Simultaneous enhancement of phenolic compound degradations by Acinetobacter strain V2 via a step-wise continuous acclimation process
Abstract
Phenol degradation enhancement of Acinetobacter strain V2 by a step-wise continuous acclimation process was investigated. At the end of 8 months, three stable adapted strains, designated as R, G, and Y, were developed with the sub-lethal concentration of phenol at 800, 1100, and 1400 mg/L, respectively, from 400 mg/L of V2 parent strain. All strains degraded phenol at their sub-lethal level within 24 h, their growth rate increased as the acclimation process continued and retained their degradation properties even after storing at −80 °C for more than 3 years. All adapted strains appeared coccoid with an ungranulated surface under electron microscope compared to typical rod-shaped parental strain V2. The adapted Y strain also possessed superior degradation ability against aniline, benzoate, and toluene. This study demonstrated the use of long term acclimation process to develop efficient and better pollutant degrading bacterial strains with potentials in industrial and environmental bioremediation.
Introduction
Phenol and phenolic compounds are used in many industries and these compounds pollute industrial effluents and therefore, surface water. Concentration of phenol in these effluents can be up to 17,500 mg/L 1. Bioremediation has become a popular approach of cleaning up hazardous environmental pollutants because it is simple to maintain, applicable over large areas, and cost effective 2, 3. The search for effective and efficient microorganisms in bioremediation has intensified in recent years. Different genera of bacteria are known for their potential phenol degradation ability 2. However, substrate inhibition and low degradation rate has limited their applications. Many strategies have been tested to overcome these limitations in order to degrade phenol at high concentrations 4, 5.
The catabolism of phenol has thus far provided a suitable paradigm for analyzing the mechanism of bacterial evolution and adaptation to degrade such compounds 5, 6. Ray and Peters 7 showed that microorganisms can develop resilience after multiple exposures to chemical stressors. Although the original bacterial population contained identical genetic information, the phenotypic heterogeneity within populations had increased leading to differences in gene expression 8. The higher phenolic stress resulted in higher activity and tolerance under higher concentration of phenol in activating sludge process of aerobic Sequential Batch Reactors (SBR) 9. Some individuals within a bacterial population can adapt and survive in a stress environment 8. Kwon and Yeom 5 demonstrated that phenol degradation rate of Pseudomonas fluorescence KNU417 was enhanced by adapting at high concentration of phenol. Elias et al. 10 further demonstrated that the preliminary acclimation of the biomass to the pollutant is a suitable strategy to shorten the start-up period of biofilters and for ensuring longer successful operation. The activated sludge acclimated to 140 mg/L of phenol could successfully treat up to 1050 mg/L of phenol without experiencing complete inhibition during the degradation process 11.
The impact of sub-lethal exposure of phenol degrading bacteria on phenol degradation seems clear. It has not been demonstrated whether the acquired phenol degradation ability of the bacteria can be further enhanced by stepwise increases the sub-lethal concentration after the bacteria have adapted to a higher chemical stressor concentration. In addition, can the acquired bacterial ability have similar advantages against other stressor with similar structure? Here we report that the phenol degrading ability of Acinetobacter strain V2 has been dramatically enriched by the long term exposure of sub-lethal concentration against not only phenol as well as aniline, benzoate, and toluene. The present study showed potential of acclimatised mutants for phenol degradation and environmental bioremediation.
Materials and methods
Acclimation of V2 isolate to increasing phenol concentrations
Acinetobacter strain V2 was originally isolated from the used engine oil contaminated soil 12 and was able to degrade up to 400 mg/L of phenol in 24 h at 30 °C, 160 rpm, but failed to replicate at 500 mg/L. A step-wise acclimation process to enhance phenol degradation of Acinetobacter strain V2 was developed as depicted below. One milliliter of standardized overnight culture (1 OD600nm) of V2 strain in nutrient broth was transferred in fresh Bushnell Haas medium supplemented with 400 mg/L phenol. Every 24 h, 1 mL of overnight culture was transferred into a fresh Bushnell Haas (BH) medium (per litre: K2HPO4 1.0 g, KH2PO4 1.0 g, NH4NO3 1.0 g, CaCl2 2H2O 0.02 g, MgSO4 7H2O 0.2g, and FeCl3 0.05g) 13 with 400 mg/L phenol. Growth of bacterial strain was monitored spectrophotometrically at 600 nm. Propagation was conducted over a period of 80 days with sampling every 16 days. Cell growth and phenol concentration were measured at 0, 8, 12, 18, and 24 h as described below. Adapted Acinetobacter strain that was found to degrade 800 mg/L of phenol in 24 h was designated as Strain “R”. A similar acclimation process was applied to Strain R and subsequently Strain G under increased sub-lethal concentration (800 and 1000 mg/L of phenol, respectively) for 80 days and generated strain Y that was able to degrade phenol up to 1400 mg/L. All parent and adapted Actinetobacter strains were used for further studies. All bacterial strains were maintained under −80 °C.
Determination of phenolic compound degradation and cell growth
Phenol degradation ability of each Acinetobacter strain was assessed at a phenol concentration range of 100 to 3000 mg/L. Residual phenol concentrations were determined by a direct photometric method based on rapid condensation with 4-amino antipyrene followed by oxidation with potassium ferric cyanide 14. Phenol concentration (mg/L) was determined against a standard curve made from known concentration of phenol standard measured at 505 nm. The growth of the bacteria was monitored by an optical density at 600 nm and confirmed using plate count techniques. The degradation abilities of Acinetobacter V2 and acclimated strain Y against aniline, benzoate, and toluene were also determined under the same experimental condition using different substrate concentration (100–1200 mg/L).
Scanning electron microscopy (SEM)
Samples were collected at the exponential phase and passed through 0.22 μm filter and immersed in 2.5% gluteraldehyde. Filters were rinsed with phosphate buffer and treated with osmium tetroxide for 1 h. Filters were then rinsed with deionized water followed by dehydration through an alcohol series (25–100%) before immersion in hexamethyldisilazane for 5 min. Filters were dried on a petri dish followed by mounting on double-sided carbon tape and gold sputter coating. Samples were examined with Zeiss Ultra Plus Field Emission SEM.
Statistical analysis
All values were presented as the mean ± the standard deviation. Student t-tests were used to examine the statistical significance (SPSS version 22) between different strains with various substrates. Probability was set at p = 0.05.
Results
A step-wise acclimation of Acinetobacter strain V2 with the sublethal concentrations of 400 mg/L of phenol was employed daily. After 16 days (approximate 500 generations), the sub-lethal concentration of Acinetobacter strain V2 gradually increased to 800 mg/L of phenol. Further inoculation at 400 mg/L phenol, did not significantly increase their degradation ability. This stably adapted Acinetobacter strain was engendered (designed as strain R). Subsequently the adapted strain was propagated for another 80 days at 800 mg/L of phenol to generate a new adapted strain after 64-day incubation, designated as strain “G” with the sub-lethal concentration at 1100 mg/L phenol. Strain G was propagated for another 80 days at 1000 mg/L to generate a subsequent strain as “Y” strain with sub-lethal concentration at 1400 mg/L phenol. The cell growths of all strains were tested on a range of phenol concentrations from 100 to 3000 mg/L. Figure 1 demonstrates the cell growth of each strain under various concentrations. It is clear that the sub-lethal concentration of bacterial strain increases as the acclimation process continued. The parent strain sustained well only at 400 mg/L of phenol, while the sub-lethal concentrations of R strain, G strain, and Y strain were increased to 800, 1100, and 1400 mg/L of phenol, respectively.
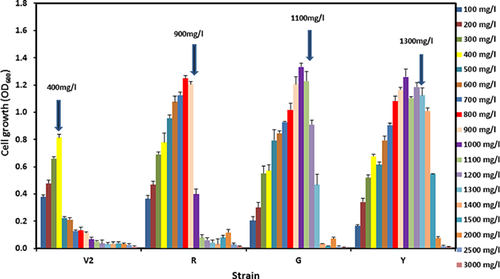
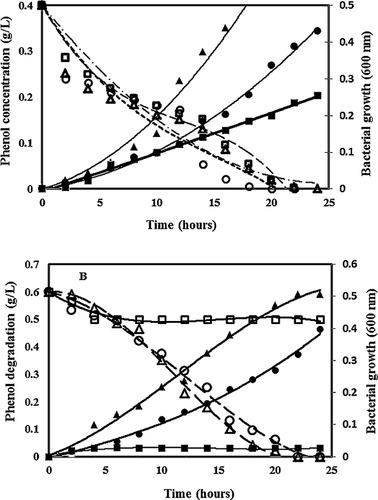
All strains failed to propagate when higher than their tolerance level of phenol concentration was applied indicating the substrate inhibition became the dominant factor in cell growth (Fig. 1). In addition, all the adapted strains grew faster and achieved higher cell population after 24 h. The degradation results in Fig. 2A shows that the parent strain V2, Strain R and Strain G could utilize 400 mg/L phenol as carbon source within 20–24 h. Furthermore, the cell growth of Strain G was almost 3× faster (0.027 OD600/h) than that of the parent strain (0.008 OD600/h), while R strain grew 2× faster (0.018 OD600/h) (Fig. 2A). When the phenol concentration was increased to 600 mg/L, the parent strain failed to replicate even after 24 h with little phenol loss and two adapted strains R and G showed the similar growth pattern as seen with 400 mg/L phenol (R: 0.016 OD600/h; G: 0.027 OD600/h) (Fig. 2B). Strain Y was capable of degrading 90% of phenol with the growth rate of 0.059 OD600/h at 800 mg/L of phenol (data not shown). The phenol degradation abilities of various Acinetobacter adapted strains retained their properties at the same level even after storing at −80 °C for more than 3 years indicating that the acquired properties were stable.
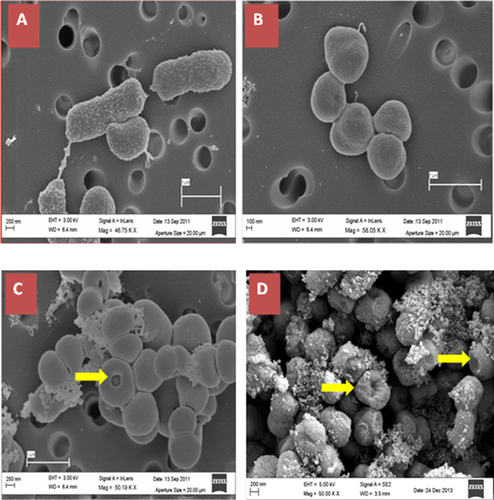
In order to study the impact of acclimation process on the morphology of Acinetobacter strains, all four strains were observed under scanning electron microscope. Figure 3 shows electron microscope images of all four Acinetobacter strains. Acinetobacter strain V2 depicted a typical rod-shaped connected by a peritrichate fibril-type appendage while acclimated strains appeared coccoid in shape with an ungranulated surface. The adapted strains showed more aggregation behavior compared to the parent strain (V2). Central depressions appeared in all (Fig. 3B), except parent strain (Fig. 3A).
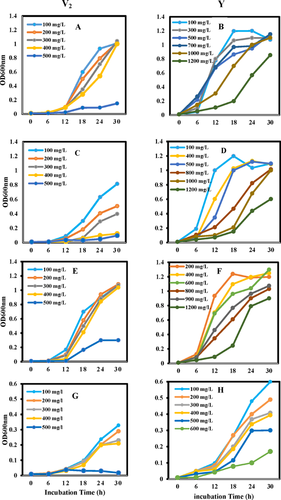
The abilities of Acinetobacter parent V2 and acclimated strain Y to degrade phenol and other phenolic compounds are shown in Fig. 4. The results in Fig. 4A and B confirm the previous results that acclimated Acinetobacter strain Y possesses higher capacity in degrading phenol compared to that of parental Acinetobacter strain V2. When different phenolic compounds replaced phenol as the substrate, acclimated strain Y also demonstrated better degradation capacity (data not shown), growth rates and had less impact on the substrate inhibition in higher concentration than the parent strain (Fig. 4C–H). Figure 4G shows that Acinetobacter strain V2 demonstrated slower growth rate and lower tolerance level using toluene as the substrate and the acclimated strain Y possessed more effective toluene utilization as the carbon and energy source.
Discussion
Phenol and its derivatives are one of the major groups of environmental pollutants due to its broad application in industries. Intensive research to obtain species with high phenol degradation ability has been one of the main focuses of the researchers throughout the world 15, 16. Higher phenolic stress promotes bacteria to possess a higher degradation ability and tolerance under higher phenol concentration 5, 9. Adapted cultures of Bacillus brevis and Pseudomonas cepacia have been shown to degrade 1750 and 2500 mg/L of phenol in 144 h, respectively 17. In this study, Acinetobacter V2 was acclimated to the sub-lethal concentration of phenol, managed to adapt to and degrade higher concentration of phenol and finally resulting into a new stable strain as observed in other studies 5, 7, 17. Repeated acclimation processes at higher phenol concentrations, resulted in Acinetobacter strains continuing enhancing its degradation level up to 1300 mg/L of phenol (strains R, G, and Y respectively; Fig. 1). The results of this study demonstrated that repeated acclimation of a biomass to the pollutant towards higher concentration is a suitable strategy to develop more effective and efficient microorganisms for bioremediation. Acinetobacter sp. Strain Y in this case have dramatically enhanced its phenol degradation ability more than 3-folds (from 400 mg/L of V2 strain to 1300 mg/L of strain Y, Fig. 1) within a period of 8 month. The adapted strains also managed to have a shorter generation times with a similar degradation rate compared to the parent strain under the same conditions (Fig. 2). Acclimated Pseudomonas putida MTCC 1194 with in the period of 3 months from 100 to 1000 mg/L was only able to degrade 1000 mg/L phenol in 162 h 18. Even though Acinetobacter Y strain could not degrade higher concentration of phenol as reported by Arutchelvan et al. 17 (1.75 and 2.5 g/L), all the Acinetobacter strains could degrade phenol within 24 h that was faster than all other reports. Stability and degradation rate of the adapted organism are important for its applications.
All adapted strains (R, G, and Y strains) possessed a change of bacterial shape from rod (parent strain V2) to round as demonstrated by electron microscope images (Fig. 3). This is in concordance with other researchers 19 who reported the morphology changes of Streptobacillus and Corynebacterium spp. from rod shape to coccoid when the bacteria were grown in the presence of phenol. Apart from morphological changes, presence of extracellular polymeric substances (Fig. 3) was also seen in response to stress as observed by other researchers 20.
Farrell and Quilty 21 suggested a link between substrate toxicity and autoaggregation when P. putida CP1 was grown on phenolic compounds. Toxic compounds can induce numerous cellular responses to combat stress and enhance survival abilities 22. Alternating biosynthetic and morphogenetic modifications including alterations in membrane and/or cell-wall composition for counteracting the toxicity due to chemical stresses have been observed by various researchers 23. Other studies 22, 24, 25 report that bacterial cell responds to the solvent stress by inducing general or specific stress responses such as heat shock proteins and chaperones 26, 27 and by inducing metabolic and transport based detoxification mechanisms that counteract the toxic effects 23, 28. Ahsan Habib et al. 19 further demonstrate that long-term adaptive responses alter biosynthetic and morphorgenetic/differentiation program. Our study also support that the growth ability of acclimated strains has been strengthened (Fig. 2) and the morphology of acclimated strains appeared coccoid in shape from a typical rod-shape (Fig. 3).
Most importantly, the results in Fig. 4 further demonstrate the acclimated strain possesses an enhanced ability toward degrading other aromatic compounds. The bioremediation processes of phenol-like compounds are often hindered by slow conversion rates and toxic effects such as membrane or protein damage caused by xenobiotics within the cell 29. Higher rate of degradation of other phenolic compounds such as benzoate, toluene, and aniline suggests that the process of acclimation does not apply to a particular compound but follow a more universal approach leading to more robust and versatile strain. The findings are significant in environmental bioremediation due to the complexity of environmental pollutants. The degradations of many aromatic compounds by bacteria often share a common degradation pathway such as the ortho-(meta-) and β-ketoadipate pathway 30, 31. Aniline, benzoate, phenol, and toluene can be independently converted to catechol and derivatives by aniline dioxygenase, benzoate dioxygenase, phenol hydroxylase, toluene monooxygenase, and others respectively 30, 32, 33. Even in the low concentration, the interactions between catechol and derivatives and the different biomolecules have the deleterious effect on the survival of bacteria 34, 35. Kwon and Yeom 5 suggested that the mechanism of adaptation related to aromatic ring cleavage at the higher concentrations was due to the early induction of catechol 1,2-dioxygenase. Each aromatic compound is degraded via individual parallel sequences of reactions that converge at the β-ketoadipate pathway 36. Studies revealed that phenol induced a heat-shock response while catechol predominantly induced oxidative stress proteins in A. calcoaceticus 37, 38. Many stress proteins protect the cell against the deleterious effects of the toxic compounds by conferring resistance mechanisms against the aromatic compounds 37-40. These enhanced mechanisms of acclimated strains can strengthen their biotechnological application potentials significantly. This method could be used to generate highly efficient and superior phenotypic strains for various applications such as bioremediation and biodegradation of various toxic compounds as well as superior tolerant stains such as thermotolerant, acid tolerant, pH tolerant etc. This method could also be used in increasing the efficiency of strains for green, environment friendly, sustainable, and economical agriculture.
Conclusion
This study shows that step wise continuous acclimation of phenol degrading strain of Acinetobacter sp. V2 could lead to well-adapted strains. The well adapted strains designated as R, G, and Y could degrade sub-lethal concentration of phenol at 800, 1100, and 1300 mg/L respectively compared to 400 mg/ml by parent strain V2. These strains showed higher growth rate and faster phenol degradation ability and this character were stably inherited. The acclimated strains enhanced the abilities to degrade a range of aromatic pollutants. Therefore this concept could be used for developing efficient bacterial strains for bioremediations.
Acknowledgments
This research was funded by the National Research Foundation of South Africa. The authors are grateful to the NRF for the award of Masters bursary to RM.
Conflict of interest
The authors have declared no conflict of interests.




