Fluid levity of the cell: Role of membrane lipid architecture in genetic sphingolipidoses
SIGNIFICANCE: Sphingolipidoses are severe and, currently, incurable neurological diseases in which sphingolipids accumulate to high and toxic levels. Little is known about the mechanism by which sphingolipids (SLs) elicit toxicity. This Commentary proposes that the insertion of SLs in biological membranes disrupts functional lipid domains and/or induces changes in membrane fluidity and curvature, leading to deregulation of cellular signaling and function.
Abstract
Sphingolipidoses arise from inherited loss of function of key enzymes regulating the sphingolipid (SL) metabolism and the accumulation of large quantities of these lipids in affected cells. Most frequently, toxicity is manifested in the nervous system, where survival and function of neurons and glial cells are most affected. Although detailed information is available on neuroglial alterations during terminal stages of the disease, the initial pathogenic mechanisms triggering neuropathology are largely unclear. Because they are key components of biological membranes, changes in the local concentration of SLs are likely to impact the organization of membrane domains and functions. This Commentary proposes that SL toxicity involves initial defects in the integrity of lipid domains, membrane fluidity, and membrane bending, leading to membrane deformation and deregulation of cell signaling and function. Understanding how SLs alter membrane architecture may provide breakthroughs for more efficient treatment of sphingolipidoses. © 2016 Wiley Periodicals, Inc.
HYPOTHESIS
Sphingolipidoses represent a large group of neurologically handicapping diseases caused by inherited loss of function of key enzymes in lipid catabolism and the consequent accumulation of undigested lipid material (for review see Platt, 2014). Although promising results are being obtained from gene therapy and hematopoietic replacement studies, the field has remained largely stalled with respect to curing most of these diseases (Cox and Cachon-Gonzalez, 2012). This shortage of effective treatments could be due, in part, to an incomplete understanding of how sphingolipids (SLs) initiate cellular dysfunction (Schulze and Sandhoff, 2011). Emerging roles of SLs in different physiological processes may hold the key for understanding their toxicity under nonphysiological conditions such as sphingolipidoses. For example, several SLs have been reported to play major roles as bioactive lipids (Bartke and Hannun, 2009), suggesting that they are not mere bricks in the big wall of biological membranes but rather integral, active, and functional constituents. It is important to note that the organization of SLs in cellular membranes is no longer regarded as random and inert (Singer and Nicolson, 1972). Rather, we now know that SLs are key components arranged into plasma membrane (PM) realms, ranging from nanometer lipid rafts (Simons and Ikonen, 1997; Lingwood and Simons, 2010) to submicrometer domains (Carquin et al., 2015). Furthermore, it is now accepted that SLs play roles in the macroscopic arrangement and shaping of cell membranes (Cooke and Deserno, 2006). These SL features are essential for host proteins and for regulating physiological events such as surface tension, force sensing (Mollinedo and Gajate, 2015), or cell signaling (Gomez-Mouton et al., 2004; Iwabuchi et al., 2010).
Under pathological conditions, when SL homeostasis is compromised, specific SLs accumulate at one or more orders of magnitude. This alteration in the local stoichiometry of the membrane could lead to rapid changes in lipid aggregation, disruption of membrane architecture, changes in membrane curvature and stability, and consequently impairment of vital membrane-dependent cellular processes (Carquin et al., 2015). This Commentary postulates that, in sphingolipidoses, the insertion of abnormal quantities of SLs in cell membranes destabilizes lipid domains and impairs the associated functions, inducing membrane changes (e.g., bending, vesiculation, and others) incompatible with normal cellular activities and leading to toxicity and cellular dysfunction.
EXPERIMENTAL CHALLENGES TO THE STUDY OF MEMBRANE LIPID DOMAINS
Three major lipid classes define the lipid organization of the PM (Fig. 1A). Cholesterol is the most abundant membrane lipid, with a nonpolar backbone inserted within the membrane bilayer and outwardly exposing a single hydroxyl group. Glycerophospholipids (phosphaticylcholine [PC], phosphatidylserine [PS], and phosphatidylethanolamine [PE]) are abundant lipids in PM, composed of saturated straight and unsaturated bent fatty acyl chains and a polar head group. SLs have a hydrophobic core formed by the sphingoid base sphingosine, which in most cases is acetylated with a fatty acyl chain to form a ceramide. Ceramides are bound to different head groups, including choline (i.e., sphingomyelin [SM]); sugars (i.e., glycosphingolipids [GSLs], such as in galactosylceramide [GalCer]; or glucosylceramide) or even more complex and larger head groups (i.e., sulfatides, gangliosides). Lysolipids are also formed by sphingosine and a sugar (i.e., galactosyl-sphingosine or psychosine).
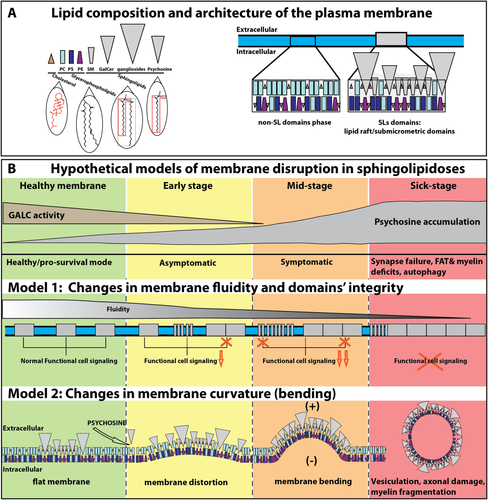
Potential pathological mechanisms initiated by SL accumulation illustrated in Krabbe's disease. A: The PM is composed of lipid domains, as illustrated for the SL domains (rafts and submicrometer domains) and the nonraft phase. Note the cone-shaped cholesterol (brown triangle), truncated cone-shaped PE (magenta), cylindrical PC (light blue), and PS (dark blue), whereas the SLs (gray) such as SM (truncated inverted cone shape), GalCer, gangliosides, and psychosine have inverted cone-shaped structures. The SL tail is composed of a sphingosine base (red polygon in psychosine) and binds to a long and saturated fatty acyl chain to form ceramide (green rectangle). In contrast, glycerophospholipids contain shorter single or polyunsaturated acyl chains (as illustrated by the bent acyl chain). B: Two hypothetical effects of abnormal levels of SLs are presented, taking as an example the increased levels of psychosine observed in Krabbe's disease. The deficiency of the galactosylceramidase (GALC) leads to increasing levels of psychosine over time. In model 1, the accumulation of SLs disrupts the architecture of lipid domains and impairs cellular signaling. The integrity of lipid domains is affected in early stages by either disruption or enlargement of these domains, leading to a decrease of functional signaling and, eventually, irreversible toxicity on a variety of potential processes (synapse failure; deficiencies in fast axonal transport [FAT] impairment, myelin, or autophagy). In model 2, the accumulation of inverted cone-shaped SLs induces an increase of the membrane curvature. The insertion of many molecules of psychosine at the outer leaflet of the PM promotes a distortion of the membrane and a positive (+; toward the extracellular space) bending. Ultimately, membrane bending may promote vesiculation, impacting axonal and myelin membrane integrity. These two models are not mutually exclusive; it is likely that a combination of both and other alternatives not discussed in this Commentary takes place during sphingolipidoses.
These lipids spontaneously assemble to form membrane bilayers in a nonrandom fashion. The biophysical mechanisms and dynamics of membrane lipid organization are essential for understanding how SLs modulate lipid domains and the consequent impact during disease. Artificial membrane models have provided a major contribution in understanding lipid organization by facilitating study of the effects of lipid content in a controlled environment. One of the basic models is the ternary lipid-phase diagram, a chart displaying thermodynamically distinct phases that coexist at equilibrium, namely, liquid-disordered, liquid-ordered (Lo), and solid-ordered (So) phases (Goni et al., 2008). Most mixtures consist of low-melting-temperature lipids (glycerophospholipids) with high-melting-temperature lipids (SLs) and cholesterol (de Almeida et al., 2003). The lipid composition is key in the formation and maintenance of membrane domains, particularly for modulating their shape and size (for review see Bagatolli et al., 2010). SLs form Lo or So domains in the glycerophospholipid fluid phase (for review see Westerlund and Slotte, 2009). Studies using simple planar lipid bilayers (Fidorra et al., 2006), giant unilamellar vesicles (Dietrich et al., 2001; Kahya et al., 2003; Pinto et al., 2008) and cell-derived PM (Bernardino de la Serna et al., 2004; Baumgart et al., 2007; Plasencia et al., 2007) have been critical in determining that SL–cholesterol interactions are essential for domain formation (Ramstedt and Slotte, 2002), which is the cornerstone of the lipid raft theory (Simons and Ikonen, 1997). Among SLs, ceramide was shown to exert striking effects on domain formation with high lateral separation at low concentration with atypical shape capable of altering the packing of the fluid phase (Castro et al., 2014).
The lipid domain concept is well established in artificial systems, but its occurrence in PM cells has been unclear (Munro, 2003; Bagatolli, 2006). Innovative imaging approaches are rapidly contributing to address this. Submicrometer domains can be observed with optical techniques such as high-resolution confocal, two-photon microscopy and total internal reflection microscopy. These microscopy techniques are versatile and can be combined with other approaches to study dynamics of lipid and the lipid–lipid or lipid–protein interactions such as fluorescence recovery after photobleaching, fluorescence lifetime imaging, and fluorescence correlation spectroscopy. The advent of super-resolution microscopic techniques has overcome the limitation of the resolution limit (∼200 nm) for analyzing the nanometer lipid rafts. These include 1) photoactivation localization microscopy, 2) structured illumination microscopy, 3) stimulated emission depletion microscopy, 4) atomic force microscopy, 5) near-field scanning optical microscopy, 6) scanning ion mass spectrometry, and 7) single dye tracing. Nanometer molecular interactions can also be measured by Förster resonance energy transfer combined with super-resolution microscopes. Altogether these techniques are becoming gold standards for studying micrometer (Carquin et al., 2015) and nanometer (Castro et al., 2013) dynamic parameters of lipid domains in biological membranes.
ALTERATION OF MEMBRANE DOMAINS IN SPHINGOLIPIDOSES
Brain cells are highly polarized and specifically enriched in various types of SLs (e.g., gangliosides in neurons; GalCer, sulfatides, and SM in myelin; for review see Aureli et al., 2015). A growing body of evidence shows that the pathological accumulation of SLs affects the highly organized architecture of brain membranes. For example, Krabbe ‘s disease is a sphingolipidosis with one the most severe neurodegenerative phenotype, caused by the deficiency of the lysosomal enzyme galactosyl-ceramidase and the accumulation of high amounts of psychosine. Psychosine is present in very low levels under healthy conditions, but its physiological function remains undetermined, being considered a biologically irrelevant intermediate (Suzuki, 1998). We have actively investigated the physicochemical properties of psychosine, seeking to understand its physiological function in health and disease. Psychosine accumulates in lipid rafts in central and peripheral nervous tissue from the murine twitcher model of Krabbe's disease as well as in the brain of Krabbe's disease patients (White et al., 2009). Increased levels of psychosine promoted significant changes in rafts, with enrichment of cholesterol, altered flotillin-2 and caveolin-1 distribution, and abnormal activation of protein kinase C (White et al., 2009). In neurons, psychosine negatively impacts fast axonal transport and neurofilament cytoskeleton via deregulation of raft-associated protein phosphatase 1 and 2 and glycogen synthase kinase-3β (Cantuti-Castelvetri et al., 2012, 2013). Additional evidence that psychosine alters membrane architecture via destabilization of lipid domains was also reported recently (Hawkins-Salsbury et al., 2013). New evidence from our laboratory underscores that psychosine disrupts endogenous submicrometer lipid domains but promotes the formation of new and likely aberrant high-order submicrometer domains, leading to an increase of PM rigidity (D'Auria and Bongarzone, unpublished data).
Similar effects have been found in other sphingolipidoses. For example, the accumulation of SM in lipid rafts impairs membrane RhoA targeting and activation in Niemann-Pick type A disease (Galvan et al., 2008). Furthermore, accumulation of SM at synaptic membranes disrupted synaptic plasticity dependent on the phosphoinositide pathway (Trovo et al., 2015). In these studies, exposure of neurons to exogenous SM reproduced those effects, suggesting that SM membrane accumulation was sufficient to induce PM defects. GM1 is another lipid raft component that can lead to major alteration of membranes and cellular processes. For example, neurons from GM1 gangliosidosis (Purpura and Baker, 1977; Purpura, 1978) or after exogenous administration of GM1 (Byrne et al., 1983) exhibited enlarged neurites. In metachromatic leukodystrophy, the accumulation of sulfatides (sulfated GalCer) decreased the content of MAL in myelin rafts (Saravanan et al., 2004) and affected the association of platelet-derived growth factor receptor-α with lipid rafts, impacting oligodendrocyte formation (Pituch et al., 2015). A recent work on immune cells analyzed the perturbation of specific SL metabolism on Toll-like receptor-dependent immune signaling, leading to a specific inflammation phenotype. Furthermore, predictions of inflammatory states in cells of patients affected by lipid storage disorders could be elaborated (Koberlin et al., 2015). As stated previously, ceramide represents a SL with a particular behavior. Generated after the hydrolysis of SM by sphingomyelinase, ceramide forms submicrometer domains also called ceramide-rich platforms. These platforms participate in membrane fragility (Montes et al., 2008) and changes in normal physiological functions, such as transmembrane signaling, clustering of specific proteins, and membrane destabilization by flip-flop diffusion (for reviews see Stancevic and Kolesnick, 2010; Castro et al., 2014) and may be relevant in neuronal deficits in sphingolipidoses (Prinetti et al., 2001).
DESTABILIZATION OF MEMBRANE ARCHITECTURE: A LETHAL KICKOFF FOR SPHINGOLIPIDOSES
The physiological and structural architecture of cell membranes is highly dependent on two key properties, fluidity and membrane curvature. Fluidity is a biophysical parameter of membranes that refers to the average membrane viscosity generated by the rotational and lateral mobility of individual molecules and their interactions with surrounding molecules (Lenaz, 1987). Fluidity is a fundamental property of cell membranes that influences the correct positioning of key proteins, receptors, and even lipids. Fluidity within the PM depends on its composition. For example, short and unsaturated lipid will promote highly fluid membranes, whereas packing of long and saturated fatty acid chains of SLs with cholesterol will decrease membrane fluidity (i.e., increase order, rigidity, or viscosity). Most SLs, such as GM1 (Nishio et al., 2004), SM (Koike et al., 1998; Galvan et al., 2008; von Einem et al., 2012) and even psychosine (D'Auria and Bongarzone, unpublished data), appear to alter cell membranes by decreasing fluidity. It is then conceivable that one of the first pathological changes in sphingolipidoses results from focal decreases in membrane fluidity, impairing the mobility of proteins and lipids essential for vital cellular functions.
Intimately linked to fluidity, membrane curvature is fundamental for regulating cell shape, endo- and exocytosis, process formation, and even synaptic activity. The shapes of different lipids influence how much a membrane can curve (i.e., bend). The intrinsic molecular volume of the head group and the composition of the fatty acyl chains (i.e., saturated vs. unsaturated) in lipids will determine steric dimensions and distinct shapes for each lipid species. From this consideration, lipids can be classified as cylinders (head group is similar to the tail; PC, PS), truncated cones (head group is smaller than the tail; PE), cones or triangles (head group is minimal; cholesterol), truncated inverted cones (head group is larger than the tail; SM), and inverted cones (tail is minimal; GSLs, psychosine, and LPC; Fig. 1A). The type of lipid and its abundance are major factors influencing morphological features of cell membranes (McMahon and Boucrot, 2015). The degree of membrane curvature of a bilayered membrane is determined by the difference resulting from the combined curvatures of the inner vs. the outer monolayer leaflets. For example, a monolayer of inverted cone lipids promotes a positive curvature, whereas conical lipids favor negative curvatures. Cylindrical lipids or associations among cones with inverted cone lipids tend to produce flat monolayers (Cooke and Deserno, 2006). The asymmetric distribution of lipid species across the bilayer (i.e., SLs are located mainly in the outer leaflet, whereas PS and PE are associated mainly with the inner leaflet) influences the resulting membrane curvature (Devaux et al., 2008). Although specialized proteins play active roles in membrane curvature (for review see McMahon and Boucrot, 2015), the strong dependence between shape/composition and membrane curvature underscores the idea that increased SLs in sphingolipidoses change the composition of lipid domains, inducing positive (outward) membrane bending (Fig. 1B). This structural alteration is likely to impact membrane topology and the activity of associated signaling pathways.
Changes in membrane curvature could explain some of the phenotypic changes, such as membrane swelling, observed in various sphingolipidoses. For example, neurons from GM1 and GM2 gangliosidoses displayed large axonal and dendritic swellings (Purpura and Baker, 1977; Purpura, 1978). For Krabbe's disease, our group reported in vivo axonal swelling in spinal cords and peripheral nerves of twitcher mice and also in cultured mutant neurons and motoneurons incubated with psychosine (Castelvetri et al., 2011). In sphingolipidoses, in which there is a progressive accumulation of SLs in the membrane, increased bending could lead to exacerbated shedding of the membrane, an emerging mechanism with significant roles in pathophysiology (Herring et al., 2013; see also Scesa et al., 2016). Altogether these data underscore the activation of common pathological changes of membranes, including increased rigidity, bending, swelling, and vesiculation, promoted by the presence of SLs (Fig. 1B).
CONCLUSIONS
The successful treatment of sphingolipidoses has been hampered not only by the rapidity and severity of their phenotype but also by an incomplete mechanistic understanding of how SLs inflict damage on brain cells. This Commentary has discussed the idea that SLs could impair physiological signals through 1) disruption of functional lipid domains, 2) membrane rigidification, and 3) cellular deformation by changes in membrane curvature (Fig. 1B). The consequences that these early changes in membrane architecture impose on brain physiology are major. Decreases of membrane fluidity and curvature may have far-reaching effects, from reducing the efficiency of docking of synaptic vesicles and their release to alterations in internodal myelin to the aberrant activation of microglia and formation of the glial scar (Seyfried et al., 1984; Paintlia et al., 2003; Jana and Pahan, 2010). Furthermore, it is probable that these early alterations are limiting factors in current treatments (White et al., 2011), which in most cases require long periods to elicit the first clinical improvements. Future therapies may take this aspect into consideration by addressing, for example, protection of membrane curvature by the use of cone-shaped lipids or membrane-stabilizing proteins (for review see McMahon and Boucrot, 2015). Although our discussion has opted for a more simplistic view of singular lipids, future work should complement our analysis by including other, more complex lipids and proteins (Koberlin et al., 2015). Decoding toxicity mechanisms in sphingolipidoses also represents a unique opportunity for understanding the physiological function and organization of SLs implicated in diseases other than sphingolipidoses (Gulbins and Petrache, 2013).
CONFLICT OF INTEREST STATEMENT
E.R.B. is a consultant with Lysosomal Therapeutics, Inc.