Glucose released by hydrolytic activity of amylase influences the pigment synthesis in Penicillium sp NIOM-02
Abstract
Carbon catabolite repression is generally considered as a regulatory mechanism to ensure sequential synthesis of secondary metabolites. In this study we made an attempt to understand the influence of amylase activity on pigment synthesis in Penicillium sp NIOM-02. The amylase activity is inversely proportional to pigment production. The high performance liquid chromatography analysis of amylase reaction revealed glucose as the major product of starch hydrolysis. The fungus grown in acarbose (inhibitor of amylase) incorporated media produced higher quantities of pigments. Apparently, glucose released due to amylase activity influenced the pigment synthesis by cAMP signaling pathway.
Introduction
Microorganisms are key organisms for production of useful biological active secondary metabolites like- antibiotics, pigments and toxins. Microorganisms have a major effect on the health, nutrition and economics of the society 1. Fungi are well known for production secondary metabolites, including important antibiotics, such as penicillin produced by Penicillium and Aspergillus species 2. The pharmaceutical applications of polyketides and monacolins produced by Monascus species are evidenced by their antioxidant, antibacterial, anti-inflammatory and hypocholesterolemic properties 3-6. These natural products represent broad structural and biosynthetic diversity, including terpenoids, polyketides, peptides and alkaloids 7.
The synthesis of these metabolites is typically regulated and closely related to developmental regulation of organisms. Some transcriptional regulator genes associated with biosynthesis genes act specifically on individual pathways and the modifications in master regulators have pleiotropic effects 2, 8. In Aspergillus nidulans, Ga protein regulates synthesis of penicillin and sterigmatocystin through its effect on brlA and aflR pathway-specific regulators 9. Biosynthesis of secondary metabolites is influenced by low molecular mass compounds, tRNA, sigma factors and gene products formed during post-exponential development 1. The wide domain regulatory mechanism that controls the synthesis of a wide range of metabolites is carbon catabolite repression. The mechanisms involved in glucose repression have been extensively investigated in Saccharomyces cerevisiae 10. Glucose also influences the formation of many aminoglycoside antibiotics produced by actinomycetes via repression of biosynthetic enzymes 11. In bacteria, catabolite repression is known to be subject to the growth rate on glucose and cells are relieved from repression at a low concentration 12. Similarly, the glucose-dependent inactivation of respiratory metabolism in Saccharomyces cerevisiae does not occur below a certain growth or dilution rate. It means that, regulatory phenomena caused by glucose may be caused by high growth rates on glucose 13. In M. purpureus red pigment production is influenced by L-asparaginase and L-glutaminase activities in the extracellular fluids 14. These studies have revealed the importance of the regulatory enzymes in secondary metabolite production. Unlike primary metabolism, the secondary metabolism pathways are still not understood to a great extent and thus provide openings for basic research of enzymology, control and differentiation 1. The specific objective of this study is to understand the repression of pigment synthesis in Penicillium sp. NIOM-02 by a catabolite (glucose) produced by the action of amylase.
Materials and methods
Culture conditions and enzyme assay
Penicillium sp. NIOM-02 previously isolated in this laboratory was cultured in media containing corn flour (2%), peptone (1%), yeast extract (0.5%). The culture conditions and enzyme assay method were followed as described by Dhale et al. 15. Protein concentrations were determined according to the method of Spector 16.
Electrophoresis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed in 10% acrylamide gels 17. Amylase activities were detected as enzyme zymograms after separating the proteins by SDS-PAGE in 10% acrylamide gel containing 0.1% Lintner's soluble starch. After electrophoresis, the amylase proteins were re-natured by incubating the gel in acetate buffer (0.1 M, pH 4.3) at 30 °C for 90 min. Amylase activities were detected accordingly Ravi-Kumar et al. 18 as zone of clearance after staining with iodine/iodide solution (0.6 g I2 and 6 g KI l−1).
Western blotting
After electrophoresis, proteins blotted on nitrocellulose membranes 19 were blocked with 10% skim milk solution. The amylase proteins were identified using antibody (1:2000 dilution) and anti-rabbit goat IgG conjugated with peroxidase (1:2000 dilution) in Tris-HCl buffer saline (10 mM, pH 8.0). Tris-HCl buffer (50 mM, pH 7.6) containing hydrogen peroxide (0.025%) and 4-chloro-1-napthol (0.04%) was used as substrate for enzyme reaction. The antibodies used in this study were raised previously to the 125 kDa starch-hydrolyzing enzyme of Aspergillus niger 20.
Estimation of pigments
The cultures were filtered through Whatman No. 1 filter paper and the filtrates were directly used for the quantification of pigment. The absorbance was determined using UV-visible spectrophotometer (UV-Visible 2450, Shimadzu Spectrophotometer, Japan) at 485 nm to quantify the pigments as optical density (OD) Units ml−1.
HPLC analysis
Sugars were determined in enzyme hydrolyzed starch by HPLC analysis. The products were separated in a Supelco LC-NH2 column (25 × 4.6 mm, 5 μm) by injecting the sample to Shimadzu SCL-6A system fitted with a RID-6A detector. Acetonitrile and water (80:20) was used as the mobile phase at a flow rate of 1.0 ml min−1 21.
Results and discussion
The microorganisms act in response to environmental conditions by varying the patterns of gene and protein expression. These variations involve adaptative responses of intracellular catabolic pathways and synthesis of molecules capable of modifying the environment. The Penicillium sp. NIOM-02 cultivated under submerged fermentation produced water soluble red pigment and the pigments, PP-V and PP-R produced by Penicillium are structurally similar to monascorubrine and monascuscorubramine pigments of Monascus 22. The pigment production and amylase activity were estimated in culture media of different pH. Even though the Penicillium NIOM-02 isolated form marine sediment, the fungi have a growth capacity although in acidic and alkaline conditions and have a preference to produce amylase in acidic conditions 23. The Aureobasidium pullulans isolated from deep sea of Pacific ocean was cultured at acidic pH for the production of amylase 24. At pH 3, amylase activity and pigment production was 48 U/mg and 0.37 OD Units/ml respectively. At pH 7, higher quantities of pigments were quantified and lower amylase activity was observed. The pigment synthesis and amylase activity are inversely proportional (Table 1) and suggested that, activity of amylase is influencing the pigment synthesis.
| pH | Amylase activity (U/mg protein) | Pigment (OD Units/ml) | Glucose (µg/ml) |
|---|---|---|---|
| 3 | 48.49 ± 0.75 | 0.37 ± 0.01 | 18.12 ± 0.53 |
| 4 | 26.13 ± 1.06 | 0.45 ± 0.01 | 10.85 ± 0.64 |
| 5 | 15.27 ± 0.54 | 0.52 ± 0.02 | 7.26 ± 0.26 |
| 6 | 9.82 ± 1.21 | 0.54 ± 0.01 | 4.61 ± 0.49 |
| 7 | 1.07 ± 0.05 | 1.88 ± 0.03 | 1.78 ± 0.03 |
At high pH values, extensive formation of swollen cells and pH-dependent change in hypal wall structure takes place in P. chrysogenum, resulting in a decrease in the resistance of hypae to impeller shear 25. But we did not observe any changes in the hypal wall structures in the used Penicillium strain. However, the changes in concentration of glucose and pigment production were observed in the media. After 10 days of fermentation, the glucose concentration was 1.78 and 8.12 μg ml−1 in media at pH 7 and 3, respectively. Similar effects were observed in Acremonium chrysogenum, in which glucose represses the biosynthesis of the β-lactum antibiotic cephalosporin C. Glucose reduces the formation of transcripts derived from the pcbC and cefEFbiosynthetic genes by means of carbon repressor CRE1 protein 26.
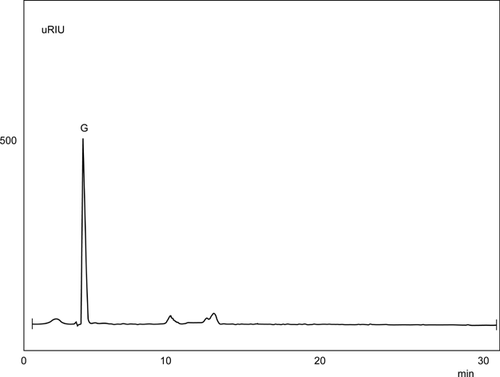
The product analysis of amylase action on starch was performed to identify the sugars released in the reaction mixture. Glucose was determined as a major product formed by hydrolytic activity of amylase (Fig. 1). The increase in the concentration of glucose molecules lead to repression of α-ketoglutarate dehydrogenase, explaining the effect of sugar concentration on enzyme repression 27. Similarly in Bacillus licheniformis mild alkaline pH favours bacitracin production 28 and the neutralizing agent CaCO3 prevents the inhibitory effect of glucose metabolism-derived acidification 29.
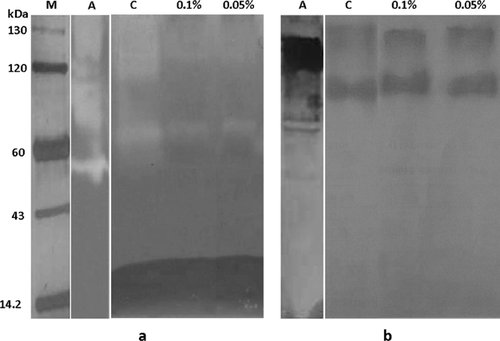
To further confirm the above results, the Penicillium sp. NIOM-02 was grown in medium containing acarbose (0.05 and 0.1%), a potent inhibitor of amylases. The higher quantities of pigment production (1.265 OD Units/ml) and lower amylase activity (0.34 U/mg) were observed in the culture medium containing 0.1% acarbose. Lower quantities of pigment production (0.631 OD Units/ml) and higher amylase activity (256 U/mg) were observed in the culture medium not containing acarbose. The higher concentrations of acarbose inhibited the growth of fungus and pigment secretion. The amylase activity could not be detected using the higher concentrations of acarbose. Amylase activities in culture filtrate were visualized as zymogram reaction after electrophoresis (non-reducing condition). The culture filtrate of control experiment (Fig. 2, lane C) hydrolyzed more starch than culture filtrate containing acarbose (Fig. 2, lane 0.05 and 0.1%). The western blot reactions (Fig. 2) revealed, that acarbose inhibited the amylase activity in the culture medium but not its secretion 30. In the culture medium containing acarbose, the amylase activity was inhibited and resulted in release of lower concentration of glucose molecules. In our earlier study the significant difference in the pigment production was not observed among culture media containing different concentration of acarbose 15. While the comparison of pigment production and amylase activity in control medium and acarbose containing medium suggested the catabolite repression of pigment synthesis. These results indicated that, glucose released by amylase activity is playing important role in the regulation of pigment synthesis. Hence the production of lower quantities pigment can be reasoned for the repression of biosynthetic enzymes by high concentration glucose in the media. Similar observations were made in the production of many amino-glycoside antibiotics produced by actinomycetes via repression of biosynthetic enzymes 11, 31. The glucose activates adenylate cyclase to convert ATP to cAMP by G-protein signaling pathway. Heterotrimeric G-proteins are acting within G-protein signaling pathways regulate multiple physiological processes and respond to nutrition are found to be involved in the regulation of secondary metabolite production 32. Stimulation of protein kinase activity by cAMP accumulation leads to phosphorylate the downstream targets resulting in various biological functions. Apparently, the pigment production in Penicillium sp. NIOM-02 may be regulated by cAMP signaling induced by the presence of glucose.
Conclusions
For successful production of Penicillium pigment for their application in food and nutraceutical industries, it requires a comprehensive knowledge of physiology and growth characteristics of the fungus. It is not only that, productions of different metabolites require different physiological conditions but also each fungus has its unique anatomical, morphological and physiological development. Therefore for maximum product yield, defined physiological conditions and appropriate stage of developments are important.
Acknowledgements
Mohan-Kumari, H. P, thanks CSIR (Council of Scientific Industrial Research), New-Delhi, India for financial support through research fellowship. We thank Dr. S. Umesh-Kumar, Head, Dept of Food Microbiology, CFTRI, Mysore, India for providing the facilities to carry out the work.




