Efficacy and safety of non-vitamin K antagonist oral anticoagulants versus warfarin in atrial fibrillation and kidney failure under hemodialysis: A meta-analysis of controlled randomized trials
Abstract
Background
Atrial fibrillation (AF)-related strokes are associated with disability and mortality. Stroke prevention with non-vitamin K antagonist oral anticoagulants (NOACs) and vitamin K antagonists (VKAs) is the cornerstone of holistic management of AF. However, the safety and efficacy of NOACs in patients with AF on hemodialysis remain uncertain. This meta-analysis aimed to evaluate currently available data to determine the potential utility of NOACs in AF patients with kidney failure receiving hemodialysis.
Methods
We searched the literature for randomized clinical trials comparing NOACs to VKA therapy in this population.
Results
About the Principal Efficacy Outcome, NOACs Did Not Decrease the Risk Compared to Warfarin (Relative Risk [RR] 0.79, 95% CI 0.45–1.37) while a Significant Heterogeneity Was Noted (p = 0.03). In the Valkyrie Study, Rivaroxaban Had better Cardiovascular Outcome than Warfarin (RR 0.57, 95% CI 0.43–0.75). For the Principal Safety Outcome, the Risk Was Similar between NOACs and Warfarin (RR 0.81, 95% CI 0.52–1.27) without Significant Heterogeneity (p = 0.11). The Pooled Event Rate of 3 Trials Disclosed a High Risk of all-Cause Mortality (39.9% for NOACs, 34.6% for Warfarin) and Cardiovascular Mortality (10.1% for NOACs, 8.5% for Warfarin) for AF Patients with Kidney Failure Receiving Hemodialysis Even on Oral Anticoagulants.
Conclusion
Our results suggest that NOACs (rivaroxaban or apixaban) are as safe and effective as VKAs in patients with AF and kidney failure with hemodialysis. Even on oral anticoagulants, these patients remain at high risk of cardiovascular events and all-cause mortality. Integrated care and holistic management are important for this high-risk population.
1 INTRODUCTION
Atrial fibrillation (AF), the most prevalent arrhythmia encountered in clinical practice, is associated with an increased risk of blood stasis and thrombus formation, leading to a heightened risk of stroke and thromboembolism.1 The prevalence of AF is projected to reach 4% by 2050, with a lifetime risk of approximately 1 in 7 for individuals aged ≥20 years.2 AF is known to elevate the risk of ischemic stroke, dementia, heart failure, myocardial infarction, and overall mortality.2
The AFNET study has identified that the primary causes of mortality in AF patients are peripheral artery disease, heart failure, diabetes mellitus, chronic obstructive pulmonary disease, and renal failure.3 Notably, AF-related strokes carry a significantly higher risk of disability and mortality compared to non-AF-related strokes.4 As such, preventing stroke is essential to the comprehensive management of AF.5
Stroke prevention in AF can be achieved through the use of oral anticoagulants, including vitamin K antagonists (VKAs) such as warfarin.5, 6 Four major randomized trials comparing warfarin to non-vitamin K antagonist oral anticoagulants (NOACs) have shown that NOACs are at least as effective as warfarin in preventing stroke and carry a markedly lower risk of intracranial hemorrhage.7 However, these studies excluded patients with kidney failure on hemodialysis, a population in which up to 25% have concomitant AF.8, 9 Advanced kidney failure is characterized by enhanced platelet aggregation and endothelial dysfunction, which create a prothrombotic environment. As a result, patients with AF on hemodialysis have a threefold increased incidence of ischemic stroke.10
With the aging population and increasing rates of AF and cardiovascular comorbidities, the number of individuals with end-stage renal disease is rising rapidly.11, 12 However, the safety and efficacy of NOACs in patients with AF on hemodialysis remain uncertain.13, 14 Due to the lack of robust prospective data on NOACs, if the decision to initiate anticoagulation has been made for patients with AF and kidney failure undergoing hemodialysis, warfarin has typically been the anticoagulant of choice in clinical practice. Nonetheless, interest in alternative anticoagulants has grown due to the high bleeding risk associated with VKAs in this population.14-18
Recent randomized controlled trials assessing the use of NOACs for stroke prevention in AF patients with kidney failure on hemodialysis have been published. However, these studies were limited by small sample sizes, which resulted in insufficient statistical power and hindered the ability to draw definitive conclusions. This meta-analysis aims to review the currently available data to assess the potential utility of NOACs in patients with AF and kidney failure on hemodialysis, providing further insight into this important clinical issue.
2 METHODS
2.1 Data sources
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols Statement of 2015.19 The review protocol was registered with the International Prospective Register of Systematic Reviews (http://www.crd.york.ac.uk/PROSPERO; registration number CRD42022374674). Two authors (TYC, CYL) independently searched the PubMed, Medline, and Cochrane Collaboration Central Register of Controlled Trials databases for studies published between January 2005 and June 2022, and any discrepancies were resolved by a third author (TFC). A combination of keywords or medical terms related to hemodialysis (e.g., dialysis, kidney failure), AF, and anticoagulation (e.g., oral anticoagulation, NOAC, Direct oral thrombin inhibitors, factor Xa inhibitors, dabigatran, rivaroxaban, apixaban, and Edoxaban) was used.
2.2 Selection criteria and data extraction
We included only registered randomized controlled trials (RCTs) comparing the efficacy and safety outcomes of NOACs and VKAs among AF patients with kidney failure receiving hemodialysis. Two authors (TYC, CYL) extracted the data independently, and any discrepancies were resolved via a third author (TFC) (Figure 1).
The principal efficacy and safety outcomes of the three analyzed studies are listed in Table 1. Individual outcomes included ischemic stroke, hemorrhagic stroke, clinically relevant non-major bleeding (CRNB), major bleeding, acute coronary syndrome (ACS), CV mortality, and all-cause mortality.
| Study, year | AXADIA-AFNET 8 study, 2022 | RENAL-AF trial, 2022 | Valkyrie study, 2021 |
|---|---|---|---|
| Enrolled patients | 97 | 154 | 132 |
| Age (mean) | 74.7 | 68 | 79.9 |
| Male | 68 (70.1%) | 98 (63.6%) | 88 (66.7%) |
| BMI | 28.6 | - | 24.9 |
| CHA2DS2-VASc score | 4.5 | 4 | 4.7 |
| HAS-BLED score | 4.2 | - | 4.7 |
| ESRD | 100% | 100% | 100% |
| Type of NOAC | Apixaban | Apixaban | Rivaroxaban |
| Dose (Apixaban, twice/day, Rivaroxaban, daily) | 2.5 mg |
55 patients (71%) 5 mg 22 patients (29%) 2.5 mg |
10 mg |
| Target INR | 2–3 | 2–3 | 2–3 |
| TTR | 50.7% | 44% | 48% |
| Antiplatelet drug | 33 (34%) | 64 (42.9%) | 46 (34.8%) |
| Hypertension | - | 146 (94.8%) | - |
| Old MI | 21 (21.7%) | 38 (24.7%) | 61 (46.2%) |
| Heart failure | - | 84 (54.5%) | 41 (31.1%) |
| Diabetes Mellitus | - | 89 (57.8%) | 62 (47%) |
| Paroxysmal AF | — | 85 (55.2%) | 60 (45.5%) |
| Old stroke | - | 29 (18.8%) | 40 (30.3%) |
| Principal efficacy endpoint | MI, ischemic stroke, all-cause death, and deep vein thrombosis and/or pulmonary embolism | All-cause death | fatal cardiovascular disease and non-fatal stroke, cardiac events, and other vascular events |
| Principal safety endpoint | All-cause death, major bleeding events and CRNB | Major bleeding events and CRNB | Life-threatening and major bleeding events |
| CRNB | ISTH consensus | ISTH consensus | Non-fatal, non-major bleeding |
| Follow-up (years) | 1.27 | 0.92 | 1.88 |
- Abbreviations: AF, atrial fibrillation; BMI, body mass index; CRNB, clinically relevant non-major bleeding; ESRD, end-stage renal disease; INR, international normalized ratio; ISTH, International Society on Thrombosis and Haemostasis; MI, myocardial infarction; NOAC, Non-vitamin K oral anticoagulants; TTR, time in therapeutic range.
2.3 Statistical analysis
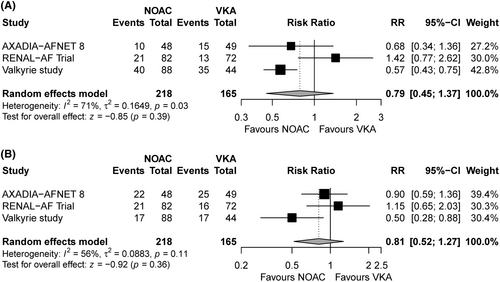
To explore the associations between NOAC/warfarin and outcomes of interest, we calculated relative risks (RRs) and related 95% confidence intervals (CIs) for binary variables, and mean differences and related 95% CIs for continuous variables. Pooled estimates with 95% CIs were estimated using inverse-variance weighted random-effects models with the DerSimonian and Laird estimators for interstudy variances (τ2). Continuity correction was used to calculate individual study results and conduct a meta-analysis based on the inverse-variance method. To quantify heterogeneity, I2 statistics and τ2 were assessed. Studies were considered heterogeneous if I2 was >50% or p was <0.05 for τ2. The results of the meta-analysis are displayed visually using forest plots (Figure 2A,B). All analyses were performed using RStudio (version 1.2.1335).
3 RESULTS
Table 1 summarizes the characteristics of enrolled studies.20-22 The three trials included a total of 383 patients (88 on rivaroxaban, 130 on apixaban) with a mean age ranging from 68 to 80 years. The mean CHA2DS2-VASc score was 4.7 in the Valkyrie study, 4 in the RENAL-AF trial, and 4.5 in the AXADIA-AFNET 8 study.20-22 The RENAL-AF trial and the AXADIA-AFNET 8 study adopted the standard CRNB criteria defined by the International Society on Thrombosis and Hemostasis. The Valkyrie study defined minor bleeding as bleeding that was neither fatal nor a major event. The mean follow-up duration ranged from 0.9 to 1.9 years.
The results of this study are presented in Figures 2 and 3. No significant differences were observed in the principal efficacy or safety endpoint between hemodialysis patients receiving NOACs versus VKAs. Regarding individual outcomes, no significant intergroup differences were observed in the risks of hemorrhagic stroke, major bleeding, CRNB, ACS, CV mortality, or all-cause mortality. Although a trend toward decreased risk of ischemic stroke was observed in patients receiving NOACs, the difference did not reach statistical significance (RR, 0.42; 95% CI, 0.17–1.04).
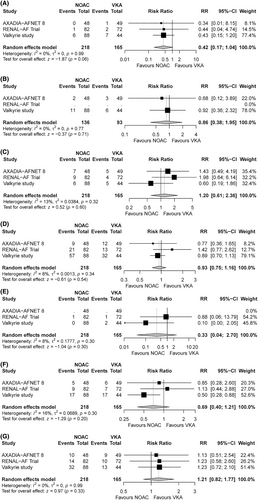
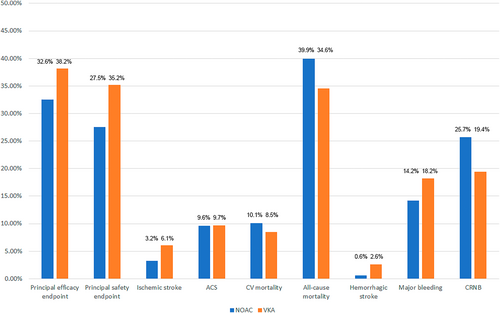
Figure 4 shows the event rates of the pooled NOAC and VKA groups. The percentages of all-cause mortality (NOACs 39.6%, VKA 34.6%) and major bleeding (NOACs 14.2%, VKA 18.2%) were higher than those of ischemic stroke (NOACs 3.2%, VKA 6.1%).
4 DISCUSSION
The main study findings were1 NOAC use did not increase bleeding rates or mortality in AF patients with kidney failure receiving hemodialysis compared with VKA use,2 NOAC use displayed a trend toward a decreased ischemic stroke rate compared with VKA use, and3 the high number of deaths and bleeding events relative to ischemic stroke events raises the need for more holistic and integrated AF management to improve clinical outcomes in patients with AF and kidney failure receiving hemodialysis.
To the best of our knowledge, this is the first meta-analysis of RCTs comparing the efficacy and safety profiles of NOACs with VKAs in patients with AF and kidney failure on hemodialysis, providing a detailed comparison of event rates between pooled NOAC- and VKA-treated cohorts. Unlike previous meta-analyses that incorporated observational studies and patients at various stages of chronic kidney disease, the present study exclusively focused on RCTs involving patients with end-stage renal disease on hemodialysis, a population that has been underrepresented in earlier research. Our findings suggest that the efficacy and safety of NOACs are comparable to those of warfarin in preventing acute coronary syndrome (ACS), cardiovascular mortality, all-cause mortality, hemorrhagic stroke, major bleeding, and clinically relevant non-major bleeding (CRNB). Furthermore, while there was a trend toward a reduction in ischemic stroke with NOACs compared to warfarin, this difference did not reach statistical significance.
4.1 Use of VKAs in patients on hemodialysis
VKAs, such as warfarin, are commonly prescribed for patients with AF. However, the use of VKAs in dialysis patients is associated with an increased risk of bleeding. Previous studies investigating the effects of VKAs in this population have yielded conflicting results.10, 14, 17, 23-28 Some studies suggested a reduced risk of stroke14 or a survival benefit23, 29, 30 with VKA use compared to no oral anticoagulant therapy, while other studies found no protective effect of VKAs on stroke risk or all-cause mortality.17, 25-28, 31
A meta-analysis involving more than 9000 dialysis patients with AF found that VKA treatment was associated with a 1.2-fold increased risk of stroke (95% CI, 0.8–1.9).24 Additionally, several studies have reported an increased risk of bleeding with VKA use.28, 32 Contributing factors such as pre-existing platelet dysfunction, routine heparinization during hemodialysis, and suboptimal time in therapeutic range (TTR) may exacerbate this bleeding risk.33, 34 In the current RCTs included in our analysis, the mean TTR in the VKA groups was consistently low, ranging from 44% to 50.7% (AXADIA-AFNET 8 study, RENAL-AF trial, and Valkyrie study), with patients being three times more likely to be subtherapeutic (international normalized ratio [INR] <2.0) than supratherapeutic (INR >3.0). The efficacy of warfarin may be compromised by a low TTR, and bleeding risks could increase if conventional TTR targets were achieved. Nonetheless, the low TTR observed in these trials also underscores the potential advantage of NOACs in this population.
4.2 Use of NOACs in patients on dialysis
In a large retrospective cohort study that included over 25,000 patients from the United States Renal Data System, standard-dose apixaban (5 mg twice daily) was associated with significantly lower rates of stroke, systemic embolism, and mortality compared to both warfarin and low-dose apixaban (2.5 mg twice daily). Furthermore, both dosing regimens of apixaban (5 mg or 2.5 mg twice daily) demonstrated lower rates of major bleeding compared to warfarin, with the standard-dose apixaban (5 mg twice daily) also being associated with reduced rates of thromboembolic events and mortality.
A recently published meta-analysis, which included data from three RCTs and three observational studies, reported comparable outcomes between patients treated with NOACs and those treated with VKAs. The rates of ischemic stroke or systemic embolism (relative risk [RR]: 0.65, 95% confidence interval [CI]: 0.38–1.10), major bleeding events (RR: 0.79, 95% CI: 0.49–1.28), and all-cause mortality (RR: 0.79, 95% CI: 0.56–1.12) were similar between the two groups. As compared with our study, in addition to ischemic stroke and bleeding events, we further reported the principal efficacy and safety endpoints, cardiovascular events including acute coronary syndrome and cardiovascular death. Moreover, we demonstrated the high number of deaths and bleeding events relative to ischemic stroke events in the result of pooled NOAC and VKA study groups, which raises the need for more holistic and integrated AF management in hemodialysis patients, such as shared decision making in the prescription of anticoagulants.
The current meta-analysis demonstrated that NOACs provide efficacy and safety outcomes comparable to warfarin. However, interstudy heterogeneity was observed in the analysis of trial-defined efficacy endpoints, largely driven by the reduced efficacy outcomes reported in the Valkyrie study, which included fatal cardiovascular disease, non-fatal stroke, cardiac events, and other vascular events as principal efficacy endpoints. In contrast, the AXADIA-AFNET 8 study defined efficacy endpoints as thromboembolic events, including myocardial infarction, ischemic stroke, all-cause mortality, and deep vein thrombosis and/or pulmonary embolism. Since the RENAL-AF trial was not designed to evaluate efficacy, the current analysis used all-cause mortality as a surrogate endpoint.
4.3 Recommendations of guidelines about oral anticoagulants in AF patients on hemodialysis
The JCS/JHRS 2024 Guideline Focused Update on Management of Cardiac Arrhythmias stated that warfarin is not recommended for patients on dialysis (class III recommendation).35 Also, the prior JCS/JHRS 2020 Guideline on Pharmacotherapy of Cardiac Arrhythmias stated that NOACs are contraindicated in patients on dialysis, and so is warfarin except in some cases, such as the perioperative period of AF ablation, mechanical valves, and for secondary stroke prevention.36 The “weak” recommendations of routine use of OACs (either warfarin or NOACs) in AF patients on dialysis are mainly due to the lack of high-quality trials demonstrating the positive net clinical benefits of OACs compared to non-anticoagulation in this population at a high bleeding risk. In 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation, the use of warfarin or apixaban in AF patients on dialysis was a class IIb recommendation.37 Apixaban was listed based on the results of some retrospective analyses of databases and the trials of apixaban compared to warfarin, rather than any randomized trials of apixaban compared to non-anticoagulation. This issue would remain controversial until the randomized trials are available which directly compare NOACs to warfarin in AF patients on hemodialysis. Based on current guideline recommendations and the findings of the present study, NOACs may be considered only for secondary stroke prevention in AF patients on hemodialysis (as suggested by the 2020 JCS/JHRS guidelines), with apixaban and rivaroxaban being the preferred choices among NOACs—and likely preferred over warfarin—once the decision to initiate anticoagulation is made.
4.4 Holistic management of AF patients with kidney failure receiving hemodialysis
Among patients across three RCTs, 144 individuals (37.6%) died, 61 (15.9%) experienced a major bleeding event, 88 (23%) suffered a CRNB event, and 17 patients (4.4%) experienced an ischemic stroke (Figure 4). A recently published meta-analysis further corroborated these findings, highlighting a high incidence of both minor and major bleeding events as well as all-cause mortality.38 The substantial burden of deaths and bleeding complications compared to ischemic stroke events underscores the necessity of a more comprehensive and integrated approach to AF management, particularly for patients with AF and kidney failure undergoing hemodialysis. In this context, the ABC (Atrial fibrillation Better Care) pathway has been proposed as an integrated management strategy aiming at reducing AF-related mortality, morbidity, and hospitalizations.39
4.5 Study limitation
We included three RCTs in our analysis: the VALKYRIE study, the RENAL-AF trial, and the AXADIA-AFNET 8 study. There were several limitations of the present study. First, the limited number of patients, which may lead to an underpowered analysis, is a major limitation of our study. However, only these three RCTs are currently available in the literature for a meta-analysis assessing the efficacy and safety of NOACs versus VKAs in AF patients on hemodialysis. Therefore, despite the small sample size, we believe the findings remain important, as this is a clinical issue we frequently encounter in daily practice. At the very least, the available data can help inform our discussions with patients and support shared decision making. Second, apixaban and rivaroxaban are distinct drugs with differing pharmacokinetics, administration methods, and clinical indications, so these differences might have influenced the pooled results. However, separate analysis would be difficult to provide more information due to the limited enrolled trials and patient number (1 rivaroxaban trial and 2 apixaban trials). Furthermore, the consistently low time in the therapeutic range observed in these trials reflects the real-world quality of VKA therapy in this patient population. To address these limitations, a meta-analysis was conducted to integrate findings across the studies, offering a more comprehensive perspective that may inform future clinical care.
5 CONCLUSION
Our results suggest that NOACs (rivaroxaban or apixaban) are as safe and effective as VKAs in patients with AF and kidney failure receiving hemodialysis. Even on oral anticoagulants, these patients remain at high risk of cardiovascular events and all-cause mortality. Integrated care and holistic management are important for this high-risk population.
ACKNOWLEDGMENTS
None.
FUNDING INFORMATION
This study was supported, in part, by research grants from the Taipei Veterans General Hospital (V108C-055, V109C-008, V110C-004, V111C-075), Ministry of Science and Technology of Taiwan (MOST 111-2314-B-075-007-MY3, 113-2628-B-075-003-MY3, 113-2314-B-075-029-MY3), SZU-YUAN RESEARCH FOUNDATION OF INTERNAL MEDICINE (108010, 109024, 110008, 111003, 111050), and “Yin Yen-Liang Foundation Development and Construction Plan” of the College of Medicine, National Yang Ming Chiao Tung University.
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data are incorporated into the article and its online supplementary material.




