Corticostriatal synaptic plasticity alterations in the R6/1 transgenic mouse model of Huntington's disease
Funding information
For this study, PC received research support from Ricerca Corrente IRCCS Fondazione Santa Lucia
Abstract
Huntington's disease (HD) is a genetic neurodegenerative condition characterized by abnormal dopamine (DA)–glutamate interactions, severe alterations in motor control, and reduced behavioral flexibility. Experimental models of disease show that during symptomatic phases, HD shares with other hyperkinetic disorders the loss of synaptic depotentiation in the striatal spiny projection neurons (SPNs). Here we test the hypothesis that corticostriatal long-term depression (LTD), a well-conserved synaptic scaling down response to environmental stimuli, is also altered in symptomatic male R6/1 mice, a HD model with gradual development of symptoms. In vitro patch-clamp and intracellular recordings of corticostriatal slices from R6/1 mice confirm that, similar to other models characterized by hyperkinesia and striatal DA D1 receptor pathway dysregulation, once long-term potentiation (LTP) is induced, synaptic depotentiation is lost. Our new observations show that activity-dependent LTD was abolished in SPNs of mutant mice. In an experimental condition in which N-methyl-d-aspartate (NMDA) receptors are normally not recruited, in vitro bath application of DA revealed an abnormal response of D1 receptors that caused a shift in synaptic plasticity direction resulting in an NMDA-dependent LTP. Our results demonstrate that corticostriatal LTD is lost in R6/1 mouse model and confirm the role of aberrant DA–glutamate interactions in the alterations of synaptic scaling down associated with HD symptoms.
Significance
The paper explores bidirectional corticostriatal plasticity alterations in R6/1 mice, an animal model of Huntington's disease with gradual development of symptoms, during a symptomatic phase. The results support the role of an abnormal interaction between dopamine D1 and glutamate NMDA receptors activities in the loss of long-term depression, the principal synaptic downscaling mechanism of the dorsolateral striatum. Given its complex regulation and its dependence on different neurotransmitter systems, this form of plasticity could be a useful tool to explore new therapeutic targets.
1 INTRODUCTION
Huntington's disease (HD) is an autosomal-dominant neurodegenerative disease characterized by selective neurodegeneration of striatal neurons and abnormal motor control associated with lack of behavioral flexibility. Studies on animal models of HD have shown that accumulation of intracellular proteinaceous aggregates are caused by expansions of polyglutamine tracts at the amino-terminal fragments of a mutant form of the protein huntingtin (mHTT) (Bates, 2003). The presence of mHTT is associated with toxicity and neurodegeneration leading to corticostriatal synapse instability and alterations in bidirectional plasticity mainly caused by abnormal dopamine (DA)–glutamate interactions (Andre, Cepeda, & Levine, 2010; Rangel-Barajas & Rebec, 2016). Analyses of brain alterations in transgenic R6/2 and R6/1 mice have provided insights into the HD pathophysiology over the course of the disease. Most of the electrophysiological studies were performed in hippocampal and cortical preparations while few studies have investigated synaptic plasticity in corticostriatal slices (Cepeda, Cummings, Andre, Holley, & Levine, 2010; Ghiglieri, Bagetta, Calabresi, & Picconi, 2012). R6/2 transgenic mice, which carry exon 1 of the HD gene with about 150 CAG repeats, display a very rapidly progressing phenotype similar to juvenile form of HD in humans while R6/1 mice carry about 116 CAG repeats and present a slower progression with a more gradual development of motor and cognitive alterations, modeling adult-onset form of disease (Ferrante, 2009; Li, Popovic, & Brundin, 2005).
In R6 lines striatal DA content is reduced (Ortiz, Kurth, Osterhaus, & Johnson, 2011; Ortiz et al., 2012; Petersen, Puschban, et al., 2002) as well as expression of DA transporter (DAT) and tyrosine hydroxylase (TH), a rate-limiting step enzyme of DA synthesis (Miller et al., 2014; Yohrling et al., 2003). Interestingly, despite a reduced dopaminergic tone, expression and phosphorylation state of intracellular substrates downstream DA D1 receptor (D1R) activation – cAMP, ERK, and DARPP32 – are abnormally increased, indicating chronic dysregulation of D1R pathway (Fusco et al., 2012; Roze et al., 2008). A resulting DA D1R supersensitivity has been associated with abnormal N-methyl-d-aspartate receptor (NMDAR) phosphorylation, which in turn brings to persistent activation of downstream enzymatic cascades that triggers pathways of neurodegeneration in different HD models (Rangel-Barajas & Rebec, 2016). DA D1R activation also directly induces mHTT aggregation, playing an independent role in mHTT-associated neurodegenerative events (Kim et al., 1999; Robinson, Lebel, & Cyr, 2008). In maintaining a vicious circle, mHTT is able to interfere with the stabilizing actions of scaffolding protein PSD-95, leading to a lack of control in the surface expression of NMDA and other receptors (Fan, Cowan, Zhang, Hayden, & Raymond, 2009; Sun, Savanenin, Reddy, & Liu, 2001). DA D2 receptors were also found altered in function and in their expression (Andre, Fisher, & Levine, 2011). In fact, while spiny projection neurons (SPNs) of the direct pathway, bearing DA D1R, seem to play a main role in the abnormal control of movements, striatal neurons of the indirect pathway, more frequently bearing DA D2 and adenosine A2a receptors, have higher vulnerability to glutamatergic-mediated excitotoxicity than direct SPNs in experimental HD (Albin et al., 1992; Reiner et al., 1988; Richfield, Maguire-Zeiss, Vonkeman, & Voorn, 1995; Tyebji et al., 2015). In the corticostriatal synapse, DA D2 receptors play a critical role in the modulation of glutamatergic neurotransmission that involves striatal interneurons (Tozzi et al., 2011) and interneuronal plasticity is hampered in symptomatic HD (Picconi et al., 2006). D2 receptors also play a key role in the production of anandamide (Giuffrida et al., 1999) and in the functioning of endocannabinoid system (reviewed in Fernandez-Ruiz, Hernandez, & Ramos, 2010),
The result of defective cell-specific D2-mediated functions is therefore a progressive increase of glutamatergic activity which triggers a more rapid degeneration of indirect pathway striato-pallidal neurons. A decreased D2 expression is associated with altered synthesis of glycogen synthase kinase-3 (Beaulieu, Del'guidice, Sotnikova, Lemasson, & Gainetdinov, 2011) and consequent dysfunctions in the ubiquitine-proteasome system that reduces the ability to cleave mHTT aggregates (Fernandez-Nogales et al., 2015), endangering cellular homeostasis. In presymptomatic R6/2 mice, modeling a very rapidly progressing phenotype, early dysfunctions of cholinergic interneurons bring specific alterations in bidirectional synaptic plasticity of SPNs associated with motor and cognitive deficits (Picconi et al., 2006), whereas symptomatic stages are characterized by a loss of corticostriatal long-term potentiation (LTP) (Kung, Hassam, Morton, & Jones, 2007). Here, using a model with a slower development of HD symptoms over time, we tested the hypothesis that altered NMDAR–D1R interactions may impact on the ability of SPNs to express bidirectional plasticity. To this end, we analyzed corticostriatal long-term depression (LTD), LTP, and synaptic depotentiation in striatal SPNs of symptomatic 27-week-old R6/1 mice.
2 METHODS
2.1 Mice models and ethics statement on animal use
Experiments were performed on 27-week-old male R6/1 transgenic mice (n = 16) and their age-matched wild-type (Wt) (n = 14) littermate controls. Mice were obtained from The Jackson Laboratory. Animals were kept under regular lighting conditions (12 hr light/dark cycle), and food and water were provided ad libitum. All procedures were conducted in conformity with the European Communities Council Directive of September 2010 (2010/63/E), in accordance with protocols approved by the Animal Care and Use Committee at the Italian Ministry of Health. All efforts were made to minimize the number of animals used and their suffering.
2.2 Electrophysiology
2.2.1 Slice preparation
All mice were decapitated by cervical dislocation and the brain was rapidly removed and coronal corticostriatal slices (thickness, 240–280 μm) were cut in Krebs' solution (in mmol/L: 126 NaCl, 2.5 KCl, 1.2 MgCl2, 1.2 NaH2PO4, 2.4 CaCl2, 10 glucose, and 25 NaHCO3), bubbled with a 95% O2–5% CO2 gas mixture, to allow recovery. After at least 1 hr, a single slice including the cortex and the striatum was transferred to a recording chamber and submerged in continuously flowing Krebs' solution (room temperature; 2.5–3 ml/min).
2.2.2 Whole-cell patch-clamp recordings
Whole-cell patch-clamp recordings were performed in current clamp mode from SPNs visualized using infrared differential interference contrast microscopy in the dorsal striatum (Eclipse FN1, Nikon) (Bagetta et al., 2011, 2012). Borosilicate glass pipettes (4–6 MΩ) were filled with the following internal solutions (in mM): 120 K-gluconate, 0.1 CaCl2, 2 MgCl2, 0.1 EGTA, 10 N-(2-hydroxyethyl)-piperazine-N-s-ethanesulfonic acid, 0.3 Na-guanosine triphosphate, and 2 Mg-adenosine triphosphate (Mg-ATP), adjusted to pH 7.3 with KOH. Signals were amplified with a Multiclamp 700B amplifier, recorded, and stored on PC using pClamp 9 (Molecular Devices, USA).
Whole-cell access resistance was 15–30 MΩ. Picrotoxin (50 μM) was added to the perfusing solution to block GABAA-mediated transmission (routine procedure to exclude the possible inhibitory interference of the GABAergic system). The recording electrodes were invariably placed within the striatum. Input resistances and injected currents were monitored throughout the experiments. Variations in these parameters (>20%) led to rejection of the experiment.
2.2.3 Intracellular recordings with sharp electrodes
For intracellular recordings, sharp electrodes were filled with 2M KCl (30–60 MΩ). Signals were recorded using an Axoclamp 2B amplifier (Molecular Devices), displayed on a separate oscilloscope, stored, and analyzed on a digital system (pClamp 9, Molecular Devices). Offline analysis was performed using Clampfit (Molecular Devices, USA).
In both patch-clamp and intracellular recordings, for synaptic stimulation, bipolar tungsten electrodes were used (Word Precision Instruments, Friedberg, Germany). The stimulating electrode was placed in the white matter of corpus callosum, between the cortex and the striatum, to activate corticostriatal fibers. In both techniques, glutamatergic excitatory postsynaptic potentials (EPSPs) were evoked by electrical stimulation every 10 s; and to induce LTD and LTP, we used a high frequency stimulation (HFS) protocol, consisting in three trains of 100 Hz, 3 s of duration, and 20 s of interval. During tetanic stimulation, the intensity of stimulation was increased to suprathreshold levels. For the LTP protocol, at the beginning of intracellular recordings, magnesium ions were omitted from the medium (Mg2+-free medium) to increase the NMDA-mediated component of EPSP (Cacace et al., 2017). This protocol is needed to induce corticostriatal LTP in SPNs in response to HFS. To depotentiate previously induced LTP, a low-frequency stimulation (LFS) protocol was delivered at 2 Hz for 10 min. Quantitative data on EPSP modifications induced by HFS protocol are expressed as a percentage of control, the latter representing the mean of responses recorded during a stable period (15–20 min) before the tetanus. Current–voltage relationships were obtained by applying steps of current of 200 pA in both hyperpolarizing and depolarizing direction (from −800 to +200 pA) in order to measure the membrane ability to accommodate and fire in response to hyperpolarizing and depolarizing current steps. Firing frequency was calculated as mean number of spikes in response to a step of 500 pA and shown as averaged values in whisker plots.
Both patch-clamp and intracellular recordings with sharp electrodes were used for intrinsic membrane properties and synaptic plasticity experiments. When results were similar, and the extent of changes comparable, normalized values obtained from the two approaches were pooled and plotted together.
2.3 Drugs
Drugs were bath applied by switching the flowing solution to Krebs' solution containing known concentrations of each compound. In order to prevent oxidation, a stock solution of DA (30 mM) was prepared fresh daily and stored in dark glass bottles at 4°C. During the cell recording, an aliquot of the stock solution was diluted in aCSF to 30 μM and kept in a syringe wrapped in aluminum foil for the entire duration of the experiment. DA and MK801 were purchased from Sigma Aldrich (Milan, Italy), picrotoxin and SKF38393 were from Tocris-Cookson (Bristol, UK).
2.4 Statistics
Values given in the text and in the figures are presented as mean ± SEM of changes in the respective cell populations. Paired t-test was used for the electrophysiological analysis of the pre- versus post-HFS protocol within the same cell/animal. Two-way ANOVA or unpaired Student's t-test was used to analyze statistical differences between experimental groups over time or at a specific time point, respectively. When time × group interaction was significant, group means for each time point were compared using Bonferroni's post hoc test. When a nonparametric test was required, Mann–Whitney test was used. Sample size for experiments has been calculated taking into account a 5% type I error and a 80% power using G*Power software (Faul, Erdfelder, Buchner, & Lang, 2009). The analyses were done using Prism 6.0 software (GraphPad Software, San Diego, CA, USA).
3 RESULTS
3.1 Bidirectional plasticity in R6/1 and Wt mice
We investigated corticostriatal bidirectional plasticity (LTD, LTP, and depotentiation) in R6/1 transgenic mice and age-matched Wt animals. First, the analysis of the intrinsic membrane and firing properties of SPNs revealed, as expected, the absence of any difference in firing discharge and current–voltage relationships (Figure 1a). Resting membrane potential was similar in most of the cells and the average values were −87.60 ± 35.7 mV for Wt and −82.22 ± 19.9 mV for R6/1 (Mann–Whitney test p > 0.05, Wt n = 6, R6/1 n = 17). In vitro patch-clamp and intracellular recordings of identified SPNs from corticostriatal acute slices show that a HFS protocol, applied over the corticostriatal fibers in Mg2+-free medium, a condition that favors removal of a physiological blockade of NMDAR at resting membrane potential, was able to induce LTP in most of the SPNs, with comparable amplitude and time course in both R6/1 and Wt age-matched mice (Figure 1b, left panel; paired t test pre- vs. 40 min post-HFS, Wt n = 4, t = 31.02 df = 3 ###p < 0.0001, R6/1 n = 6, t = 4.153, df = 5; ##p = 0.0089).
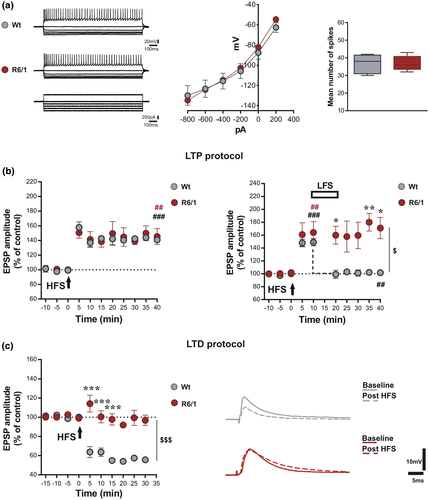
In order to study synaptic depotentiation, LTP was induced in SPNs of both genotypes (Figure 1b, right panel; paired t test pre- vs. 10 min post-HFS, Wt n = 5, t = 8.54 df = 4, ###p = 0.0010; R6/1 n = 9, t = 3.42, df = 8, ##p = 0.0091). Once induced, enhanced EPSP responses could be depotentiated by a LFS protocol only in Wt mice (Figure 1b, gray circles, paired t test–post-HFS 10 min vs. 35 min, n = 5, t = 5.36, df = 4, ##p = 0.0058). In contrast, in R6/1 mice, LFS exerted no effect on previously induced LTP (Figure 1b, dark red circles, unpaired t test at 35 min, R6/1 vs. Wt, t = 6.63, df = 7, p = 0.0003). Two-way ANOVA analysis shows a significant time × group interaction (F(9,72) = 3.287, p = 0.0021) with a significant group effect (F(1,8) = 8.013, $p = 0.0221) and post hoc comparison indicates significant differences at 20 and 40 (*p < 0.05, t = 2.903, and t = 3.072, respectively) and at 35 min post-stimulation (**p < 0.01, t = 3.722).
In a separate set of experiments carried in a control condition, in which the bathing solution contained a physiological concentration of magnesium ions, application of a HFS protocol was able to exert a robust LTD in Wt mice (Figure 1c, gray circles, paired t test pre vs. 15 min post-HFS, Wt n = 5, t = 4.83, df = 4, ###p < 0.0001). This form of plasticity could not be induced in the same conditions in SPNs of R6/1 mice (Figure 1c, dark red circles, paired t test pre vs. 15 min post-HFS, R6/1 n = 6, t = 0.18, df = 5, p = 0.08619) indicating a marked difference in LTD expression between the two genotypes (unpaired t test at 15 min post-HFS, R6/1 vs. Wt, t = 6.37, df = 9, p = 0.0001). Grouped analysis of first 15 min after HFS shows a significant time × group interaction (Two-way ANOVA F(6,60) = 18.02, p < 0.0001) with a significant group effect (F(1,10) = 34.36, $$$p = 0.0002) and post hoc comparison indicates significant differences at 5, 10, and 15 min post-stimulation (***p < 0.001, t = 8.378, 6.119, and 7.039, respectively).
3.2 Pharmacological modulation of LTD
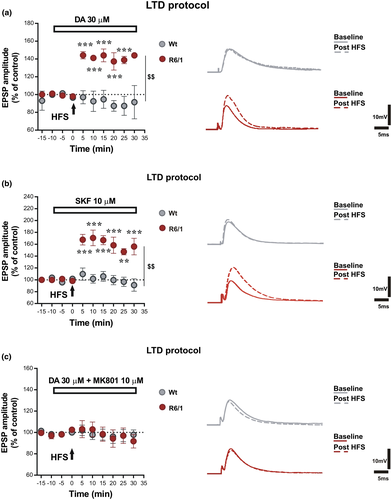
Given that corticostriatal LTD depends on concomitant activation of D1 and D2 receptors (Calabresi, Maj, Mercuri, & Bernardi, 1992) and that striatal DA release and DA content are reduced in R6 lines during symptomatic phases of disease (Ortiz et al., 2011, 2012; Petersen, Chase, et al., 2002), we explored the possibility to rescue LTD by in vitro application of DA exerting a tonic stimulation of DA receptors.
Delivery of HFS on corticostriatal fibers in physiological conditions (using a standard aCSF solution) in the presence of DA (30 μM) was not able to rescue LTD in SPNs of R6/1 animals, which, instead, showed a shift of plasticity toward LTP (Figure 2a, dark red circles, paired t test pre vs. 25 min post-HFS, R6/1 n = 5, t = 7.15, df = 4, p = 0.0020). Interestingly, bath application of DA at the same concentration prevented the induction of LTD in SPNs of Wt mice (Figure 2a, gray circles, n = 5) showing a clearly different response to DA application between the two experimental groups (Figure 2a, unpaired t test at 25 min, R6/1 vs. Wt, t = 4.53, df = 8, p = 0.0019; Two-way ANOVA time × group interaction F(7,56) = 8.606 p < 0.0001; group effect F(1,8) = 22.91, $$$p = 0.0003, Bonferroni post hoc: ***p < 0.001 at 5, 10, 15, 20, 25 min post-HFS, t = 4.747, 4.897, 5.632, 5.895, 5.880). These effects were mimicked by bath application of 10 μM D1R agonist SKF38393 (Figure 2b, dark red circles, paired t test pre versus 25 min post-HFS, R6/1 n = 5, t = 11.47, df = 4, p = 0.0003) that exerted no effect in SPNs of Wt mice (Figure 2 B, gray circles, paired t test pre versus 25 min post-HFS, Wt n = 5, t = 0.30, df = 4, p = 0.7811; Two-way ANOVA time × group interaction F(7,56) = 8.606 p < 0.0001; group effect F(1,8) = 22.91, $$p = 0.0014; Bonferroni post hoc: ***p < 0.001 at 5, 10, 15, 20, 30 min post-HFS, t = 4.309, 4,980, 4.706, 4.646, 5.116, respectively, **p < 0.01 at 25 min post-HFS, t = 4.0), while application of a D2 agonist did not induce similar responses (data not shown). Since D1R signaling is an important modulator of NMDAR activity, we explored whether this D1-mediated abnormal form of plasticity was relying on NMDAR activation even in a standard condition that should not favor the involvement of these receptors due to a physiological magnesium-dependent blockade of the receptor channel. Co-application of DA and the noncompetitive NMDAR antagonist MK801 (10 μM) was able to block the shift in synaptic plasticity direction in R6/1 mice that was no longer able to respond with changes in EPSP amplitude after HFS (Figure 2c, dark red circles, paired t test pre vs. 25 min post-HFS, R6/1 n = 5, t = 0.71, df = 4, p = 0.5146). NMDAR antagonism on SPNs of Wt mice upon delivery of HFS under the same condition exerts no effects on their response to 30 μM DA application (Figure 2c, gray circles, paired t test pre vs. 25 min post-HFS, Wt n = 4, t = 0.64, df = 3, p = 0.5692) that was similar to their response to DA alone (Figure 2a).
4 DISCUSSION
In this study, we provide evidence that the two main forms of synaptic downscaling were lost in 27-week-old R6/1 mice, modeling symptomatic HD. Our results confirm and extend to a model with a slower development of disease features, previous observations obtained in R6/2 mice, showing loss of bidirectional synaptic plasticity (Picconi et al., 2006) in a presymptomatic phase.
In 27-week-old R6/1 mutants and their Wt littermates, we first explored the ability of SPNs to express DA D1R- and NMDAR-dependent LTP. The R6/1 mice used in this study were tested during a symptomatic but not disease end stage, in which a reliable response of SPNs to depolarizing and hyperpolarizing current steps and their ability to express LTP was preserved. These features were selected as main criterion for animal viability.
In corticostriatal acute slices, manipulation of aCSF solution to relieve striatal NMDA receptors from physiological blockade allowed to observe a robust LTP in response to HFS in both genotypes. Mutant mice showed a complete loss of synaptic depotentiation (reversal of LTP) despite normal intrinsic membrane properties and LTP similar to that observed in Wt mice.
Other animal models of hyperkinetic disorders have shown a similar pattern of alteration and inability of the synapse to downscale its activity upon cortical stimulation (Calabresi et al., 2016; Martella et al., 2009; Picconi et al., 2003; Quartarone & Pisani, 2011). Lack of synaptic depotentiation in corticostriatal synapses of R6/2 mice was found dependent on cell-type–specific alterations in synaptic plasticity (Picconi et al., 2006). This pattern of aberrant plasticity is in line with many observations reporting a persistent increase of cyclic AMP and enhanced phosphorylation cAMP-regulated phosphoprotein-32 (DARPP-32) (Ariano et al., 2002; Bibb et al., 2000) in HD models, leading to inactivation of one of its preferential targets phosphatase-1 (PP1), which is required for synaptic depotentiation (Picconi et al., 2003).
Based on these observations, our next objective was to analyze the ability of SPNs to reduce synaptic activity with an alternative strategy of the dorsolateral striatum. Although LTP and LTD are equally expressed in the dorsolateral striatum, depending on the order of activity received (Fino, Glowinski, & Venance, 2005), in our experimental setting, LTD can be induced with less physical and chemical manipulation of the recording conditions. This D1/D2-dependent form of plasticity can be observed in SPNs when a HFS protocol is delivered in physiological conditions over the corticostriatal fibers projecting to dorsolateral striatum. According to many studies reporting an imbalance between striatal D1 and D2 receptor functions (Andre et al., 2011; Chen, Wang, Cepeda, & Levine, 2013), SPNs of R6/1 mice were not able to respond to a HFS stimulation of the corticostriatal pathway with a LTD, while this form of plasticity was still observed in age-matched Wt mice. Since evoked DA release (Ortiz et al., 2011) and striatal DA levels (Petersen, Chase, et al., 2002) are decreased in R6/1 mice and corticostriatal LTD strictly depends on concurrent activation of D1 and D2 receptors (Calabresi, Maj, Mercuri, et al., 1992), we explored the possibility that tonic DA receptor stimulation was able to rescue activity-dependent LTD in mutant mice. However, bath application of 30 μM DA before HFS and throughout the recording not only was unable to rescue LTD in SPNs of mutant mice but it also induced an unexpected effect of synaptic potentiation resulting in a robust LTP. This DA-induced effect was mimicked by D1R agonist SKF38393 suggesting an abnormal responsivity of D1Rs during HFS. This result is in agreement with several studies reporting increase in ERK signaling (Fusco et al., 2012; Roze et al., 2008) and associated CREB phosphorylation in R6 line mice at the late phase of disease (Fusco et al., 2012). D1-mediated cAMP activity was also found increased in R6/1 mice (Tyebji et al., 2015) and evidence of D1R hyperactivation in HD models has been provided by several studies (Rangel-Barajas & Rebec, 2016).
Given that D1R regulates activity, surface expression, and phosphorylated state of NMDAR through activation of protein kinase A (Snyder, Fienberg, Huganir, & Greengard, 1998), we explored the possibility that this abnormal response of D1R stimulation was also NMDAR dependent. In line with this hypothesis, NMDAR antagonism was able to prevent the induction of abnormal D1-dependent LTP, suggesting that enhanced D1–NMDAR interactions may underlie this pathological response. A similar effect can be seen in the presence of unbalanced D2 and D1 receptors activation leading to a shift from LTD to LTP, as observed either in D2 KO mice or upon pharmacological blockade of D2 receptors (Calabresi, Maj, Pisani, Mercuri, & Bernardi, 1992; Calabresi et al., 1997).
Accordingly, in R6/1 mice functional interaction between D1 and D2 receptors activity is dysregulated and the glutamatergic synapse is more prone toward an uncontrolled D1R–NMDAR interaction that brings to destabilization and excitotoxicity (Rangel-Barajas & Rebec, 2016). Mutant HTT further contributes to this instability by interfering with the binding between scaffolding protein PSD-95 and NMDAR, which form ternary complexes with D1Rs (Fiorentini, Gardoni, Spano, Luca, & Missale, 2003) to promote and sustain sensitization of these glutamatergic receptors.
Taken together our results demonstrate that an abnormal enhancement of D1R–NMDAR interaction coupled with a possible D2 receptor defective function are linked to a typical pattern of corticostriatal plasticity alterations in symptomatic R6/1 mice before disease end stage. Our data provide a rationale for exploring LTD in these models as a useful tool to test distinct contribution of D1 and D2 and other neurotransmitter systems (Calabresi, Picconi, Tozzi, & Filippo, 2007) specific alterations to the overall pattern of dysregulation observed.
Further experiments are required to target specific steps in the multiple degenerative pathways activated by mHTT and track the disease progression in symptomatic models with a gradual development of disease phenotype.
Understanding the D1R- and D2R-dependent mechanisms of tardive degeneration of the striatal function in symptomatic HD is currently an urgent need in clinical research. Despite the difficulties to translate insights from animal models into clinical settings, it is crucial to design new therapeutic strategies that integrate multiple approaches focusing on DA modulation as a promising option (Cepeda, Murphy, Parent, & Levine, 2014; Koch & Raymond, 2019) to ease a complex management of the triad of motor, cognitive, and psychiatric symptoms in HD patients.
ACKNOWLEDGMENTS
We thank Dr Vincenza Bagetta for her technical contribution and input in developing the original experimental design and Dr Alessandro Tozzi for intellectual discussions and proofreading.
CONFLICT OF INTEREST
Only relevant conflict of interest for each author that is relevant to this manuscript is reported here. All authors reported no biomedical financial interests or potential conflict of interest.
AUTHORS CONTRIBUTIONS
Conceptualization, V.G., P.C. and B.P.; Data Curation, V.G., F.C., G.M. and G.N.; Formal Analysis, V.G., F.C., G.M. and G.N.; Investigation, V.G., F.C., G.M. and G.N.; Project Administration, V.G.; Supervision, V.G. and B.P.; Writing – Original Draft, V.G.; Data Curation, V.G., F.C., G.M. and G.N.; Methodology, V.G., F.C., G.M. and G.N.; Funding Acquisition, P.C.; Resources, V.G., B.P. and P.C.; Writing – Review & Editing, V.G., B.P. and P.C.