation have made E. coli an attractive host for the heterologous expression of functional genes; therefore, it is important to eliminate its ability to accept vectors. However, plasmids are eliminated from host cells in the absence of selection pressure such as antibiotics. Copy number fluctuations in plasmids have been studied extensively in order to measure gene expression because the magnitude and effects of these fluctuations remain unclear 18, indicating that flexibility in selecting the insertion location of transgenes is markedly reduced. Therefore, the development of stable gene expression tools is important for the introduction of transgenes.
In the present study, we examined an E. coli chromosome, into which multiple genes had been introduced, that was constructed with self-ligated fragments, namely, non-replicative closed circular DNA fragments, which eliminated the unnecessary nucleotide sequences derived from a plasmid, such as a replication origin, according to the R4 integrase system. The validity of novel sequential integration method based on the site-specific R4 integrase system was confirmed in the most versatile E. coli.
Materials and methods
DNA manipulation and PCR amplification
Standard molecular biological techniques were used for all DNA manipulations 16. E. coli strain JM109 (Toyobo, Osaka, Japan) and strain DH5α MCR (Cosmo Bio, Tokyo, Japan) were used as hosts for plasmid construction, transformation, and preparation. All primers for PCR amplification were listed in Table 1. A Thermal Cycler Dice (Takara Bio, Shiga, Japan) and KOD-plus-DNA polymerase (Toyobo) were used to generate PCR fragments for the construction of plasmids. The amplified DNA fragments were electrophoresed and purified using 0.8–2.0% agarose LO3 gels (Takara Bio).
| Primer pair | Primer sequences (5′→3′) |
|---|---|
| F-attB/Bg | TTAGATCTGACGGGGCCGGGATGACGAT |
| R-attB/SpSm | TAGCATGCCCGGGGTCGGCTACACGGAGCAGGA |
| F-attB/CSp | TTATCGATGCATGCGACGGGGCCGGGATGAGAT |
| R-attB/Hd | TAAAGCTTGTCGGCTACACGGAGCAGGA |
| lacZα-mu+ | TAGAAGCTTACATGCCTGCAGGTCGACTCTGGAGGATCCGAT |
| lacZα-mu– | ATCGGATCCTCCAGAGTCGACCTGCAGGCATGTAAGCTTCTA |
| F-lacZα | CTAGCCATGGTAATGACCATGATTACGCC |
| R-lacZα | TAGAGATCTATGCGCCGCTACAGGGCG |
| F-GFPuv2 | TAGGGTACCTGAGTAAAGGAGAAGAACTT |
| GFP-RII | AAGTAGTGACAAGTGTTGGCC |
| 5′-tet | CGAATTTACGACACAACCAA |
| 3′-tet | GACTGATCAACTTCCATAGG |
| attB-E | TAGGTACCAGCAGGACCGGGTGTGTCTG |
| 5′-Cm2 | TTTATTCGGCGCAAAGTGCG |
- Underlined areas indicate the restriction enzyme recognition site.
Isolation of attB-inserted E. coli
The DNA fragment containing the attB-site ligated to the kanamycin (Km) resistance gene (kan) was constructed. A 1.4-kb BglII and SmaI fragment containing the attB region from S. parvulus 2297 was cloned into the BamHI and SmaI site of the plasmid pTZ18R (Toyobo), generating pAT26 11. pAT26 was digested with XbaI and treated with the Klenow Fragment (Nippon Gene, Toyama, Japan) in order to obtain a DNA fragment with blunt ends. The kan DNA fragment was obtained from the plasmid pDG780 (The Bacillus Genetic Stock Center, Columbus, OH) 20 after digestion with HindIII. The resulting 1.4-kb DNA fragment was treated with the Klenow Fragment and cloned into the blunt-ended XbaI site of pAT26 in order to generate pKB. The 2.9-kb attB-kan DNA fragment was isolated from pKB by digestion with EcoRI and HindIII and then the resultant DNA fragment was inserted into the EcoRI and HindIII sites of pMODTM-2<MCS> (Epicentre, Madison, WI), resulting in the transposome plasmid pMOD-KB.
Random transposon mutagenesis was performed using an EZ::TN Transposome kit, according to the manufacture's instruction (Epicentre). The attB-kan DNA fragment was isolated from pMOD-KB by digestion with PvuII, and the resultant transposome DNA fragment and transposase were mixed with the JM109 strain cell suspension. The mixture was subjected to electroporation using Gene Pulser II (Bio-Rad, Hercules, CA) at 12.5 kV cm−1, 600 Ω, and 25 μF. Electroporated cells were allowed to recover in SOC medium at 37 °C for 1 h. In order to select transformants, 1 ml of the incubated cells was spread on a 2×YT agar plate containing a final concentration of 15 μg ml−1 Km. The plates were cultured at 37 °C for an appropriate period of time, normally 18 h. The attB-inserted E. coli genomic DNA was extracted and digested with HindIII. HindIII-digested DNA fragment sizes from 3 to10 kb were purified and cloned into the HindIII site on pUC119 to yield a clone library, and then transformed to DH5αMCR strain cells. Transformants were spread on a 2×YT agar plate containing 15 μg ml−1 Km and 75 μg ml−1 ampicillin (Ap). The nucleotide sequence flanking the transposon was determined using the BigDyeTM Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA) using a PE Applied Biosystems Automated DNA Sequencer (model 3130xl).
Construction of plasmids and non-replicative closed circular DNA fragments
The integration plasmid pEQGFP 12 harboring two attP sites, the gfp fused C-terminal hexahistidine (His6)-tag fragment as the reporter gene (gfpHis, approximately [ap.] 1.8 kb), and the Cm resistance gene (cat, ap. 1.8 kb) was used for the first integration. In order to remove the ori gene from the integration plasmid, the DNA fragment containing two attP sites, gfpHis, and cat between the SphI sites on pEQGFP, was obtained by digestion with SphI and self-ligated by T4 DNA ligase (Nippon Gene) to generate a non-replicative closed circular DNA (nrccDNA) fragment.
The second integration plasmid containing the 122-bp attB (attBa) and 123-bp attB (attBb) sites was then constructed. The attBb PCR fragment was generated with the primers F-attB/Bg and R-attB/SpSm (Table 1) using pAT26 as the template. The resulting PCR amplicon was digested with BglII and SphI and inserted into the BglII and SphI sites of pET-15b (Novagen Inc., Madison, WI), yielding pETB6. The attBa PCR fragment was also generated with the primers F-attB/CSp and R-attB/Hd using pAT26 as the template. The resulting PCR amplicon was digested with ClaI and HindIII and inserted into the ClaI and HindIII sites of pETB6, yielding pEWB7. The tetracycline resistance gene (tet, ap. 2.1 kb) DNA fragment was obtained from pDG1515 (The Bacillus Genetic Stock Center) after digestion with EcoRI and PstI. The resulting DNA fragment was treated with Mung Bean Nuclease (Nippon Gene) and the Klenow Fragment and inserted into the SmaI site of pEWB7 to generate pEWBTr. The lacZα gene was generated in the PCR reaction using pUC119 (Takara Bio) as a template with the primers lacZα-mu+ and lacZα-mu–. The amplifying 1.4-kb lacZα DNA fragment was digested with HindIII and BamHI and inserted into the HindIII and BamHI sites of pUC119, yielding pUC119mu. The lacZα gene PCR fragment generated with the primers F-lacZα and R-lacZα using pUC119mu as a template was cloned into the NcoI and BglII sites of pQE60 (Qiagen, GmbH, Germany), yielding pQElacZα. The lacZα DNA fragment containing the T5 promoter and terminator was isolated from pQElacZα by digestion with XbaI and XhoI and inserted into the XbaI and XhoI sites of pEWBTr to generate pEQB-lacZα-T. The second integration DNA fragment containing two attPs, gfp, and cat between the SphI sites on pEQB-lacZα-T, was obtained by digestion with SphI and self-ligated by T4 DNA ligase, thereby generating the second nrccDNA.
In vivo integration assay
The R4 integrase expression vector pSre4 11 was introduced into E. coli cells containing the attB site and spread on a 2×YT agar plate containing 75 μg ml−1 Ap and 100 μg ml−1 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (X-gal). An in vivo integration assay was performed as previously described 11. In brief, 2 μg DNA precipitated by ethanol was mixed with 100 μl of the cell suspension of E. coli harboring pSre4, and was then electroporated at 12.5 kV cm−1, 600 Ω, and 25 μF. The electroporated cells were allowed to recover in SOC medium at 37 °C for 1 h. In order to select transformants, 1 ml of the incubated cells was spread on a 2×YT agar plate containing appropriate antibiotics (12.5 μg ml−1 chloramphenicol (Cm), 5 μg ml−1 tetracycline (Tc), 75 μg ml−1 Ap, and/or 15 μg ml−1 Km). Growing colonies were randomly selected, and inoculated into 2×YT medium containing appropriate antibiotics. Since pSre4 gave negative effects by the induction of IPTG on the E. coli strain 11, E. coli harboring pSre4 was incubated in 2×YT medium at 37 °C for 2 days, and spread onto a 2×YT agar plate containing appropriate antibiotics with isopropyl-β-d-thiogalactopyranoside (IPTG). The competencies (per microgram DNA) of these electrocompetent cells were measured by electroporation with pACYC184 (Nippon Gene). Transformation frequency (%) was calculated using the following equation: transformation frequency (%) = the number of integrants per the total number of cells before transformation × 100.
Taq DNA polymerase (Gene Taq, Nippon Gene) was used to amplify the site-specific integration site in the Thermal Cycler Dice. The colony-PCR reaction was performed under the following conditions; first integration, 3 min at 95 °C, then 30 cycles of 95 °C (30 s), 54 °C (30 s), and 72 °C (45 s), and 72 °C (2 min); second integration when using 3′-tet and F-GFPuv2 primers, 3 min at 95 °C, then 30 cycles of 95 °C (30 s), 52 °C (30 s), 72 °C (2 min), and 72 °C (4 min); second integration when using 5′-Cm2 and 5′-tet primers, 3 min at 95 °C, then 30 cycles of 95 °C (30 s), 52 °C (45 s), 72 °C (45 s), and 72 °C (2 min). These amplified DNA fragments were electrophoresed and examined using 1.0–1.5% agarose LO3 gels (Takara Bio).
Western blot analysis
An overnight culture of recombinant E. coli (100 µl) was inoculated into 10 ml of 2×YT medium containing 12.5 µg ml−1 Cm and cultured with reciprocal shaking at 130 rpm at 37 °C until optical density (600 nm) (OD600) reached 0.5–0.6. After the addition of a final concentration of 1 mM IPTG to the culture, growth was continued for 20 min and 1 ml of the culture was transferred into a 1.5-ml microcentrifuge tube. Cells were harvested after centrifugation at 15,000×g at 4 °C for 10 min. Cells were suspended in [absorbance value at OD600 of culture × 100] µl of 2 × sample buffer (0.05 M Tris–HCl [pH6.5], 2% sodium dodecyl sulfate [SDS], 10% glycerol, 0.1% [w/v] bromophenol Blue, 2% [v/v] 2-mercaptoethanol). Cells were then incubated at 98 °C for 2 min. SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was performed using 16.5% (w/v) polyacrylamide gels. The supernatant proteins prepared for the Western blot analysis were separated on SDS-PAGE gels. After electrophoresis, the proteins were transferred to a Hybond-ECL nitrocellulose membrane (GE Healthcare Bio-Sciences, Buckinghamshire, UK) in a transfer buffer (25 mM Tris, 192 mM glycine, 20% (v/v) methanol, 0.1% (w/v) SDS) using a semidry blotting system (TAITEC, Saitama, Japan). Immunoblots were detected as described in the manufacturer's instructions for the ECL Plus Western blot detection system (GE Healthcare Bio-Sciences) using a His6-tag antibody (Qiagen). The signals hybridized were quantified by densitometry using 1DscanEX (M&S Instruments Inc., Osaka, Japan). A two-sided unpaired Student's t-test was performed to statistically compare the intensity of chemical fluorescence.
Green fluorescence and β-galactosidase activity
In order to examine GFP expression and β-galactosidase activity, transgene-integrated E. coli strains were grown on a 2×YT agar plate containing 15 µg ml−1 Km, 0.2 mM IPTG, and 0.004% (w/v) X-gal at 37 °C for 18 h. Green fluorescence was displayed using a UV transilluminator (Cosmo Bio).
Results
Characterization of the attB-inserted E. coli strain
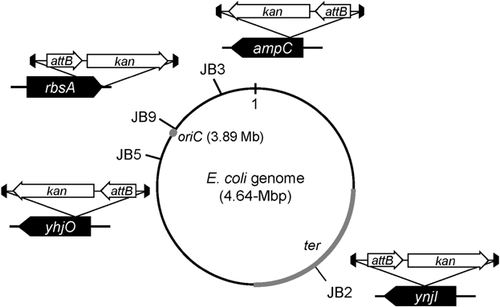
In order to generate an attachment site for gene integration into the E. coli JM109 strain chromosome, the transposome containing the attB site was introduced into random points of the JM109 strain chromosome and Km-resistant transformants via the transposon were isolated. In this study, nine Km-resistant strains were isolated and designated as JB strains. The integration site of the transformant was confirmed to be a single copy by Southern hybridization using the attB probe (data not shown). The insertion location of the attB site on the JB strain chromosomes was identified by shotgun cloning and sequencing (Table 2). No significant differences were observed in the growth of the Km-resistant transformants, JB2, JB3, JB5, and JB9 or strain JM109, whereas the growth of the remaining strains, JB1, JB4, JB6, JB7, and JB8, was markedly slower than that of strain JM109 (data not shown). Accordingly, strains JB2, JB3, JB5, and JB9 were used for site-specific integration. The attB–kan DNA fragment was inserted into the ynjI, ampC, and yhjO genes on the chromosomes of strains JB2, JB3, and JB5, respectively (Fig. 1). In the case of strain JB9, the fragment was located downstream of the stop codon of the rbsA gene.
| Strain | Position (kb) | Locus | Function and gene description |
|---|---|---|---|
| JB1 | 4061.9 | yshA | Putative outer-membrane protein |
| JB2 | 1842.9 | ynjI | Putative inner-membrane protein |
| JB3 | 4376.5 | ampC | β-lactamase |
| JB4 | 4056.0 | typA | Phosphorylated protein |
| JB5 | 3693.6 | yhjO | Cellulose synthase catalytic subunit |
| JB6 | 4220.1 | arpC | Regulator of acetyl-coenzyme A synthetase gene expression |
| JB7 | 1098.1 | ycdX | Putative metallo-dependent hydrolase |
| JB8 | 3841.4 | yicO | Putative xanthine/uracil permease |
| JB9 | 3931.4 | rbsA | d-ribose high-affinity transport system |
Site-specific integration of the gfpHis gene into attB-inserted JB strains: first integration
The Sre expression plasmid, pSre4, was introduced into attB-inserted JB strains using electroporation. The transformants JB2[pSre4], JB3[pSre4], JB5[pSre4], and JB9[pSre4] were isolated. No significant difference was observed in host competency among the JB[pSre4] strains (Table 3), indicating that it was possible to compare transformation frequencies between JB strains. The first nrccDNA fragment containing two attPs, gfpHis, and cat was introduced into JB strains harboring pSre4 by electroporation, and integrant strains were selected based on Ap, Cm, and Km resistances (Fig. 2). A total of 8 antibiotic resistant strains in JB2[pSre4], 22 in JB3[pSre4], 12 in JB5[pSre4], and 32 in JB9[pSre4] grown on the plate, respectively. The site-specific integration event mediated by Sre occurred at a transformation frequency of 1.7 – 3.4 × 10−6% (Table 3). In contrast, no integrant strain was obtained in the absence of pSre4 or when using strain JM109 harboring pSre4 (data not shown).
| Strain | Competency (%) [per µg pACYC184] | Transformation frequency (%) [per µg nrccDNA fragment]a |
|---|---|---|
| JM109[pSre4] | >1.0 × 10−1 | n.d.b |
| JB2[pSre4] | 2.8 × 10−2 | 2.0 ± 0.2 × 10−6 |
| JB3[pSre4] | 3.2 × 10−1 | 3.4 ± 2.6 × 10−6 |
| JB5[pSre4] | 2.6 × 10−2 | 1.7 ± 0.8 × 10−6 |
| JB9[pSre4] | 1.5 × 10−1 | 2.8 ± 1.9 × 10−6 |
- a Transformation frequency (%) was calculated using the following equation: Transformation frequency (%) = the number of integrants per the total number of cells before transformation × 100. Data represent triplicate determinations. nrccDNA, non-replicative closed circular DNA.
- b Not detected.

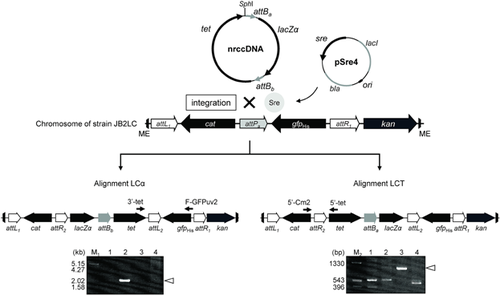
Although the first nrccDNA contained the attPa and attPb sites, two alignments of 5′-attL1–gfpHis–attPb–cat–attR1–kan-3′ (alignment LG) and 5′-attL1–cat–attPa–gfpHis–attR1–kan-3′ (alignment LC) were generated when site-specific recombination occurred between one attP site on nrccDNA and the attB site on the JB strain chromosomes (Fig. 2). A PCR amplification analysis was performed based on the attR1 site region in order to confirm the site-specific integration site (Fig. 2). The integrant strains harboring the alignments LG and LC accounted for 62.5 and 37.5% in JB2[pSre4], 81.8 and 18.2% in JB3[pSre4], 70.0 and 30.0% in JB5[pSre4], and 89.5 and 10.5% in JB9[pSre4], respectively, indicating that the integration event almost occurred between attPa and attB. The nrccDNAs were integrated into the attB site on the chromosome (Fig. 2).
Detection of GFP expression by a Western blot analysis
A Western blot analysis was performed in order to verify the expression of GFP in alignment LG- and LC-inserted integrant JB strains, termed JB2LC, JB2LG, JB3LC, JB3LG, JB5LC, JB5LG, JB9LC, and JB9LG. A Western blot analysis of protein extraction supernatants revealed that all strains expressed GFP (data not shown). GFP expression levels in strains JB3LC, JB3LG, JB5LC, JB5LG, JB9LC, and JB9LG were 2.0- to 2.7-fold higher than those in JB2LC and JB2LG (Fig. 3). However, no significant differences were observed in the mean chemical fluorescence intensities of the alignment LC and LG strains.

Site-specific integration of the lacZα gene into attP-inserted strain JB2LC: second integration
Strain JB2 had the highest integration frequency among the integrant strains (Table 3). In order to integrate the second reporter gene, lacZα, into the attP site (Fig. 4), strain JB2LC was used for electrocompetent cells. The second nrccDNA was introduced into strain JB2LC harboring pSre4, yielding strain JB2LC[pSre4], and integrants were selected based on Ap, Cm, and Tc resistances, indicating that the site-specific integration event occurred between the attPa site on the chromosome and one attB site on the second nrccDNA. As with the first integration, two alignments of 5′-attL1–cat–attR2–lacZα–attBb–tet–attL2–gfpHis–attR1–kan-3′ (alignment LCα) and 5′-attL1–cat–attR2–tet–attBa–lacZα–attL2–gfpHis–attR1–kan-3′ (alignment LCT) were generated when the integration event occurred between attBa and attPa and between attBb and attPa, respectively (Fig. 4). A PCR amplification analysis was performed to verify the second site-specific integration site. Gene alignments were detected by PCR amplification of the attL2 region using the primers of 3′-tet and F-GFPuv2 for the alignment LCα and by amplification of the attR2 region using primers of 5′-Cm2 and 5′-tet for the alignment LCT (Fig. 4). A total of eight integrant strains harboring the alignments LCα and LCT accounted for 37.5% and 62.5%, respectively, indicating that an integration event frequently occurred between attPa and attBb.
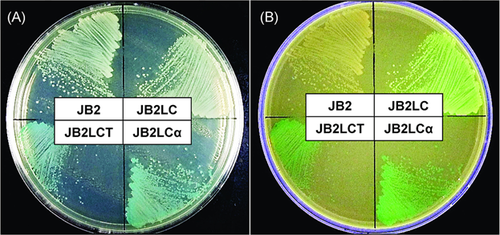
Expression of two transgenes
Regarding strains JB2, JB2LC, JB2LCα, and JB2LCT, the resultant integrant cells clearly exhibited β-galactosidase activity and green fluorescence. Strains JB2LCα and JB2LCT turned blue (Fig. 5A), while strains JB2LC, JB2LCα, and JB2LCT turned green (Fig. 5B), suggesting the co-expression of LacZα and GFP introduced in strains JB2LCα and JB2LCT.
Discussion
The serine-type R4 integrase-based site-specific integration system may contribute to the development of biotechnology for the engineering of host chromosomes. The typical serine integrase catalyzes an integration event regardless of the conformation of plasmid DNA. Morita et al. 21 previously reported that efficiency when introducing a linearized plasmid DNA fragment was half or less than that when introducing a super-coiled plasmid DNA, presuming that open-circular plasmid DNA reduces the transformation frequency rather than super-coiled plasmid DNA. Our method requires only gene integration, regardless of frequency. The practical transformation frequency was sufficient for the R4 integration system. Therefore, the removal of unnecessary DNA elements appeared to be practically effective.
The stable retention of functional genes introduced into a chromosome may avoid the disadvantages of inherent issues associated with plasmid expression, such as adjusting the direction, position, and number of attachment sites, which affect the efficiency of the integrase system 22. Moreover, the antibiotic resistance gene as a selection marker is still an issue that requires further study when introducing multiple genes into the E. coli chromosome. In the present study, we showed that the insertion location of the attB site on the E. coli chromosome needed to be selected in consideration of cell growth, indicating the existence of an appropriate insertion location on the host chromosome (Fig. 1). Moreover, nrccDNA containing attachment sites, a transgene, and a selection marker gene may overcome incompatibility, namely, plasmids harboring the same replicon not being maintained in E. coli cells 16. In the present study, we provided a standard E. coli host-vector system for R4 integrase-based site-specific recombination in order to optimize the expression of a selection marker and facilitate routine molecular genetic engineering.
The first and second site-specific integration events frequently occurred between the attPa and attB-inserted JB strain chromosomes and between the attBb and attPa-inserted JB2LC strain chromosome. The sequence lengths of the attPa and attPb sites and attBa and attBb sites only differed by two base-pairs and one base-pair, respectively. The attPa site sequence was placed downstream of gfpHis and upstream of the cat gene (Fig. 2). These transcriptional directions were identical and both DNA fragment lengths were approximately 1.8-kb. Meanwhile, the attBb site sequence was located downstream of lacZα and upstream of the tet gene (Fig. 4). The transcription of these genes was in the same direction, while the tet fragment length was approximately 0.65 kb longer than that of lacZα. In the present study, we were unable to elucidate any clear principle regarding the preferential selection of the att site in the first and second integration reactions, presuming that R4 integrase-based site-specific recombination inadvertently occurred independently by the arrangement and direction of the assembled genes in the present study.
Strategies for gene discovery need to be guided by synthetic biology and metabolic/genetic engineering 23-25. With the advent of the genomic era, we are now able to obtain intractable genetic information by comprehensive metagenomic sequencing to discover bacteria that naturally produce bioactive compounds and can also identify more as yet undiscovered biosynthetic pathways related to nonribosomal peptide synthetase (NRPS) and polyketide synthetase (PKS) 26, 27. Although a large proportion of the metabolic gene repertoire is only expressed at very low levels or is inactive in bacterial cells 28, our method should be applicable to the identification of novel NRPS and PKS genes, and cluster reconstruction for combinatorial chemistry.
In conclusion, we developed a method for efficiently integrating a DNA vector from which regions unnecessary for gene expression were removed. The present study is the first to integrate an nrccDNA fragment generated by self-ligation into a host chromosome using an R4 integration system. We also demonstrated that two transgenes and two antibiotic-resistant genes integrated into the host chromosome had the ability to function at the same time.
Acknowledgments
We thank M.D. Yan-Zhuo Yang for his technical support and kind provision of the requested plasmid. We also thank Dr. Hideo Takahashi for useful discussions and suggestions.
Conflicts of interest
The authors declare that they have no conflict interest.




