Number needed to treat for net clinical benefit of oral anticoagulants in Asian patients with atrial fibrillation
Abstract
Background
Oral anticoagulants (OAC) can reduce ischemic stroke/systemic embolism (SSE) in patients with non-valvular atrial fibrillation (AF) while increasing the risk of major bleeding. We aimed to analyze the number needed to treat for the net benefit (NNTnet) of warfarin and non-vitamin K antagonist oral anticoagulants (NOACs).
Methods
We analyzed the results from multicenter national AF registry from 27 hospitals in Thailand. Follow-up data were collected every 6 months until 3 years. Main outcomes were SSE, major bleeding, and intracranial hemorrhage (ICH). NNT was calculated from the absolute risk reduction (ARR) of SSE or absolute risk increase (ARI) of major bleeding or ICH. We compared NNTnet of warfarin versus no OAC, NOACs versus no OAC, and NOACs versus warfarin. Warfarin was also categorized into time in therapeutic range (TTR) < and ≥65%.
Results
We studied a total of 3405 patients (mean age 67.8 ± 11.3 years, 1424 (41.8%) were female). The incidence rates of SSE, major bleeding, and ICH were 1.51, 2.25, and 0.78 per 100 person-years, respectively. Warfarin had negative NNTnet −37 compared to no OAC. NOACs had positive NNTnet 101 and 27 compared to no OACs and warfarin. Warfarin with TTR 65% had positive NNTnet 42 compared to no OAC. NOACs had comparable NNTnet as warfarin with TTR ≥65%.
Conclusion
Warfarin had a negative NNTnet compared to no OAC. Only warfarin with TTR 65% has positive NNTnet. NOACs had positive NNTnet compared to no OAC and when compared to warfarin.
1 INTRODUCTION
Patients with non-valvular atrial fibrillation (AF) are at risk of thromboembolic complications such as ischemic stroke. Hence, most patients with AF had at least one additional risk factor for ischemic stroke are recommended oral anticoagulants for stroke prevention.1, 2 OACs significantly reduce ischemic stroke risk by 64% and all-cause mortality by 26% compared to placebo of control,3 but increase risk of major bleeding including intracranial hemorrhage (ICH), which is of particular concern in Asians.4 More recently, the non-vitamin K antagonist oral anticoagulants (NOACs; also referred to as Direct Oral Anticoagulants, DOACs) are at least as good as warfarin for the reduction of ischemic stroke with the rate of ICH approximately half compared to warfarin.5
However, the relative risk reduction does not necessarily translate into the absolute benefit to the patients and also the impact on healthcare policy. A treatment that had relative risk reduction of greater than 50% might have only a small absolute risk reduction and thereby should not be necessarily recommended to be used in the population as a whole. Despite the superior recommendation for the use of NOACs compared to warfarin for AF patients who are at risk of ischemic stroke, the rate of warfarin use still remains high especially in low-income or low-to-middle-income countries (LIC and LMIC) mainly because of the high costs of medications.1, 6 Besides, Asian population have an increased risk of major bleeding and ICH compared to non-Asians, irrespective of whether warfarin or NOACs are used.7-10
In this study, we aimed to determine the number needed to treat for the net clinical benefit of warfarin and NOACs in patients with AF using the data from the nationwide prospective COhort of antithrombotic use and Optimal INR Level in patients with non-valvular atrial fibrillation in Thailand (COOL-AF) registry.
2 METHODS
2.1 Study population
The COhort of antithrombotic use and Optimal INR Level in patients with non-valvular atrial fibrillation in Thailand (COOL-AF) registry is a prospective nationwide registry involved 27 hospitals in Thailand. Patients with a diagnosis of non-valvular AF with age at least 18 years were enrolled. The enrollment period was 2014–2017. The exclusion criteria were (1) rheumatic valve disease (2) mechanical valve (3) ischemic stroke within 3 months (4) AF from transient reversible cause (5) ongoing participation in clinical trial (6) life expectancy less than 3 years (7) hematologic disease that increased bleeding risk such as thrombocytopenia (platelet <100 000/mm3) (8) current hospitalization (9) refusal to participate and (10) inability to come for follow-up visits. This study was approved by the Institutional Review Board of the Central Research Ethic Committee (CREC)(Certificate of Approval number 003/2014). All patients gave written informed consent before participation. The study was performed following the principles of the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice Guidelines. The study protocol has been previously published.1
Written informed consent was obtained from every patient. All methods were conducted in accordance with the principles set forth in the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice Guidelines.
2.2 Study protocol
Investigators were instructed to enroll consecutive AF patients according to the inclusion and exclusion criteria. After the informed consent process, investigators reviewed medical record, interviewed patients and entered the data into the case record form (CRF) and into the web system. All data were doubled entry by the data management site to ensure the correctness of the data. Verification process was performed by communication between the central site and the study site, and the mistake was corrected accordingly. Site monitoring was performed for every study site to ensure that the study was conducted in concordance with the Good Clinical Practice (GCP) guideline and to maintain good data quality.
2.3 Data collection
Data were collected at baseline and every 6 months until 3 years. The following data were collected; age, sex, comorbid conditions and cardiovascular risk factors such as hypertension, cigarette smoking, type 2 diabetes (T2D), hypercholesterolemia, coronary artery disease, chronic kidney disease, investigation data such as ECG, laboratory results, eachocardiography, medications. Components of CHA2DS2−VASc score [C = congestive heart failure (1 point); H = hypertension (1 point); A = age > 75 years (2 points); D = diabetes (1 point); S = stroke (2 points); V = vascular disease (1 point); A = age 65–74 (1 point); and Sc = female sex category (1 point)]11 and HAS-BLED score [uncontrolled Hypertension, Abnormal renal, or liver function; history of Stroke; history of Bleeding; Labile INR; Elderly (age above 65 years); and, Drugs or alcohol (1 point each)]12 were recorded.
2.4 Outcomes
Main outcomes of this study were ischemic stroke or systemic embolism (SSE), major bleeding, and intracranial hemorrhage (ICH). Ischemic stroke was defined as sudden-onset neurologic deficit lasting greater than 24 h or transient ischemic attack (TIA) for the duration of the neurologic deficit less than 24 h. Systemic embolism was defined as a clinical and objective evidence of the sudden loss of end-organ perfusion. Major bleeding was defined using criteria published by the International Society of Thrombosis and Haemostasis (ISTH), which includes at least one of the following (1) fatal bleeding, (2) bleeding in critical area or organs, or (3) bleeding that results in a decrease in hemoglobin level of 20 g/L or more, or that requires a transfusion of 2 units of red cells or more. ICH was defined as the bleeding within the skull (may be intracerebral bleeds, subarachnoid bleeds, subdural bleeds, or epidural bleeds). According to the Bleeding Academic Research Consortium (BARC) definition, microbleeds and secondary hemorrhagic transformation were not counted as ICH.13
All events were reviewed and validated by the study investigator team at the data management unit. After validation, all data were sent to the adjudication committee for confirmation. In cases with inconclusive evidence or further questions, additional data or explanation was requested from the study site for clarification.
2.5 Statistical analysis
Description of continuous data were made by mean and standard deviation (SD) and categorical data by count and percentages. Incidence rates of clinical outcomes are shown as rate per 100 person-years. Test for the differences of continuous data were made by the student's t-test for unpaired data and for categorical data by the chi-square test. Actual 3-year risks of outcomes were displayed as percentages and 95% confidence interval (CI). The relative risk reduction (RRR) or increase (RRI) was calculated from the proportion of the change in absolute risk in treatment group compared to no treatment group. Absolute risk reduction (ARR) or increase (ARI) was calculated from the change in actual risk between treatment group and no treatment group. Number needed to treat (NNT) for benefit (NNTbenefit) and harm (NNTharm) was calculated from the reciprocal of ARR and ARI. NNT of net clinical benefit (NNTnet) derived from the reciprocal of difference in ARR and ARI.14 The calculation for relative risk reduction or increase, absolute risk reduction or increase, and number needed to treat for net clinical benefit is shown in Table S1. In this study, we calculate NNTnet of warfarin compared to no OAC, NOAC compared to no OAC, and NOAC compared to warfarin. A separate subgroup analysis of patients with time in therapeutic range (TTR) <65% and ≥65% for warfarin group. The sensitivity analysis was performed on NNTnet for patients in the low-risk and intermediate to high-risk category.
Low risk was defined by CHA2DS2-VASc score = 0 in male or 1 in female, intermediate to high-risk was defined as CHA2DS2-VASc score ≥1 in male or ≥2 in female. All statistical analysis was performed by the SPSS Statistics software (SPSS, Inc., Chicago, IL, USA) and MedCalc Statistical Software version 12.5.0.0 (MedCalc Software bv, Ostend, Belgium). A p-value <0.05 was considered statistical significance.
3 RESULTS
3.1 Study population
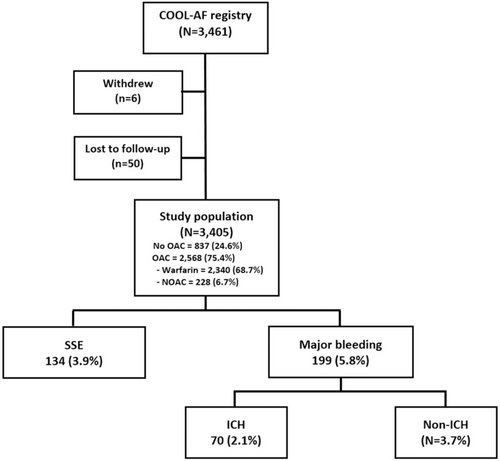
We studied a total of 3405 patients (mean age was 67.8 ± 11.3 years, 1424 (41.8%) were female). Patients were classified into a low-risk group and intermediate to high-risk group. Table 1 shows baseline characteristics of study population and details in low-risk and intermediate to high-risk groups. Patients in the intermediate to high-risk group are older, more likely female, and had more co-morbid conditions compared to the low-risk group. A flow diagram of the study population is shown in Figure 1.
| Variables | All (n = 3405) | Low risk (n = 287) | Intermediate to high risk (n = 3118) | p-value |
|---|---|---|---|---|
| Age (years) | 67.8 ± 11.3 | 54.6 ± 8.0 | 69.0 ± 10.7 | <.001* |
| Female gender | 1424 (41.8%) | 91 (31.7%) | 1333 (42.8%) | <.001* |
| BMI (kg/m2) | 25.2 ± 4.7 | 24.7 ± 3.9 | 25.2 ± 4.8 | .020* |
| Time after diagnosis of AF (years) | 3.4 ± 4.3 | 2.9 ± 3.8 | 3.4 ± 4.4 | .037* |
| Atrial fibrillation | <.001* | |||
| Paroxysmal | 1148 (33.7%) | 148 (51.6%) | 1000 (32.1%) | |
| Persistent | 645 (18.9%) | 46 (16.0%) | 599 (19.2%) | |
| Permanent | 1612 (47.3%) | 93 (32.4%) | 1519 (48.7%) | |
| Symptomatic AF | 2620 (76.9%) | 245 (85.4%) | 2375 (76.2%) | <.001* |
| History of heart failure | 913 (26.8%) | 1 (0.3%) | 912 (29.2%) | <.001* |
| History of CAD | 547 (16.1%) | 0 (0.0%) | 547 (17.5%) | <0.001* |
| CIED | 341 (10.0%) | 24 (8.4%) | 317 (10.2%) | .330 |
| History of ischemic stroke/TIA | 592 (17.4%) | 0 (0.0%) | 592 (19%) | <.001* |
| Diabetes mellitus | 839 (24.6%) | 0 (0.0%) | 839 (26.9%) | <.001* |
| Hypertension | 2330 (68.4%) | 0 (0.0%) | 2330 (74.7%) | <.001* |
| Smoking | 678 (19.9%) | 72 (25.1%) | 606 (19.4%) | .022* |
| Dyslipidemia | 1917 (56.3%) | 73 (25.4%) | 1844 (59.1%) | <.001* |
| Renal replacement therapy | 40 (1.2%) | 2 (0.7%) | 38 (1.2%) | .771 |
| Dementia | 29 (0.9%) | 0 (0.0%) | 29 (0.9%) | .169 |
| Systemic embolism | 25 (0.7%) | 2 (0.7%) | 23 (0.7%) | .938 |
| History of peripheral vascular disease | 44 (1.3%) | 0 (0.0%) | 44 (1.4%) | .048* |
| History of PCI | 253 (46.2%) | 0 (0.0%) | 253 (8.1%) | <.001* |
| History of CABG | 65 (12.0%) | 0 (0.0%) | 65 (2.1%) | .014* |
| History of bleeding | 324 (9.5%) | 5 (1.7%) | 319 (10.2%) | <.001* |
| CKD | 1756 (51.6%) | 34 (11.8%) | 1722 (55.2%) | <.001* |
| Anemia | 1293 (38.0%) | 16 (5.6%) | 1277 (41%) | <.001* |
| HAS-BLED score | <.001* | |||
| 0 | 490 (14.4%) | 165 (57.5%) | 325 (10.4%) | |
| 1-2 | 2375 (69.8%) | 121 (42.2%) | 2254 (72.3%) | |
| ≥3 | 540 (15.9%) | 1 (0.3%) | 539 (17.3%) | |
| Antiplatelet | 892 (26.2%) | 69 (24.0%) | 823 (26.4%) | .386 |
| Anticoagulant | 2568 (75.4%) | 102 (35.5%) | 2466 (79.1%) | <.001* |
| Warfarin | 2340 (68.7%) | 81 (28.2%) | 2259 (72.5%) | <.001* |
| NOACs | 228 (6.7%) | 21 (7.3%) | 207 (6.6%) | .600 |
- Abbreviations: AF, atrial fibrillation; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; CIED; cardiac implantable electronic device; CKD, chronic kidney disease; NOACs, Non-vitamin K antagonist oral anticoagulants; PCI, percutaneous coronary intervention; TIA, transient ischemic attack. Bold indicate statistical significance.
3.2 NNTbenefit, NNTharm, and NNTnet
The incidence rates of SSE, major bleeding, and ICH were 1.51, 2.25, and 0.78 per 100 person-years, respectively. The incidence rate of SSE, major bleeding, and ICH for the whole group, no OAC, and each OAC category including warfarin and NOACs are shown in Table 2. Warfarin was further subcategorized into those with TTR < and ≥65%.
| Number of patients | Number of events | 100 person-years | Rate per 100 person-years | |
|---|---|---|---|---|
| SSE—all patients | 3405 | 134 | 88.99 | 1.51 (1.26–1.78) |
| OAC | 2568 | 92 | 67.19 | 1.37 (1.10–1.68) |
| Warfarin | 2340 | 87 | 60.94 | 1.43 (1.14–1.76) |
| TTR < 65% | 1432 | 64 | 37.24 | 1.72 (1.32–2.19) |
| TTR ≥65% | 801 | 18 | 21.98 | 0.82 (0.49–1.29) |
| NOAC | 228 | 5 | 6.25 | 0.8 (0.26–1.87) |
| No OAC | 837 | 42 | 21.81 | 1.93 (1.39–2.60) |
| Major bleeding—all patients | 3405 | 199 | 88.36 | 2.25 (1.95–2.59) |
| OAC | 2568 | 174 | 66.22 | 2.63 (2.25–3.04) |
| Warfarin | 2340 | 163 | 60.03 | 2.72 (2.31–3.17) |
| TTR < 65% | 1432 | 115 | 36.56 | 3.15 (2.60–3.78) |
| TTR ≥65% | 801 | 39 | 21.74 | 1.79 (1.28–2.45) |
| NOAC | 228 | 11 | 6.19 | 1.78 (0.89–3.18) |
| No OAC | 837 | 25 | 22.14 | 1.13 (0.73–1.67) |
| ICH—all patients | 3405 | 70 | 89.85 | 0.78 (0.61–0.98) |
| OAC | 2568 | 64 | 67.56 | 0.95 (0.73–1.21) |
| Warfarin | 2340 | 59 | 61.30 | 0.96 (0.73–1.24) |
| TTR < 65% | 1432 | 43 | 37.49 | 1.15 (0.83–1.55) |
| TTR ≥ 65% | 801 | 9 | 22.06 | 0.41 (0.19–0.78) |
| NOAC | 228 | 5 | 6.26 | 0.8 (0.26–1.86) |
| No OAC | 837 | 6 | 22.29 | 0.27 (0.10–0.59) |
- Abbreviations: ICH, intracranial hemorrhage; NOACs, non-vitamin K antagonist oral anticoagulants; OAC, oral anticoagulants; SSE, ischemic stroke/systemic embolism; TTR, time in therapeutic range.
Table 3-part A shows the RRR, RRI, ARR, ARI, NNTbenefit, NNTharm, and NNTnet of SSE versus major bleeding and SSE versus ICH for (1) warfarin compared to no OAC, (2) NOAC compared to no OAC, and (3) NOAC compared to warfarin.
| SSE risk at 3-years | Warfarin vs No OACa | NOAC vs No OACa | NOAC vs warfarina |
|---|---|---|---|
| Risk of comparator group (%) | 5.02 (3.62–6.78) | 5.02 (3.62–6.78) | 3.72 (2.98–4.59) |
| Risk of treatment group (%) | 3.72 (2.98–4.59) | 2.19 (0.71–5.12) | 2.19 (0.71–5.11) |
| Relative risk reduction | 0.26 | 0.56 | 0.41 |
| Absolute risk reduction (%) | 1.30 | 2.82 | 1.52 |
| NNTbenefit | 76.92 | 35.40 | 65.58 |
| Major bleeding risk at 3-years | |||
| Risk of comparator group (%) | 2.99 (1.93–4.41) | 2.99 (1.93–4.41) | 6.97 (5.94–8.12) |
| Risk of treatment group (%) | 6.97 (5.94–8.12) | 4.82 (2.41–8.63) | 4.82 (2.41–8.63) |
| Relative risk increase | 1.33 | 0.62 | −0.31 |
| Absolute risk increase (%) | 3.98 | 1.84 | −2.14 |
| NNTharm | 25.13 | 54.42 | −46.70 |
| NNTnet | −37.33 | 101.29 | 27.28 |
| ICH risk at 3-years | |||
| Risk of comparator group (%) | 0.72 (0.26–1.56) | 0.72 (0.26–1.56) | 2.52 (2.46–2.59) |
| Risk of treatment group (%) | 2.52 (1.92–3.25) | 2.19 (0.71–5.12) | 2.19 (0.71–5.11) |
| Relative risk increase | 2.52 | 2.06 | −0.13 |
| Absolute risk increase (%) | 1.80 | 1.48 | −0.33 |
| NNTharm | 55.42 | 67.74 | −304.52 |
| NNTnet | −198.20 | 74.14 | 53.96 |
| Warfarin vs No OACa | NOAC vs No OACa | NOAC vs Warfarina | ||||
|---|---|---|---|---|---|---|
| Low risk | Intermediate to high risk | Low risk | Intermediate to high risk | Low risk | Intermediate to high risk | |
| SSE risk at 3-years | ||||||
| Risk of comparator group (%) | 2.16 (0.59–5.54) | 5.83 (4.12–8.00) | 2.16 (0.59–5.54) | 5.83 (4.12–8.00) | 1.23 (0.03–6.88) | 3.81 (3.05–4.70) |
| Risk of treatment group (%) | 1.23 (0.03–6.88) | 3.81 (3.05–4.70) | 0.00 (0.00–0.00) | 2.42 (0.78–5.64) | 0.00 (0.00–0.00) | 2.42 (0.78–5.64) |
| Relative risk reduction | 0.43 | 0.35 | 1.00 | 0.59 | 1.00 | 0.37 |
| Absolute risk reduction (%) | 0.93 | 2.02 | 2.16 | 3.41 | 1.23 | 1.39 |
| NNTbenefit | 107.81 | 49.47 | 46.25 | 29.30 | 81.00 | 71.86 |
| Major bleeding risk at 3-years | ||||||
| Risk of comparator group (%) | 0.00 (0.00–0.00) | 3.83 (2.48–5.66) | 0.00 (0.00–0.00) | 3.83 (2.48–5.66) | 4.94 (1.35–12.64) | 7.04 (5.99–8.22) |
| Risk of treatment group (%) | 4.94 (1.35–12.64) | 7.04 (5.99–8.22) | 0.00 (0.00–0.00) | 5.31 (2.65–9.51) | 0.00 (0.00–0.00) | 5.31 (2.65–9.51) |
| Relative risk increase | - | 0.84 | - | 0.39 | −1.00 | −0.25 |
| Absolute risk increase (%) | 4.94 | 3.20 | 0 | 1.48 | −4.94 | −1.72 |
| NNTharm | 20.25 | 31.21 | - | 67.58 | −20.25 | −57.99 |
| NNTnet | −24.93 | −84.54 | 46.25 | 51.73 | 16.20 | 32.09 |
| ICH risk at 3-years | ||||||
| Risk of comparator group (%) | 0.00 (0.00–0.00) | 0.92 (0.34–2.00) | 0.00 (0.00–0.00) | 0.92 (0.34–2.00) | 2.47 (0.30–8.92) | 2.52 (1.91–3.27) |
| Risk of treatment group (%) | 2.47 (0.30–8.92) | 2.52 (1.91–3.27) | 0.00 (0.00–0.00) | 2.42 (0.78–5.64) | 0.00 (0.00–0.00) | 2.42 (0.78–5.64) |
| Relative risk increase | - | 1.74 | - | 1.62 | −1.00 | −0.04 |
| Absolute risk increase (%) | 2.47 | 1.60 | 0.00 | 1.50 | −2.47 | −0.11 |
| NNTharm | 40.50 | 62.38 | - | 66.88 | −40.50 | −927.80 |
| NNTnet | −64.87 | 239.10 | 46.25 | 52.15 | 27.00 | 66.70 |
- Abbreviations: ICH, intracranial hemorrhage; NNT, number needed to treat; NOACs, non-vitamin K antagonist oral anticoagulants; OAC, oral anticoagulants; SSE, ischemic stroke/systemic embolism; vs., versus.
- a Comparator group.
Warfarin had a negative NNTnet compared to no OAC for SSE versus major bleeding and also for SSE versus ICH which meant that warfarin overall had more risk than benefit. NNTnet was smaller for SSE versus major bleeding indicated that the magnitude of differences was greater for SSE versus major bleeding compared to SSE versus ICH.
NOAC had a positive NNTnet compared to no OAC both for major bleeding and ICH. NNT was similar for major bleeding and ICH indicated that the effect of NOAC on major bleeding and ICH was relatively similar. NOACs had a positive NNTnet compared to warfarin for both major bleeding and ICH.
3.3 Warfarin versus no OAC or NOACs according to TTR < and ≥65% (Table 4-part a)
| SSE risk at 3-years | Warfarin (TTR < 65%) vs No OACa | Warfarin (TTR ≥ 65%) vs No OACa | NOAC vs warfarin (TTR < 65%)a | NOAC vs warfarin (TTR ≥ 65%)a |
|---|---|---|---|---|
| Risk of comparator group (%) | 5.02 (3.62–6.78) | 5.02 (3.62–6.78) | 4.47 (3.34–5.71) | 2.25 (1.33–3.55) |
| Risk of treatment group (%) | 4.47 (4.36–4.58) | 2.25 (2.14–2.35) | 2.19 (0.71–5.12) | 2.19 (0.71–5.12) |
| Relative risk reduction | 0.11 | 0.55 | 0.51 | 0.02 |
| Absolute risk reduction (%) | 0.55 | 2.77 | 2.28 | 0.05 |
| NNTbenefit | 182.27 | 36.09 | 43.93 | 1844.73 |
| Major bleeding risk at 3-years | ||||
| Risk of comparator group (%) | 2.99 (1.93–4.41) | 2.99 (1.93–4.41) | 8.03 (6.63–9.64) | 4.87 (3.46–6.66) |
| Risk of treatment group (%) | 8.03 (7.88–8.18) | 4.87 (4.71–5.02) | 4.82 (2.41–8.63) | 4.82 (2.41–8.63) |
| Relative risk increase | 1.69 | 0.63 | −0.40 | −0.01 |
| Absolute risk increase (%) | 5.04 | 1.88 | −3.21 | −0.04 |
| NNTharm | 19.83 | 53.13 | −31.19 | −2254.67 |
| NNTnet | −22.25 | 112.53 | 18.24 | 1014.60 |
| ICH risk at 3-years | ||||
| Risk of comparator group (%) | 0.72 (0.26–1.56) | 0.72 (0.26–1.56) | 3.00 (2.17–4.05) | 1.12 (0.51–2.13) |
| Risk of treatment group (%) | 3.00 (2.91–3.09) | 1.12 (1.05–1.20) | 2.19 (0.71–5.12) | 2.19 (0.71–5.12) |
| Relative risk increase | 3.19 | 0.57 | −0.27 | 0.95 |
| Absolute risk increase (%) | 2.29 | 0.41 | −0.81 | 1.07 |
| NNTharm | 43.75 | 245.85 | −123.49 | 93.51 |
| NNTnet | −57.56 | 42.30 | 32.40 | −98.50 |
| Warfarin (TTR < 65%) vs No OACa | Warfarin (TTR ≥ 65%) vs No OACa | NOAC vs Warfarin (TTR < 65%)a | NOAC vs Warfarin (TTR ≥ 65%)a | |||||
|---|---|---|---|---|---|---|---|---|
| Low risk | Intermediate to high risk | Low risk | Intermediate to high risk | Low risk | Intermediate to high risk | Low risk | Intermediate to high risk | |
| SSE risk at 3-years | ||||||||
| Risk of comparator group (%) | 2.16 (0.59–5.54) | 5.83 (4.12–8.00) | 2.50 (2.03–3.04) | 4.53 (4.41–4.64) | 0.00 (0.00–0.00) | 2.36 (2.25–2.47) | 2.16 (0.59–5.54) | 5.83 (4.12–8.00) |
| Risk of treatment group (%) | 2.50 (2.03–3.04) | 4.53 (4.41–4.64) | 0.00 (0.00–0.00) | 2.42 (2.21–2.64) | 0.00 (0.00–0.00) | 2.42 (2.21–2.64) | 0.00 (0.00–0.00) | 2.36 (2.25–2.47) |
| Relative risk reduction | −0.16 | 0.22 | 1.00 | 0.47 | - | −0.03 | 1.00 | 0.60 |
| Absolute risk reduction (%) | −0.34 | 1.30 | 2.50 | 2.11 | - | −0.06 | 2.16 | 3.47 |
| NNTbenefit | −296.00 | 76.78 | 40.00 | 47.38 | - | −1682.43 | 46.25 | 28.80 |
| Major bleeding risk at 3-years | ||||||||
| Risk of comparator group (%) | 0.00 (0.00–0.00) | 3.83 (2.48–5.66) | 5.00 (4.33–5.74) | 8.12 (7.97–8.27) | 5.41 (4.68–6.21) | 4.84 (4.69–5.00) | 0.00 (0.00–0.00) | 3.83 (2.48–5.66) |
| Risk of treatment group (%) | 5.00 (4.33–5.74) | 8.12 (7.97–8.27) | 0.00 (0.00–0.00) | 5.31 (5.00–5.64) | 0.00 (0.00–0.00) | 5.31 (5.00–5.64) | 5.41 (4.68–6.21) | 4.84 (4.69–5.00) |
| Relative risk increase | - | 1.12 | −1.00 | −0.35 | −1.00 | 0.10 | - | 0.26 |
| Absolute risk increase (%) | 5.00 | 4.28 | −5.00 | −2.80 | −5.41 | 0.47 | 5.41 | 1.01 |
| NNTharm | 20.00 | 23.35 | −20.00 | −35.67 | −18.50 | 212.28 | 18.50 | 99.15 |
| NNTnet | −18.73 | −33.54 | 13.33 | 20.35 | 18.50 | −188.50 | −30.83 | 40.59 |
| ICH risk at 3-years | ||||||||
| Risk of comparator group (%) | 0.00 (0.00–0.00) | 0.92 (0.34–2.00) | 5.00 (4.33–5.74) | 2.95 (2.86–3.04) | 0.00 (0.00–0.00) | 1.18 (1.10–1.26) | 0.00 (0.00–0.00) | 0.92 (0.34–2.00) |
| Risk of treatment group (%) | 5.00 (4.33–5.74) | 2.95 (2.86–3.04) | 0.00 (0.00–0.00) | 2.42 (2.21–2.64) | 0.00 (0.00–0.00) | 2.42 (2.21–2.64) | 0.00 (0.00–0.00) | 1.18 (1.10–1.26) |
| Relative risk increase | - | 2.20 | −1.00 | −0.18 | - | 1.05 | - | 0.28 |
| Absolute risk increase (%) | 5.00 | 2.03 | −5.00 | −0.53 | - | 1.24 | 0.00 | 0.26 |
| NNTharm | 20.00 | 49.38 | −20.00 | −188.70 | - | 80.81 | - | 387.95 |
| NNTnet | −18.73 | −138.35 | 13.33 | 37.87 | - | −77.11 | 46.25 | 31.11 |
- Abbreviations: ICH, intracranial hemorrhage; NNT, number needed to treat; NOACs, non-vitamin K antagonist oral anticoagulants; OAC, oral anticoagulants; SSE, ischemic stroke/systemic embolism; TTR = time in therapeutic range.
- a Comparator group.
Among 2340 patients with warfarin, TTR was calculated in 2233 patients (95.4%); 1432 (64.1%) had a TTR <65%, and 801 (35.9%) had TTR ≥65%. The NNTnet was positive for both SSE versus major bleeding and SSE versus ICH in warfarin group with TTR ≥65% compared to no OAC whereas the negative NNTnet becomes more prominent in patients with TTR <65%. NOACs were superior to warfarin with TTR <65%, but were not superior to warfarin with TTR ≥65%.
Figure 2 shows restricted cubic spline curves of adjusted hazard ratio of SSE or major bleeding (Figure 2A), and SSE or ICH (Figure 2B) on the Y-axis and TTR on the X-axis. When considering both ischemic and bleeding events, the benefit of warfarin was observed when the TTR was above 74.9% for SSE or major bleeding and 69.4% for SSE or ICH.

3.4 Sensitivity analysis
Table 3-part B shows sensitivity analysis in the low-risk group and intermediate to high-risk group of RRR, RRI, ARR, ARI, NNTbenefit, NNTharm, and NNTnet of (1) warfarin compared to no OAC, (2) NOAC compared to no OAC, and (3) NOAC compared to warfarin. NNTnet for patients with intermediate to high stroke risk was −84.5 for warfarin versus no OAC, 51.7 for NOAC versus no OAC, and 32.1 for NOAC versus warfarin.
The negative NNTnet of warfarin versus no OAC was present in both low-risk and intermediate to high-risk groups for both major bleeding and ICH, which was similar to the positive NNTnet of NOAC versus no OAC and NOAC versus warfarin. An exception was for the NNTnet of warfarin versus no OAC on ICH in which the intermediate to high-risk group which had a positive NNTnet but the magnitude of NNT was large indicating that the effect was relatively small.
Table 4-part B shows the effect of splitting warfarin according to TTR (< and ≥65%) on the primary results of NNT for net benefit of warfarin and no OAC, and warfarin and NOACs for low-risk and intermediate to high-risk group. The results were aligned with the whole population dataset.
We performed additional analysis to determine the impact of antiplatelet use on the NNTnet. The NNTnet of warfarin versus no OAC was −31.8 and −12.7 for patients with and without antiplatelet, respectively. For NOAC versus warfarin, the NNTnet was 7.4 and 36.3 in patients with and without antiplatelet, respectively.
4 DISCUSSION
In this prospective nationwide AF registry, we demonstrate that choice of OAC is important as a major determinant of NNT of net clinical benefit of the benefit and risk of OAC for stroke prevention in patients with AF. Warfarin was associated with a negative NNTnet compared with no OAC. Only warfarin with TTR ≥65% had a positive NNTnet compared to no OAC, while NOACs had positive NNTnet compared to no OAC and when compared to warfarin.
Patients with AF with at least one additional risk of ischemic stroke are recommended to use OAC for stroke prevention.2, 15, 16 In a meta-analysis, warfarin can reduce ischemic stroke by 64% and all-cause mortality by 26% when compared to placebo or control.3 From a systematic review of 16 randomized studies and 31 observational studies, the rate of major bleeding was 2.1 per 100 person-years in randomized studies and 2.0 per 100 person-years in observational studies.17 Indeed, the annual rate of ICH was 0.3%–0.6% for warfarin and 0.1%–0.2% for NOACs.18, 19 In the clinical trials, NOACs were associated with approximately 50% reduction in the rate of ICH compared to warfarin.5 It has been recommended that stroke prevention management in AF patients should depend on the balance of the benefit in stroke reduction and risk of major bleeding of OAC, and shared decision making between patient, family, and physicians is suggested.2, 15, 20
The risks of SSE were 1.51 per 100 person-years are less than the reported from some Asian countries such as Taiwan21 and Hong Kong22 but similar to the reports from the European EORP-AF registry23 and from GARFIELD registry.24
In contrast, the major bleeding rate in our study was 2.25 per 100 person-years which is greater than the report from Western populations23 and the GARFIELD-AF registry.24 Indeed, our observed rate was similar to the results from some Asian countries such as Taiwan25 and Japan.26 This is consistent with data from Asian populations with AF which had more bleeding complication compared to non-Asians.7, 27 The explanation for the increased risk of bleeding in Asians may reflect greater use of warfarin, genetic predisposition, lower body weight, and other factors.27 The rate of ICH from our study was 0.78 per 100 person-years which was higher than some other reports. The main OAC used in our study was warfarin, which accounted for 91.1% of patients using OAC. Indeed, the report rates of ICH from warfarin were 0.3%–0.6% per year.18, 19 Also, Asian population had a greater risk of ICH associated with warfarin.7
The NNTnet was positive for both SSE versus major bleeding and SSE versus ICH in warfarin group compared to no OAC which indicates that when we give warfarin the ARI of increased major bleeding was greater than the ARR of SSE reduction. If we have a good TTR control of ≥65%, the NNTnet becomes positive which strongly emphasizes that in order to have benefit of warfarin greater than increased risk, we need to have a good TTR control in patients receiving warfarin. In our study, only 35.9% of patients who were on warfarin had a TTR ≥65% which meant that most patients taking warfarin in our study may have ARI more than ARR. A previous study on the EURObservational Research Program-AF General Registry (EORP-AF) showed a positive NNTnet for OAC compared to no OAC.28 In EORP-AF study proportion of warfarin was slightly greater than NOACs which is different from our study. In our study, warfarin accounted for 91% of patients using OAC. And we found that warfarin had no benefit from NNTnet in our population unless the TTR was at least 65%. High-quality warfarin treatment based on a good TTR has been recommended in the recommendation in Asian population.2, 9
For NOACs, the results of NNTnet convincingly show that NOACs are the preferred agents for SSE reduction in our population, since NOACs had positive NNTnet compared to no OAC and when compared to warfarin. These data were consistent across the risk groups. Ding et al.29 showed that AF patients with stable dose warfarin with INR 2–3 for at least 6 months had a positive NNTnet when compared to no OAC, and the magnitude of NNTnet from real world data was smaller than the results from clinical trials suggesting that OAC had benefit that was greater than risk in the real world, more than seen in clinical trial results. Nonetheless, non-Asian and Asian populations may have a different risk and benefit profile from OAC use, as has been discussed earlier.
The rate of OAC use in our study was 35.5%. OAC use in low-risk patients is not uncommon. Data from GARFIELD-AF and ORBIT II registry showed that the rate of OAC use in low-risk group was 46% and 57%, respectively.30 The results of our survey for the reason of OAC use in low-risk group indicated that most common reason was physician preference (43.1%). Other reasons were left ventricular systolic dysfunction not qualifying CHA2DS2-VASc criteria (the criteria need left ventricular ejection fraction lower than 40% or having heart failure) (10.8%), left ventricular thrombus (4.9%), post-AF ablation (12.8%), cardioversion (8.8%), hyperthyroidism (2%), hypertrophic cardiomyopathy (2%), endomyocardial fibrosis (2%), previous OAC use from referral hospital (4.9%), and patient preference (8.8%).31
There were 24.6% of patients in our study that OAC was not used. We have reported the reason for not using OACs in 796 patients. The reasons were as follows: low stroke risk 39.9%, taking antiplatelets (26.5%), patient preference (20.9%), physician preference (10.4%), fear of bleeding (10.2%), fall risk (2.6%), OAC compliance concern (1.8%), others (1.0%).32
In our study, 24.6% of AF patients were not on OAC. A recent report from Italy showed that among patients with a hospital diagnosis of AF, 38.9% were not on OACs33 which is greater than the results from our study. An Italian study revealed that warfarin use was more common than NOAC use, and warfarin had a higher risk of ICH and major bleeding compared to NOACs.
The introduction of NOACs clearly changed the practice landscape of stroke prevention in AF.34 GARFIELD AF registry has shown a global trend of the increased use of NOACs in AF patients.35 The rate of NOAC use in our study is low. There are many reasons for the low rate of NOAC use in our study. The COOL-AF registry enrolled AF patients during 2014–2017 and may not reflect the current data. NOACs can be reimbursed only in a small proportion of AF patients. Government policy promotes the use of warfarin due to the cost concern. However, recent real-world showed that the trend of NOAC use in Thailand significantly increased.36
Our study calculated NNTnet for SSE and major bleeding due to the purpose of the comparison among each OAC strategy. We did not focus on death which is one of the major outcomes in AF patients. The incidence rate of death in our registry is 4.21 per 100 person-years. This is similar to the mortality rate of 3.8% reported from the GARFIELD AF registry and 5.5% from Fushimi registry. The result from XANTUS program which included AF patients who were on rivaroxaban demonstrated a low 1-year mortality rate of 1.7%.37 Part of the reason may be related to the use of NOAC in all cases and the patients were being carefully followed-up. A report from Italy showed a high mortality of 10% per year which may be related to the in-hospital setting as the criteria for enrollment and the mean age was high (78 years) compared to less than 70 years in other registries.33
4.1 Limitations
There are some limitations of this study. First, there were a low number of patients receiving NOACs. Therefore, interpretation of the results comparing NOACs with no OAC and NOACs with warfarin based on a relatively low number of NOACs. Second, our results were from large size hospitals and patients being managed by cardiologists. Even so, NOACs use and the proportion of TTR ≥65% were low. Third, we do not consider OAC change during follow-up into consideration. The OAC change during follow-up might have an impact on clinical outcome. Among 2568 patients with OAC at baseline, 211 (8.2%) the OAC was discontinued during follow-up whereas 270 out of 837 patients (32.3%) with no OAC at baseline changed to be on OAC during follow-up. Fourth, We did not collect data on NOAC dosing. Since it has been reported that inappropriate NOAC underdosing is common and has a negative impact on clinical outcome.38 However, despite NOAC underdosing influence the outcome, the results of our study are still positive for the benefit of NOAC compared to warfarin and no OAC.
5 CONCLUSION
Warfarin had a negative NNTnet compared to no OAC. Only warfarin with TTR 65% has a positive NNTnet. NOACs had positive NNTnet compared to no OAC and when compared to warfarin.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Chulalak Komoltri, PhD, for statistical analysis and Ahthit Yindeengam, BSc, and Poom Sairat, MSc, for data management. The COOL-AF investigators are as follows: Buddhachinaraj Hospital: Tomorn Thongsri, Central Chest Institute of Thailand: Komsing Methavigul, Charoen Krung Pracha Rak Hospital: Pattraporn Srirattana, Chiangrai Prachanukroh Hospital: Wattana Wongtheptien, Chonburi Hospital: Pornchai Ngamjanyaporn, Faculty of Medicine, Chiang Mai University: Arintaya Phrommintikul, Faculty of Medicine, Chulalongkorn University: Smonporn Boonyaratavej, Faculty of Medicine, Naresuan University: Pongpun Jittham, Faculty of Medicine, Prince of Songkla University: Treechada Wisartpong, Faculty of Medicine Ramathibodi Hospital, Mahidol University: Sirin Apiyasawat, Faculty of Medicine Siriraj Hospital, Mahidol University: Arjbordin Winijkul, and Rungroj Krittayaphong, Faculty of Medicine, Thammasat University (Rangsit Campus): Roj Rojretamphai, Faculty of Medicine Vajira Hospital, Navamindradhiraj University: Kulyot Jongpiputvanich, Golden Jubilee Medical Center: Somchai Dutsadeevettakul, Khon Kaen Hospital: Chaisit Wongwipaporn, Lampang Hospital: Tanita Bunyapipat, Nakorn Ratchasima Hospital: Weerapan Wiwatworapan, Nakornping Hospital: Khanchai Siriwattana, Phramongkutklao College of Medicine: Tharanit Chantrarat, Police General Hospital: Kasem Rattanasumawong, Prapokklao Hospital (Chanthaburi): Wiwat Kanjanarutjawiwat, Queen Savang Vadhana Memorial Hospital: Sakaorat Kornbongkotmas; Ratchaburi Hospital: Thanasak Patmuk, Sapphasitthiprasong Hospital: Praprut Thanakitcharu, Surat Thani Hospital: Suchart Arunsiriwattana, Surin Hospital: Thaworn Choochunklin; Udon Thani Hospital: Sumon Tangsuntornwiwat.
FUNDING INFORMATION
This study was funded by grants from the Health Systems Research Institute (HSRI) (grant no. 59-053), and the Heart Association of Thailand under the Royal Patronage of H.M. the King. None of the aforementioned funding sources influenced any aspect of this study or the decision of the authors to submit this manuscript for publication.
CONFLICT OF INTEREST STATEMENT
GYHL: Consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, and Daiichi-Sankyo. No fees are directly received personally. Other authors hereby declare no personal or professional conflicts of interest relating to any aspect of this particular study.
ETHICS STATEMENT
This study was approved by the Institutional Review Board of the Central Research Ethic Committee (CREC)(Certificate of Approval number 003/2014). The study was performed following the principles of the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice Guidelines.
PATIENT CONSENT STATEMENT
All patients gave written informed consent before participation.
CLINICAL TRIAL REGISTRATION
The trial has been registered with the Thai Clinical Trials Registry (TCTR) which complied with WHO International Clinical Trials Registry Platform dataset. The registration number is TCTR20160113002 (05/01/2016).
Open Research
DATA AVAILABILITY STATEMENT
The dataset that was used to support the conclusion of this study is included within the manuscript. Any other additional data will be made available upon request to the corresponding author.




