Retinoic acid reduces solvent-induced neuropathy and promotes neural regeneration in mice
Abstract
In humans, exposure to organic solvents (OS) is frequent in work activities or as a recreational inhalant, inducing severe neuropathy (secondary to demyelization of peripheral nerves). We have previously shown that all-trans retinoic acid (ATRA) increases local content of neural growth factor (NGF), improving peripheral neuropathy of diverse origins. In this study, we evaluated the effect of ATRA on OS-induced peripheral neuropathy in experimental mice. Two simultaneous experiments were performed. The first one aimed to evaluate ATRA for the prevention of damage induced by OS, the second to test ATRA as an OS-induced neuropathy treatment. Nociceptive threshold latency and NGF concentration in serum and in peripheral nerves were determined. Morphological changes and evidence of sciatic nerve regeneration were evaluated. Mice exposed to OS developed neuropathy and axonal degeneration. ATRA diminished the effects of OS inhalation on sensorial changes and nerve morphology. Treatment with ATRA reversed sensorial and nerve morphological changes of OS-induced neuropathy, and this was associated with increased contents of NGF. Similar to previous experiences on diabetic and toxic neuropathy, ATRA reduced and partially reversed the peripheral neuropathy caused by OS exposure. These favorable effects apparently are due to local production of NGF induced by neural regeneration in response to the administration of retinoic acid. © 2014 Wiley Periodicals, Inc.
Organic solvents (OS) are chemical mixtures containing at least 20 different aromatic and aliphatic compounds, such as toluene, benzene, acetone, methanol, and hexane (Baelum, 1991). They are used in textiles, paints, and solvent-based cleaning fluids, which are ubiquitous in labor and home-cleaning settings. Intentional inhalation of chemical fumes from OS to achieve euphoric effects is a popular method of substance abuse throughout the world because of their ready availability, low cost, and uncomplicated administration (Yamada, 1993; National Institutes of Health, 2005).
Chronic abuse of OS results in structural and functional impairment of a variety of organs, particularly in the nervous system (Zaidi et al., 2007). Electrophysiological studies have demonstrated that chronic inhalation of OS causes a decrease in nerve conduction velocity, muscle denervation, prolonged latencies with decreased amplitudes on visually evoked potentials, and slow waves on the electroencephalogram (Seppalainen, 1985). Several studies have shown histological changes in the central and peripheral nervous systems after long-term exposure to OS inhalation. The damage includes cerebellar and brainstem atrophy, diffuse focal white matter atrophy, and neural demyelization (Yamanouchi et al., 1995). A major chemical agent in OS is the neurotoxin toluene, which represents 60–70% of OS composition (Baelum, 1991). Long-term toluene abuse causes permanent changes in brain structures, which correlate with neural dysfunction (Baelum, 1991; Yamanouchi et al., 1995). Moreover, chronic exposure to benzene produces peripheral neuropathy, mental impairment, irreversible changes in cerebellar morphogenesis, and brain atrophy. Additionally, benzene shows a potential synergism when combined with toluene, which occurs in the case of paint thinner (Juntunen et al., 1980; Damasceno and de Capitani, 1994). Paint thinner inhalation is an important cause of brain damage and peripheral neuropathy, and it leads to irreversible neural injuries (Yamanouchi et al., 1995).
Morphological, functional, and molecular studies of OS-induced neuropathy are scarce. However, some evidence from experimental animals suggests that central nervous system alterations resulting from chronic inhalation of paint thinner could be secondary to defective neurotransmitter release at the synaptic space, specifically increasing the gap between membranes (Carabez et al., 1998). Moreover, it is known that toluene inhalation stimulates oxidative stress in cells, which elicits reactive gliosis through the generation of reactive oxygen species and induces the synthesis of gliosis-related proteins, such as glial fibrillary acidic protein (GFAP), and a decrease in glutathione levels in the brain tissue (Mattia et al., 1993).
On the other hand, retinoids are derivatives of vitamin A that play a role in a variety of biological functions, particularly in epithelial and neural differentiation (Zhong et al., 2008). They produce changes in gene expression through binding to specific families of intracellular receptors known as retinoid acid receptors (RARs) and retinoid X receptors (RXRs). Different types of retinoids possess a variable selectivity to individual retinoid receptors, such as ATRA, which directly transactivates downstream target genes through the activation of its nuclear RARs and the heterodimers RARs–RXRs (Ortiz et al., 2002). ATRA participates with neural growth factor (NGF) in eliciting the survival and differentiation of various neuronal cell lines (Corcoran and Maden, 1999; Halifeoglu et al., 2000). In vivo and in vitro studies have shown that NGF promotes survival and differentiation of sympathetic neurons, and it prevents cisplatin- and paclitaxel-induced neuropathy (Apfel et al., 1992; Hayakawa et al., 1998). Also, ATRA stimulates NGF and the expression of its receptor; thus, the feedback of ATRA and NGF may potentiate each other. Additionally, it is known that NGF induces the synthesis of retinoic acid by expression of retinaldehyde deshydrogenase type 2, whereas the inhibition of the synthesis of retinoic acid blocks neural growth-dependent NGF (Corcoran and Maden, 1999). Moreover, ATRA is capable of activating local retinoic acid responses in adult rat nerves after peripheral nerve injury, stimulating neurite outgrowth and promoting functional regeneration of sensory axons in the spinal cord (Corcoran and Maden, 1999; Zhelyaznik et al., 2003; Hernandez-Pedro et al., 2008). Previously, we reported that ATRA administration prevents and partially reverses sensitivity and morphological changes in both diabetic and chemotherapy-induced neuropathy in experimental animals as well as in humans (Arrieta et al., 2005, 2011; Hernandez-Pedro et al., 2008). This study evaluated the effects of ATRA administration as a preventive and therapeutic agent in experimental OS-induced peripheral neuropathy.
MATERIALS AND METHODS
Animal Care
Two hundred ten male Swiss NIH albino mice, weighing 20 g each, were housed in plastic containers (50 × 20 cm) and kept at room temperature under artificial lighting on a 12-hr light–dark cycle. Mice were fed with pellets (Laboratory Rodent Diet; Harlan Laboratories, Madison, WI) and water ad libitum. All experiments were reviewed and approved by the Research and Experimental Animal Care Committees of the Instituto Nacional de Neurología y Neurocirugía de México.
Experimental Design
Experiment I
To evaluate whether ATRA could have a preventive effect on the development of OS-induced neuropathy, 120 mice were randomly assigned to four groups. Group A (n = 30) received 25 μl of mineral oil subcutaneously as a placebo and was the control group. Group B (n = 30) received 20 mg/kg of ATRA (Sigma-Aldrich, St. Louis, MO) resuspended in mineral oil (Arrieta et al., 2005). Group C (n = 30) was exposed to the inhalation of industrial paint thinner for 30 min in a closed chamber (OS, 20 ppm). Group D (n = 30) received a subcutaneous injection of ATRA (as described for group B) and inhaled the OS (as described for group C). All treatments were administered daily for 90 days. The composition of the paint thinner administered, confirmed by chromatographic analysis, was determined to be 9% methanol, 13% butanol, 8% hexane, 8% benzene, 49% toluene, and 1% xylene, 1% tetradecane, and 1% pentadecane (Carabez et al., 1998; Fig. 1).
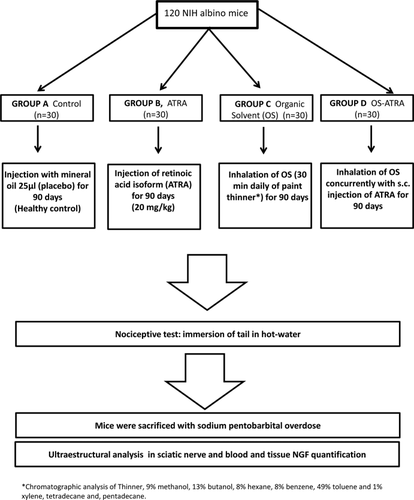
Experiment II
To evaluate the possible therapeutic effect of ATRA in animals with neuropathy induced by OS, 90 Swiss NIH albino mice were randomly assigned to two groups. Group I (n = 45) was treated with a subcutaneous injection of 25 μl mineral oil as a placebo and was the control. Group II (n = 45) inhaled OS (as described above for group C). Both treatments were administered for 90 days. After this time, 15 animals of each group were subjected to nociceptive testing and were sacrificed for NGF quantification and ultrastructural analysis, as described below. The remaining animals were treated as follows. Group I (n = 30) continued receiving a subcutaneous injection of 25 μl mineral oil (control). For group II, (n = 30) the OS inhalation was interrupted, and mice began receiving treatment with a daily subcutaneous injection of 20 mg/kg ATRA resuspended in mineral oil for another 90 days (Fig. 2). Afterward, mice underwent nociceptive testing and were sacrificed with a sodium pentobarbital overdose (200 mg/kg, i.p.) for NGF quantification and ultrastructural evaluation.
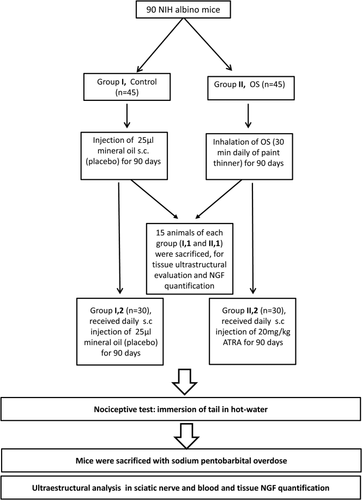
Measurement of Nociceptive Activity
All animals were subjected to nociceptive thresholds using a tail immersion test. This determination was made by immersion of the last one-third of the rat's tail into a water bath with a constant temperature of 52°C (Arrieta et al., 2005). This test was done three times with each animal, and the average of the response times was scored. The time for tail withdrawal latency (in seconds) was measured with a manual stopwatch (Hernandez-Pedro et al., 2008). This test was carried out at the end of each experiment.
NGF Levels
All mice were anesthetized with inhaled ether and sacrificed; blood samples were obtained by cardiac puncture and centrifuged at 2,500 rpm for 10 min, obtaining serum that was stored at −70°C until use. Both sciatic nerves were exposed at the midthigh and dissected toward the origin in the lumbar vertebral area and the distal portion at the ankle; one was used for electronic microscopic analysis, and the other was homogenized 1:1 (weight:volume) with a buffer (Tris-HCl 100 mmol/liter, NaCl 400 mmol/liter, 2% albumin, 0.05% sodium azide, pH 7.0) and protease inhibitors (PMSF 1 mmol/liter, aprotinin 7 μ/ml, and EDTA 4 mmol/liter). The sample was centrifuged at 100,000g for 10 min at 4°C, and the supernatant was mixed 1:1 with 0.2% Triton X-100 in CaCl2 mmol/liter. The samples were processed by enzyme-linked immunosorbent assay on microtiter plates previously coated with monoclonal antibodies against NGF (anti-mouse β [2.5S] 0.1 μg/ml NGF; Roche Molecular Biochemicals, Mannheim, Germany), which were incubated for 2 hr at 37°C and washed with Tris-HCl/Triton X-100 buffer at pH 7. Samples of 200 μl of either tissue or serum were diluted in the same buffer (1:2 for serum and 1:5 for tissue) and were incubated overnight at 4°C. Then, the plates were washed three times with Tris-HCl buffer and murine monoclonal anti-β (2.5S). NGF labeled with β-galactosidase (Roche Molecular Biochemicals) in a 1:10 dilution was added as a third reactant. After 4 hr of incubation at 37°C, the well plates were washed and the enzymatic reaction was carried out by adding a substrate solution of chlorophenol red galactopyranoside (Roche Molecular Biochemicals). The colorimetric reaction was read 30 min later with a spectrophotometer at 570 nm. The standard curve against which the NGF content was determined was made by successive dilution of NGF in buffer solution containing 5–300 pg/ml of NGF (Ordonez et al., 1994).
Ultrastructural Evaluation of Sciatic Nerve
After assays, the left sciatic nerve of treated mice was dissected and fixed overnight in 2.5% glutaraldehyde. After that, nerves were washed with PBS and postfixed with 0.5% tetroxide osmium for 1 hr, alcohol dehydrated, and Epon embedded. Ultrathin transverse sections at the silver/gray area of the spectrum of interference colors were uranyl acetate/lead citrate stained and examined via transmission electron microscopy (EM-10 A; Carl Ziess, Oberkochen, Germany). Ultrastructural study of peripheral nerve elements was conducted as previously described by Jacobs and Love (1985). Several parameters that include myelinated and unmyelinated axons, number of Schwann cell nuclei, regeneration bands (formed by plasma membrane stretching of Schwann cell arranged spirally around axons), axons in remyelination process characterized by a thin myelin band, and the percentage of myelinic axons with ultrastructural alterations, such as myelin changes, were quantified in square millimeters. Morphological evaluation was performed by an independent observer unaware of the group source of the specimens.
Statistical Analysis
Continuous variables were summarized as arithmetic means, medians, and standard deviations (SD); categorical variables were proportions. Inferential comparisons among animal groups were conducted by one-way analysis of variance (ANOVA) and post hoc (Tukey) or Kruskal-Wallis test. Statistical significance was determined at P < 0.05 in a two-sided test. Data were analyzed in SPSS software (version 17.0; SPSS, Chicago, IL).
RESULTS
Experiment I
Nociceptive tests
All mice survived the 90 days of treatment. In assays no significant differences in body weight were found among the groups during the study (control 20.4 ± 1.2, ATRA 20.4 ± 1.1, OS 18.9 ± 1.6, and OS-ATRA 20.2 ± 0.7; P = 0.83). Results of nociceptive latency by tail immersion test at day 90 are shown in Figure 3A. Inhalation of OS induced a significant increase in latency (14 ± 3 sec, 95% CI 12–15 sec) compared with control (6 ± 1 sec, 95% CI 5–6 sec) and with animals that received ATRA (6 ± 2 sec; 95% 6–7 sec). Administration of ATRA significantly reduced the effect of OS inhalation on nociceptive latency (10 ± 3 sec, 95% CI 9–11 sec; P < 0.001) compared with animals from group C that were subjected to OS inhalation.
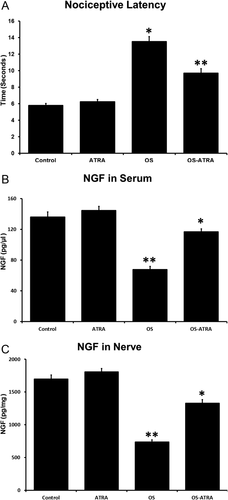
NGF levels
Inhalation of OS induced a significant reduction of NGF in serum and nerve (group C, 68 ± 22 pg/μl, 95% CI 60–76; 736 ± 192 pg/mg, 95% CI 665–808) compared with control (group A, 136 ± 36 pg/μl, 95% CI 123–149; 1,699 ± 334 pg/mg, 95% CI 1,574–1,824) and healthy animals treated with ATRA (group B, 145 ± 29 pg/ml, 95% CI 134–155; 1,807 ± 283 pg/mg, 95% CI 1,701–1,913; P < 0.001 in all comparisons). Simultaneous administration of ATRA significantly reduced the effect of OS inhalation on NGF levels both in serum and in the sciatic nerve (group D, 117 ± 21 pg/μl, 95% CI 109–125; 1,330 ± 290 pg/mg, 95% CI 1,222–1,439; P < 0.001) compared with animals exposed to OS inhalation (Fig. 3B,C).
Ultrastructural analysis
The number of the unmyelinated axons per area (square millimeters) was significantly lower in nerves from animals exposed to OS inhalation (group C, 46,008 ± 23,570; group D, 37,116 ± 8,218) compared with control animals (64,179 ± 16,993; P < 0.08) and healthy animals that received ATRA (68,372.18 ± 18,464; P < 0.036 and P < 0.001 for groups C and D, respectively). Evidence of nerve regeneration evaluated by regeneration bands was very high in group D (1,127 ± 446) compared with the other groups (group A, 90 ± 48; group B, 100 ± 79; group C, 24 ± 24; P < 0.001; Fig. 4). No differences were found among groups regarding the number of myelinic axons (P < 0.485), Schwann cells (P < 0.954), or remyelinization bands (P < 0.193).
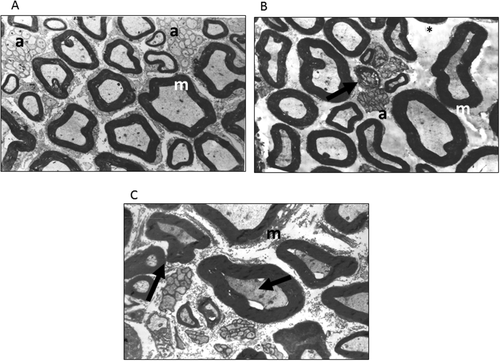
Experiment II
Nociceptive tests
All animals survived during the treatment time. The potential for reversal of OS-induced neuropathy with ATRA treatment was analyzed in experiment II. The animals did not shown significant differences in body weight between groups (group 1, 33 ± 1; group 2, 34 ± 1; OS, 28 ± 3; ATRA 33 ± 2; P = 0.85). Establishment of peripheral neuropathy after 90 days of OS inhalation was tested in group 2, which showed significantly higher withdrawal latency times (15 ± 3 sec, 95% CI 14–16 sec) compared with control in group 1 (6 ± 1 sec, 95% CI 5–6 sec; P < 0.001), showing an increased threshold for thermal hypoalgesia. Mice in group 2 that received ATRA for 90 days after chronic inhalation of OS (subgroup 2b) showed improved nociceptive latency to 9 ± 1 sec (95% CI 8–9 sec; P < 0.001, compared with nontreated ATRA group and OS exposed animals), reaching values similar to those observed from control (6 ± 2 sec, 95% CI 5–7 sec; P = 1.0; Fig. 5A).
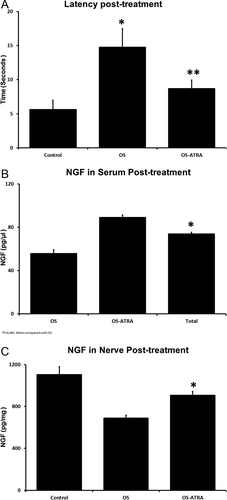
NGF levels
NGF contents in mice exposed to OS were significantly lower compared with those of control, both in serum (56 ± 9 pg/μl, 95% CI 53–59 vs. 71 ± 19 pg/μl, 95% CI 64–78; P = 0.001) and in nerve (689 ± 203 pg/mg, 95% CI 613–765 vs. 1,104 ± 405 pg/mg, 95% CI 953–1,256; P < 0.001). The treatment with ATRA resulted in an increase in serum levels of NGF, showing a mean of 89 ± 16 pg/μl (95% CI 80–98) compared with nontreated mice (P < 0.001) but did not achieve values similar to control (P = 0.235). In a similar manner, nerve contents of NGF in OS-exposed animals treated with ATRA increased to 907 ± 191 pg/mg (95% CI 801–1,012) compared with 689 ± 203 pg/mg in non-ATRA-treated mice exposed to OS (P < 0.001; Fig. 5B,C).
Ultrastructural analysis
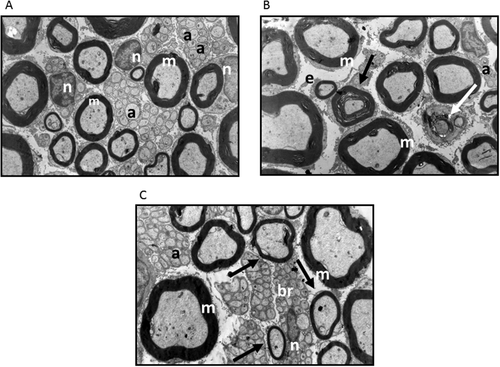
Ultrastructural analysis from treated samples is shown in Figure 6. Myelinic axons in group 2 (26,504 ± 1,612) were higher compared with control (group 1, 22,883 ± 1,046; P = 0.008). Similarly, amyelinic axons were higher in group 2b (111,598 ± 8,632) vs. control (71,805 ± 5,036; P = 0.005). Additionally, Schwann cells were increased in group 2b (2,540 ± 166) compared with group 1b (1,671 ± 187) and with control (1,415 ± 138; P < 0.001 and P = 0.005, respectively). Regeneration bands, indicative of nerve regeneration, were higher in group 2b (1,151 ± 110) compared with group 1b (207 ± 56) and with control (150 ± 46; P < 0.001). The remyelination bands were also higher in group 2b (7 ± 1) compared with group 1a (3.2 ± 0.67) and control (2.9 ± 0.66; P < 0.001 and P = 0.005, respectively).
DISCUSSION
Intense exposure to OS produces severe neural damage, according to the intensity and time of exposure. Clinical studies have shown that damage induced by OS exposure produces a decrease in cognitive functions and diverse abnormalities in the peripheral nervous system (National Institutes of Health, 2005). Because of the highly lipophilic characteristic of solvents, diffusion throughout the nervous system causes demyelination and changes in sensation and sensorimotor functions; these alterations progress after exposure and may be difficult to reverse (Seppalainen, 1985; Dick, 2006). In this study, daily exposure to 20 ppm of volatile paint thinner for 90 days induced severe nerve damage manifested by an increase in latency for thermal hypoalgesia; it is well known that exposure to OS induces an axonal diffuse degeneration, eliciting an increase in the umbral tolerance after nociceptive stimulus (Wright et al., 2004; Chentanez et al., 2009). Paint thinner has direct cytopathic effects on nerve-free terminals. Toluene produces free radicals and lipid peroxidation in the peripheral tissues, including the peripheral nerve and nerve terminals (Baydas et al., 2003). Previous studies have demonstrated that oxygen free radicals at the site of injury result from several sources, including activated neutrophils, swelling, and vacuolization of mitochondria, predominantly in axons (Kallenborn-Gerhardt et al., 2012). Also, free radicals damage many cellular components by interaction between nitric oxide and superoxide radicals, resulting in the formation of peroxynitrite, leading to nerve hypoxia, and contributes to the maintenance of thermal hyperalgesia (Althaus et al., 1993; Khalil et al., 1999).
In humans and mice, NGF plays a key role in recovery from insults of the peripheral nerve and prevention of neuropathy (Apfel et al., 1992; Hayakawa et al., 1998; Aloe et al., 2000; Arrieta et al., 2011). This study agrees with previous studies that administration of retinoic acid (RA) reverses peripheral neuropathies of different etiology, such as diabetes mellitus or cancer chemotherapy. A decrease was observed in the NGF contents both in serum and in sciatic nerves of mice exposed to chronic inhalation of OS, suggesting a ubiquitous role for NGF in peripheral neuropathy in humans. RA is capable of regulating the expression not only of NGF but also of other neurotrhopins, such as ciliary neurotrophic factor (CNF), neurotrophin-3 (NT-3), and brain-derived neurotrophic factor (BDNF), which could participate in nerve regeneration (Althaus et al., 1993; Halifeoglu et al., 2000; Baydas et al., 2003).
In experimental studies, nervous system alterations resulting from chronic inhalation of paint thinner have been related to oxidative stress, inducing reactive gliosis (Baydas et al., 2003). Toluene inhalation induces generation of reactive oxygen species and stimulation of the synthesis of gliosis-related proteins, such as GFAP, as well as the reduction of glutathione levels (Burmistrov et al., 2001). Ultrastructural evaluation of the sciatic nerve in OS-exposed mice disclosed degenerated myelinic axons with altered myelin sheaths and edematous amyelinic axons with endoneural damage.
Previously, we showed that ATRA is capable of partially reversing morphological and functional changes of diabetic and toxic peripheral neuropathy, simultaneously increasing NGF concentrations in nerve and in serum (Arrieta et al., 2005). A possible mechanism by which ATRA could exert protective effect is by the promotion of neurite outgrowth through transcription of neurotrophin receptor genes, which control actin polymerization and growth cone advancement (Hayakawa et al., 1998). In a feedback pathway, NGF induces the production of retinaldehyde dehydrogenase type 2, which synthesizes RA, stimulating intracellular retinoid pathways (Corcoran and Maden, 1999). In this study, the alterations in nerve function were partially reversed when ATRA was administered simultaneously with OS. This effect was also observed after the development of OS-induced peripheral neuropathy; in this case the observed improvement did not result in latency times similar to those of control, although the difference was not significant. This favorable change on functional tests could be related to prevention of NGF depletion, promoting nerve regeneration in both experiments.
Another mechanism by which RA could exert neuropotective effects is through the stimulation and expression of their nuclear receptors. Previously, we reported that RA stimulates the RAR by expression in the sciatic nerve of neuropathy induced by chemotherapy (Arrieta et al., 2011). Also, other studies have demonstrated that the expression of all RARs plays an important role in regeneration both in sciatic nerves and in Schwann cells (Althaus et al., 1993). Additionally, we observed that treatment with RA induces neurite outgrowth through counteracting myelin-dependent inhibition selectively by Lingo1 repression (Khalil et al., 1999).
In this study, we observed that treatment with ATRA partially prevented and reversed morphological changes characteristic of peripheral neuropathy. The main change observed was an increase in regeneration bands; this positive effect was noted in both experiments on OS-induced neuropathy. Previously, we reported a similar behavior in rats with diabetic neuropathy (Arrieta et al., 2005; Hernandez-Pedro et al., 2008) and in rats and humans with chemotherapy-induced peripheral neuropathy (Arrieta et al., 2011).
Morphological, functional, and molecular studies of OS-induced neuropathy are scarce. The present study provides experimental information on OS-induced neuropathy and gives evidence of an apparent role of ATRA in nerve regeneration in this form of experimental peripheral neuropathy. However, more exhaustive pharmacologic studies are necessary to determine the dynamics and kinetics of ATRA therapy for peripheral neuropathy of different etiologies.
We conclude that experimental neuropathy induced by inhalation of OS might be partially prevented and reversed by the administration of ATRA. These data, as well as our previous reports, show 1) that deficiency of NGF is a common pathway for the most frequent etiologies of peripheral neuropathy and 2) that administration of RA derivatives might constitute a potential therapy for peripheral neuropathy, apparently by the induction of local NGF production.




