Developmental origins of cortical hyperexcitability in Huntington's disease: Review and new observations
Abstract
Huntington's disease (HD), an inherited neurodegenerative disorder that principally affects striatum and cerebral cortex, is generally thought to have an adult onset. However, a small percentage of cases develop symptoms before 20 years of age. This juvenile variant suggests that brain development may be altered in HD. Indeed, recent evidence supports an important role of normal huntingtin during embryonic brain development and mutations in this protein cause cortical abnormalities. Functional studies also demonstrated that the cerebral cortex becomes hyperexcitable with disease progression. In this review, we examine clinical and experimental evidence that cortical development is altered in HD. We also provide preliminary evidence that cortical pyramidal neurons from R6/2 mice, a model of juvenile HD, are hyperexcitable and display dysmorphic processes as early as postnatal day 7. Further, some symptomatic mice present with anatomical abnormalities reminiscent of human focal cortical dysplasia, which could explain the occurrence of epileptic seizures in this genetic mouse model and in children with juvenile HD. Finally, we discuss recent treatments aimed at correcting abnormal brain development.
Significance
The role of neurodevelopment in the pathophysiology of Huntington's disease (HD) is becoming more and more recognized. Here, we review evidence that cortical maldevelopment contributes to hyperexcitability in severe forms of HD. We also propose that only correcting abnormal brain development can new therapies be successful.
1 INTRODUCTION
Huntington's disease (HD) can be defined by a triad of motor, cognitive, and psychiatric symptoms (Harper & Jones, 2002). The most characteristic and debilitating motor symptom is the occurrence of uncontrollable dance-like movements (chorea). Psychiatric symptoms include depression, mood swings, and suicidal ideation. Cognitive symptoms typically precede chorea and include sensory and attention deficits (Bates, Harper, & Jones, 2002; Lawrence et al., 1998). The cause of HD is a genetic mutation consisting of an expansion of CAG repeats in the huntingtin (HTT) gene, localized in the short arm of chromosome 4 (The Huntington's Disease Collaborative Research Group, 1993). When the number of CAG repeats exceeds 39, the affected individual will invariably develop HD symptoms sooner or later. In addition, there is an inverse relationship between disease onset, severity of symptoms, and the number of CAG repeats, such that the longer the repeat length the sooner symptoms manifest (Andrew et al., 1993; Penney, Vonsattel, MacDonald, Gusella, & Myers, 1997).
Histopathological studies have demonstrated that the brain regions more susceptible to degeneration in HD are the caudate nucleus/putamen and the cerebral cortex (Vonsattel & DiFiglia, 1998; Waldvogel, Kim, Tippett, Vonsattel, & Faull, 2015). Striatal and cortical projection neurons are preferentially lost, whereas diverse types of interneurons are spared, with the exception of GABAergic parvalbumin (PV)-expressing interneurons (Reiner et al., 2013). Interestingly, motor and psychiatric symptoms are tightly correlated with cell loss in the cerebral cortex (Thu et al., 2010; Waldvogel et al., 2015). Specifically, motor symptoms correlate with primary motor cortex cell loss whereas mood symptoms are associated with cell loss in the cingulate cortex (Thu et al., 2010).
Although neurodegenerative changes have long been recognized to underlie HD motor symptoms, several clinical and experimental studies have suggested that aberrant cortical development may also play an important role in the manifestation of HD symptoms (Godin et al., 2010; Paulsen et al., 2006; Tereshchenko et al., 2019). In this review, we discuss why we think that faulty cortical development is at the root of some HD functional alterations, in particular cortical hyperexcitability. Also, we provide preliminary morphological and electrophysiological evidence that in the R6/2 genetic mouse model of HD, cortical architecture, neurons, and circuits are altered very early in postnatal development.
1.1 Size matters; one mutation, two different forms of HD
HD is generally conceived as an adult-onset neurodegenerative disorder. However, another less common (5%–10%) juvenile form (JHD, known as rigid or Westphal variant) of the disease also exists, typically when the CAG triplet repeat expansion is >65 (Hunnicutt et al., 2016). Studies have shown that the sex of the transmitting parent, usually the father, exerts a major influence on CAG repeat expansion leading to earlier symptom onset (Telenius et al., 1993). The symptoms of JHD differ from those typically seen in adult-onset HD. Children with HD display mental retardation, hyperactivity, and aggressive behavior. Some of these children also have microcephaly, suggesting that this form of HD may represent a developmental rather than a neurodegenerative disorder (Hunnicutt et al., 2016; Letort & Gonzalez-Alegre, 2013). With disease progression, dystonia, rigidity, and chorea also occur. A fundamental difference between JHD and adult-onset HD is the high prevalence of epileptic seizures in the juvenile form (Gambardella et al., 2001; Rasmussen et al., 2000; Seneca et al., 2004). The cause of epileptic seizures remains unknown (Cummings et al., 2009). However, we have hypothesized that seizures could be the result of faulty development of cortical circuits, similar to those observed in malformations of cortical development (MCD), specifically focal cortical dysplasia (FCD) of Taylor (Estrada-Sánchez, Levine, & Cepeda, 2016; Taylor, Falconer, Bruton, & Corsellis, 1971). This was based on evidence that in some animal models of HD, and presumably also in human cases, the cerebral cortex progressively becomes hyperexcitable (Cummings et al., 2009).
1.2 HTT is essential for normal brain development
The HTT protein has multiple functions that include, among many others, vesicle trafficking, spindle orientation during cell division, endocytosis, transcriptional regulation, maintenance of cell morphology and survival (Saudou & Humbert, 2016). It is also known that normal HTT plays a crucial role during development, as lack of this protein is lethal (Duyao et al., 1995; Nasir et al., 1995; Saudou & Humbert, 2016; Zeitlin, Liu, Chapman, Papaioannou, & Efstratiadis, 1995). Other studies have shown that embryonic, conditional deletion of HTT from cortical pyramidal neurons (CPNs) reduced cortical volume and neuron abundance (Dragatsis et al., 2017). Similarly, loss of HTT function in subpallial lineages disrupted forebrain interneuron species early in life and also led to a number of neurological deficits reminiscent of HD (Mehler et al., 2019).
In the past few years, the idea that HD in general, and JHD in particular, is not solely a neurodegenerative but also a neurodevelopmental disease, has gained momentum (Durieux, Schiffmann, & de Kerchove d'Exaerde, 2011; Godin et al., 2010; Wiatr, Szlachcic, Trzeciak, Figlerowicz, & Figiel, 2017). Indeed, the HTT protein may alter different aspects of chromatin regulation and transcription during neural development (Durieux et al., 2011). For example, in vivo inactivation of HTT by RNA interference or deletion of the gene affects spindle orientation and cell fate of cortical progenitors in the ventricular zone of mouse embryos, altering the thickness of the developing cortex as well as the polarization and migration of newly generated neurons (Godin et al., 2010; Molina-Calavita et al., 2014). Furthermore, depletion of HTT in post-mitotic projection neurons leads to the mislocalization of layer-specific neuronal populations in the mouse neocortex, suggesting that HTT, via regulation of RAB11-dependent N-Cadherin trafficking, is critical for neuronal migration (Barnat, Le Friec, Benstaali, & Humbert, 2017). Importantly, the authors also evinced that mutant HTT (mHTT) loses its capacity to promote neuronal migration. Normal HTT also is required for the correct establishment of cortical and striatal excitatory circuits and this function is lost when the mHTT is present (McKinstry et al., 2014). When cortical HTT function is conditionally silenced from CPNs, cortical and striatal excitatory synapses form and mature at an accelerated rate through postnatal day (P)21 but exuberant synaptic connectivity is lost over time in the cortex, resulting in the deterioration of synapses by 5 weeks of age (McKinstry et al., 2014). It can thus be postulated that mHTT impairs neurodevelopmental pathways (Blockx et al., 2012; HD iPSC Consortium, 2017). Indeed, expression of mHTT during early development is sufficient to produce a permanent HD phenotype even if expression is terminated at P21. Furthermore, developmental deficits associated with HTT function render cells more susceptible to degeneration (Arteaga-Bracho et al., 2016; Molero et al., 2016).
Not only is cortical brain development compromised by the presence of mHTT, but CPN function itself is altered by the early formation of mHTT aggregates. In R6/2 transgenic mice (Mangiarini et al., 1996), intranuclear inclusions can be detected as early as 3 weeks of age (Cummings et al., 2012; Meade et al., 2002; Morton, Lagan, Skepper, & Dunnett, 2000). At this age, intranuclear inclusions are most abundant in CA1 hippocampal region and cortical layers III–V, whereas in striatum inclusions are rare (Morton et al., 2000). Using more sensitive immunohistochemical methods, diffuse proto-aggregates have been observed in developing axonal tracts during embryonic development and early postnatal brains in juvenile and adult-onset mouse models of HD (Osmand, Bichell, Bowman, & Bates, 2016). These axonal aggregates could alter synaptic physiology during early postnatal development. In support, using a corticostriatal coculture from YAC128 mice, a model of adult-onset HD (Slow et al., 2003), synaptic transmission was impaired as early as three weeks in vitro (Buren, Parsons, Smith-Dijak, & Raymond, 2016). In addition, in an in vitro model of HD based on the generation of induced pluripotent stem cells from HD patients and controls, it was observed that HD-derived cells displayed a greater number of neuronal progenitors compared with controls. This cell population showed enhanced vulnerability to brain-derived neurotropic factor (BDNF) withdrawal in the JHD lines (Mattis et al., 2015). Interestingly, increased vulnerability was due to N-methyl-D-aspartate (NMDA) glutamate receptor-mediated toxicity, suggesting that aberrant Ca2+ signaling could be involved.
The observation of aberrant migration and polarization of cortical progenitors is reminiscent of the cortical maldevelopment observed in humans with FCD, a disorder characterized by dyslamination, CPN misorientation, and the presence of dysmorphic pyramidal neurons, all of which contribute to epileptogenesis (Cepeda, Hurst, Calvert, et al., 2003; Dautan et al., 2016; Taylor et al., 1971). Thus, we could hypothesize that the presence of mHTT in neuronal progenitors affects cortical organization and induces diffuse architectural and cellular abnormalities, similar to those observed in FCD, which results in cortical hyperexcitability (Blumcke et al., 2011; Cummings et al., 2009; Estrada-Sánchez et al., 2016).
1.3 The cerebral cortex is hyperexcitable in HD brains
The central role of the cerebral cortex in the genesis of the HD phenotype has long been recognized in clinical and experimental studies (Estrada-Sanchez & Rebec, 2013; Laforet et al., 2001; Paulsen et al., 2006; Virlogeux et al., 2018). In this section we review clinical and experimental evidence.
1.3.1 Evidence from human studies
The fact that cognitive and psychiatric disturbances appear long before overt motor symptoms (Lawrence et al., 1996, 1998) is an indication that the cortex is heavily involved in striatal neuron dysfunction. Functional abnormalities are evident as revealed by changes in cortical excitability and plasticity in preclinical and early HD (Orth et al., 2010; Schippling et al., 2009). Overall, studies indicate that at least certain cortical areas become hyperexcitable with disease progression (Agarwal et al., 2019). For example, transmagnetic stimulation (TMS) studies have demonstrated increased intracortical facilitation and reduced short interval intracortical inhibition in premanifest and early manifest HD patients (Abbruzzese et al., 1997; Berardelli & Suppa, 2013; Nardone et al., 2007; Schippling et al., 2009) indicating altered excitatory/inhibitory balance. As a consequence, motor cortex plasticity has been shown to be abnormal in HD gene carriers (Orth et al., 2010). Indeed, using paired-pulse TMS paradigms, it was suggested that GABA-mediated cortical inhibition is deficient in both presymptomatic and symptomatic patients (Philpott et al., 2016). Consistent with this idea, a study conducted on a small cohort of HD patients demonstrated that inhibitory TMS (1 Hz) of the supplementary motor area (SMA) significantly reduced choreic movements, leading the authors to conclude that overactivity of the SMA plays an essential role in the generation of abnormal movements (Brusa et al., 2005).
1.3.2 Evidence from animal studies
The excitotoxicity hypothesis of cell death in HD postulates that neurodegeneration is caused by excess glutamate release at striatal synapses and/or increased sensitivity of postsynaptic glutamate NMDA receptors (DiFiglia, 1990), in particular those located extrasynaptically (Milnerwood et al., 2010; Okamoto et al., 2009; Raymond et al., 2011). Thus, it is believed that sustained activation of extrasynaptic NMDA receptors triggers an apoptotic cascade that culminates in cell death of medium-sized spiny projection neurons (MSNs). While the traditional view considered the cerebral cortex as the main contributor of glutamate release, recent work has revealed a major contribution from the thalamo-striatal pathway (Huerta-Ocampo, Mena-Segovia, & Bolam, 2014; Smith et al., 2014). This pathway is affected early and persistently in several HD mouse models (Deng, Wong, Bricker-Anthony, Deng, & Reiner, 2013; Kolodziejczyk & Raymond, 2016; Parievsky et al., 2017).
If glutamate release becomes excitotoxic to medium-sized spiny neurons (MSNs), it might be expected that reducing glutamate inputs could prevent cell loss in HD. Indeed, in animal models evidence indicates that removal of the cerebral cortex delays HD symptoms and extends life span (Stack et al., 2007). Furthermore, preventing the expression of mHTT in CPNs has been shown to ameliorate the HD phenotype (Estrada-Sanchez et al., 2015; Wang et al., 2014). There also is evidence of glutamate release dysregulation when overt symptoms emerge (Cepeda, Hurst, Calvert, et al., 2003), as well as biphasic changes in glutamate release, initially elevated, and then progressively reduced (Joshi et al., 2009). The occurrence of large synaptic events in MSNs coinciding with the onset of overt symptoms in R6/2 mice suggested cortical hyperexcitability (Cepeda, Hurst, Calvert, et al., 2003). Consistent with this idea, electrophysiological studies in the BACHD mouse model (Gray et al., 2008) found decreased layer II/III PV-interneuron excitation and decreased CPN inhibition at 6 months, when behavioral symptoms become evident (Gu et al., 2005; Spampanato, Gu, Yang, & Mody, 2008). Interestingly, it was recently shown that R6/2 mice have fewer perisomatic PV-positive terminals on CPNs than their wild type (WT) counterparts, an observation that was also consistent in HD autopsy brains (Burgold et al., 2019). Importantly, this reduced inhibition was reflected by increased cortical activity measured with in vivo calcium imaging. Increased cortical excitability also was demonstrated electrophysiologically in vivo. For example, membrane fluctuations (Down to Up state) could be evoked with smaller currents in cortical neurons from symptomatic R6/2 compared with control mice (Stern, 2011). Furthermore, the amount of coherence in the state transitions of single neurons was less correlated with global activity compared with controls. This effect was proposed to affect the ability of CPNs to participate in coordinated activity within neuronal assemblies, which could explain the diminished synchrony of spikes found in behaving R6/2 mice (Walker, Miller, Fritsch, Barton, & Rebec, 2008).
As in humans, studies in HD animal models have shown impaired cortical synaptic plasticity, likely as a result of altered excitatory/inhibitory balance. For example, presymptomatic R6/1 mice show deficits in barrel cortex plasticity in a somatosensory whisker-deprivation paradigm (Cybulska-Klosowicz et al., 2004; Mazarakis et al., 2005). In the same mouse model, electrophysiological studies demonstrated progressive derailment of long-term depression (LTD) and long-term potentiation (LTP) at perirhinal and prefrontal synapses (Cummings et al., 2006, 2007; Dallerac et al., 2011). Remarkably, alterations in LTD and LTP could be reversed by dopamine D2 and D1 receptor agonists, respectively.
Definitive corroboration of cortical hyperexcitability and loss of the excitatory/inhibitory balance was provided by our group (Cummings et al., 2009). In three different genetic mouse models of HD, the frequency of spontaneous excitatory postsynaptic currents (EPSCs) was increased whereas that of spontaneous inhibitory postsynaptic currents (IPSCs) was decreased. In support of perturbations of the excitatory/inhibitory balance in the cerebral cortex of R6/2 mice, immunohistochemistry demonstrated increased VGLUT1 expression and reduced GAT1 expression. In addition, compared with WT mice, blockade of GABAA receptors in slices from R6/2 mice induced more frequent complex, ictal-like epileptiform discharges in CPNs. This phenomenon was observed in mice as young as P21. What is not known is why and how early the cerebral cortex becomes hyperexcitable.
1.4 New observations in developing R6/2 mice point to aberrant cortical neuron development and early hyperexcitability
1.4.1 Morphological and electrophysiological findings
Recently we extended our studies on CPN excitability to developing R6/2 and control littermates (P7 and P14). Mice (n = 13) of either sex were used at P7 (6 WT and 7 R6/2). Experimenters were blind to the genotype, which was only identified a posteriori from DNA tail samples, thus ensuring unbiased results. CPNs in layers II/III and V from the motor cortex were visualized with infrared differential interference contrast (IR-DIC) optics (see detailed methods in Supplementary Material). In order to increase cortical excitability, the GABAA receptor antagonist bicuculline (BIC, 10 μM), alone or in conjunction with the type “A” K+ channel blocker 4-aminopyridine (4-AP, 100 μM), were used. Both compounds are proconvulsant and we have used them in the past to examine cortical excitability and seizure susceptibility in HD mice (Cummings et al., 2009). The patch pipette also contained biocytin (0.2%) to label the recorded cells and, following histological processing, the fine morphology of CPNs was examined.
Preliminary observations indicate that CPN membrane excitability is increased in R6/2 mice as early as P7, the earliest age we examined. Indeed, blockade of GABAA receptors with BIC induced paroxysmal discharges more often in CPNs (4 out of 8) from HD mice but not in CPNs (1 out of 7) from age-matched WT littermates. Furthermore, concurrent application of BIC and 4-AP generated ictal-like activity in R6/2 CPNs, but not in WT CPNs (Figure 1b). These observations are consistent with our previous study demonstrating the higher occurrence of complex paroxysmal discharges in R6/2 compared with WT mice at P21 (Cummings et al., 2009), and highlight the fact that changes in cortical excitability are present even earlier during postnatal life.
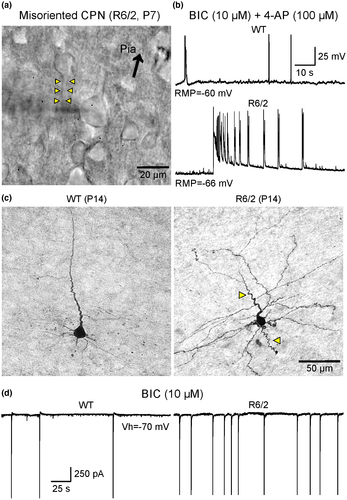
Morphological evidence of CPN maldevelopment also was observed during early development. Under IR-DIC microscopy we observed that some CPNs displayed misorientation, for example, apical dendrites pointing in the wrong direction, as well as the presence of dysmorphic dendritic and axonal processes (Figure 1a,c). While these processes extend with smooth transitions in CPNs from WT mice, in R6/2 mice some CPNs exhibit curvy, meandering dendrites and axons, suggesting that pathfinding mechanisms during fetal development had been disturbed by the presence of mHTT. At P14, signs of hyperexcitability in CPNs from R6/2 mice also were found (Figure 1d). In R6/2 CPNs, bath application of BIC induced more frequent epileptiform discharges compared with age-matched controls.
1.5 FCD-like abnormalities occur in the cerebral cortex of R6/2 mice
The cellular morphological abnormalities observed in young R6/2 mice are reminiscent of those we found in our studies of cortical tissue samples from pediatric epilepsy surgery patients with FCD histopathology (Abdijadid, Mathern, Levine, & Cepeda, 2015; Cepeda, Hurst, Flores-Hernandez, et al., 2003), suggesting that cortical maldevelopment in HD could underlie cortical hyperexcitability and seizure proclivity. According to the International League Against Epilepsy (ILAE) classification of FCD, there are at least 3 different categories (Barkovich, Dobyns, & Guerrini, 2015; Blumcke et al., 2011). FCD type 1 is characterized by cortical dyslamination with aberrant columnar and/or radial architecture. In addition, CPN misorientation and abnormal processes can be found. FCD type 2 is defined by additional features including the presence of dysmorphic, cytomegalic neurons and in some cases also balloon cells. FCD type 3 occurs only in association with other pathologies such as tumors or hippocampal sclerosis.
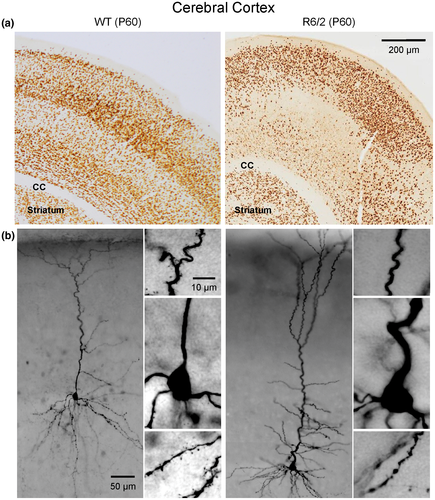
In a previous report, we speculated that one of the underlying mechanisms of cortical hyperexcitability and seizures in R6/2 mice could be a mild form of FCD, that is, type 1 (Estrada-Sánchez et al., 2016). To test this hypothesis, we recently examined potential histopathological evidence of FCD in symptomatic R6/2 mice (age P60). We chose this age group in a first approximation because spontaneous or evoked convulsive seizures are only observed in fully symptomatic mice. Six pairs of R6/2 and control mice of either sex were perfused, sliced, and stained for the specific neuronal marker, NeuN to examine cortical cytoarchitecture and CPN morphology (see Supplementary Material). Initial observations indicate that FCD-like abnormalities occur in the motor and somatosensory cortices of at least 50% of R6/2 mouse brains examined (Figure 2a). Abnormalities include cortical dyslamination, neuronal crowding in some areas but others devoid of NeuN label, all suggesting cortical maldevelopment. In addition, biocytin staining of CPNs demonstrated the presence of abnormal processes similar to those observed in R6/2 mice at P7, including misoriented neurons, recurving dendrites and extreme meandering of axonal processes (Figure 2b). Interestingly, in another model of HD with 100 CAG repeats we also documented the presence of misoriented CPNs as well as the common occurrence of dysmorphic dendrites (Laforet et al., 2001). These observations beg the question as to whether dysmorphic dendrites are the result of degenerative or abnormal neurodevelopmental processes.
1.6 Is HD a special case of MCD?
Cortical development is a delicate process that follows precise spatial and temporal rules. If, for any reason, these creodes (Waddington, 1962) are violated, the cytoarchitecture of the cortex crumbles. In particular, when CPNs terminate in ectopic positions FCD and other structural malformations ensue, leading to a growing number of neurological and psychiatric diseases (Ayoub & Rakic, 2015; Rakic, 2006; Wu et al., 2014). MCD represent a group of pathologies associated with aberrant development of the cerebral cortex and are a common etiology of epilepsy (Barkovich et al., 2015). The causes of MCD are multiple and diverse. Genetic as well as extrinsic factors play a role. As HD is a genetic disorder, we can surmise that mHTT is able to alter the rules that underlie cortical development. This is supported by experimental evidence that the presence of mHTT during embryonic development affects neuronal migration and final positioning and orientation (Barnat et al., 2017; Osmand et al., 2016).
Using Golgi impregnation of striatal neurons from HD patients, Graveland et al. described the abnormal presence of recurving terminal dendrites in MSNs (Graveland, Williams, & DiFiglia, 1985). Notably, the authors suggested that the high frequency of recurved dendrites may be a clue to the pathophysiology of HD. Degeneration and regeneration of CPNs from HD patients was also evinced by increases in the length of terminal branches and an overall greater branching complexity of the dendritic trees, somehow recapitulating normal brain development (Sotrel, Williams, Kaufmann, & Myers, 1993). Furthermore, an antibody used to detect the N-terminal region of mHTT was found in neuronal intranuclear inclusions and dystrophic neurites in the HD cortex and striatum (DiFiglia et al., 1997; Sapp et al., 1999). Dystrophic neurites, likely corresponding to distended axon terminals, were more prevalent in deep cortical layers and because they could be seen in a presymptomatic adult patient it was suggested that they precede clinical onset (DiFiglia et al., 1997). Morphological changes in the cerebral cortex include enlargement of gyral crowns and abnormally thin sulci (Nopoulos et al., 2007; Paulsen et al., 2006). Interestingly, enlargement of cortical gyri also is observed in some FCD types (Blumcke et al., 2011). Cortical thinning and white matter loss are common in pre-manifest HD subjects (Aylward, 2007; Reading et al., 2005; Rosas et al., 2005, 2006; Waldvogel et al., 2015). Notably, smaller intracranial volumes in prodromal HD patients indicate that mHTT can cause abnormal brain development (Nopoulos, Aylward, Ross, Mills, & Langbehn, 2011). Interestingly, smaller intracranial volumes can be associated with cerebellar enlargement, which could explain hypokinesia in JHD (Tereshchenko et al., 2019). Thus, HD therapies have to take into account that the goal is not only to prevent neurodegeneration in a susceptible brain but also to correct aberrant development.
1.7 Targeting cortical maldevelopment as a new strategy for the treatment of HD
There is an almost universal consensus that, in order to treat HD symptoms, interventions should start early. The question is how early? Based on the previous review of the literature and recent morphological and electrophysiological data in very young R6/2 mice, it appears that only by targeting early brain development can any treatment be successful. So the question becomes, can cortical maldevelopment be rescued? Using human HD induced pluripotent stem cell cultures it was demonstrated that the presence of mHTT negatively affects striatal and cortical neuronal progenitor specification and commitment leading to abnormal cell organization and acquisition of mature neuronal identities in cerebral organoids (Conforti et al., 2018). Notably, these defects could be rescued by down-regulating mHTT with synthetic Zinc Finger Proteins or pharmacologically by inhibiting the metalloproteinase ADAM10, which is a mHTT effector. Another study showed that very early behavioral, cellular, and molecular changes associated with the presence of mHTT can be reverted through administration of HDAC inhibitors (Siebzehnrübl et al., 2018). Finally, a recent study using a disease-on-a-chip microfluidic platform to examine the corticostriatal network in vitro, provided further evidence that cortical alterations are critical to the progression of the disease (Virlogeux et al., 2018). Further, substitution of HD cortical neurons with wild-type neurons was sufficient to rescue cellular alterations in mutant striatal neurons. Although we are still far from using these experimental approaches in human patients, these results offer a glimmer of hope.
2 CONCLUSIONS, FUTURE STUDIES AND SOME UNANSWERED QUESTIONS
Based on this review of the literature, as well as some preliminary data, we can conclude that brain development is altered in the most severe form of HD, that is, JHD, where there is strong evidence of cortical maldevelopment, similar to FCD. However, more anatomical studies using neuron-specific markers in younger animals are warranted so as to determine when the first manifestations of cortical malformation occur. In that sense, studies using cortical layer-specific markers will help a better understanding of CPN malpositioning. Future studies should also consider potential sex differences of cortical development in genetic models of HD. For example, it has been shown that the pattern of structural brain changes associated with normal HTT is remarkably different between normal male and female school-age children. Thus, within the normal range of CAG repeats (<36), cortical thickness and cognitive function were directly correlated with higher number of repeats in females but not in males (Lee et al., 2017).
Another important question for future studies is how cortical maldevelopment and hyperexcitability in HD mice affect corticostriatal synapses and MSN function. Recent studies have shown that during a critical period of mouse brain development (P10-18) corticostriatal connectivity is extremely sensitive to changes in cortical activity, suggesting that early imbalances in cortical function can impair basal ganglia circuit development (Peixoto, Wang, Croney, Kozorovitskiy, & Sabatini, 2016). Thus, we predict that altered excitatory/inhibitory balance in the cerebral cortex of HD mice will induce early changes in striatal neurons due to abnormal activity along the corticostriatal pathway. Initial support for this assumption was provided using a striatal and cortical coculture system. As early as 3 weeks in vitro differences in striatal MSNs were observed, including reduced frequency of spontaneous EPSCs as well as reduced dendritic complexity (Buren et al., 2016).
Another still unresolved question is whether GABAergic interneuron fate and positioning in the cerebral cortex are affected by the HD mutation. In addition, as mHTT is also abundant in striatum, it would be important to know if striatal compartmental organization, that is, striosome and matrix, is affected. For example, studies have shown that reduced expression of wildtype HTT during development can induce profound changes in striatal organization, including heterotopias that share striosome and matrix identities (Arteaga-Bracho et al., 2016). Finally, circuit organization in brain stem and thalamic regions, such as the inferior colliculus, should be examined to determine if abnormal development could explain the exquisite susceptibility of R6/2 mice to manifest audiogenic seizures.
A last point to consider is whether cortical maldeveloment is a general feature of HD or is only applicable to the more severe forms. At the present time it would be premature to generalize findings from JHD to adult-onset HD. However, recent morphological evidence obtained from a group of pre-manifest adult-onset HD patients, showed that the HTT mutation may indeed influence cortical neurodevelopment, although this seemed to be independent from processes leading to neurodegeneration (Kubera, Schmitgen, Hirjak, Wolf, & Orth, 2019). In addition, based on the fact that in adult-onset HD the cortex also shows hyperexcitablity, we could speculate that cortical development could be altered and that its manifestations are delayed or mitigated by compensatory mechanisms. For example, in presymptomatic (P21) and early symptomatic (P40) R6/2 mice the frequency of spontaneous IPSCs is increased in CPNs compared with WTs (Cummings et al., 2009). In addition, at P21, some cells displayed a bursting pattern of large-amplitude IPSCs. This could suggest that the intrinsic firing properties of cortical GABAergic interneurons are altered in mouse models of HD, resulting in an increased inhibitory drive onto CPNs. This upregulation of GABA activity could prevent early manifestation of HD symptoms. However, with disease progression this compensatory mechanism is no longer sufficient to prevent cortical hyperexcitability and eventual cell loss. Thus, in addition to targeting cortical maldevelopment, reinforcing cortical inhibition represents another valid strategy for therapeutic intervention.
ACKNOWLEDGMENTS
The authors would like to thank members of the Levine/Cepeda laboratory, in particular Dr. Sandra M. Holley, for fruitful discussions and suggestions. This work was funded by USPHS grant NS111316 (CC).
CONFLICT OF INTEREST
The authors declare they do not have any conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
Conceptualization, C.C.; Methodology, C.C., C.K.W. and H.V.V.; Investigation, C.C., K.D.O., D.C., J.B., V.W.Y., D.T.C., J.A., C.K.W. and H.V.V.; Formal Analysis, C.C. and K.D.O.; Writing – Original Draft, C.C. and H.V.V.; Writing – Review & Editing, C.C., K.D.O., D.C., J.B., V.W.Y., D.T.C., J.A., C.K.W. and H.V.V.; Visualization, C.C., K.D.O., D.C., J.B., C.K.W. and H.V.V.; Supervision, C.C. and H.V.V.; Funding Acquisition, C.C.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.