High doses of salicylate and aspirin are inhibitory on acid-sensing ion channels and protective against acidosis-induced neuronal injury in the rat cortical neuron
Abstract
Aspirin and its main metabolite salicylate are widely used to relieve pain, treat inflammatory diseases, and prevent ischemic stroke. Multiple pathways are responsible for the therapeutic actions exerted by these drugs. One of the pathways is targeting neuronal receptors/ion channels in the central nervous system. Correspondingly, increasing evidence has implicated acid-sensing ion channels (ASICs) in the processes of the diseases that are medicated by aspirin and salicylate. We therefore employed whole-cell patch-clamp recordings to examine the effects of salicylate as well as aspirin on ASICs in cultured cortical neurons of the rat. We recorded rapid and reversible inhibition of ASIC current by millimolar concentrations of aspirin and salicylate and found that salicylate reduced acidosis-induced membrane depolarization. These data suggest that ASICs in the cortex are molecular targets of high doses of aspirin and salicylate. In addition, the results from lactate dehydrogenase release measurement showed that high doses of aspirin and salicylate protected the cortical neuron from acidosis-induced neuronal injury. These findings may contribute to a better understanding of the therapeutic mechanisms of aspirin and salicylate actions in the brain and provide new evidence on aspirin and salicylate used as neuroprotective agents in the treatment of ischemic stroke. © 2011 Wiley Periodicals, Inc.
Aspirin and its main metabolite salicylate are widely used in the medication of chronic inflammatory diseases, pain, and cerebrovascular diseases. In those patients treated with aspirin or salicylate for inflammatory diseases, the serum concentration of salicylate can reach up to several millimoles (Insel,1996). Low-dose aspirin is routinely used in the prevention of ischemic stroke (van der Worp and van Gijn,2007). Recent studies have shown that intraperitoneal injection of high-dose aspirin reduces infarct size in the rat cerebral ischemic model, suggesting its strength as a neuroprotective agent in the treatment of ischemic stroke (Berger et al.,2004, 2008; Zheng et al.,2007). The studies in cultured neurons confirm the neuroprotective effects of aspirin or salicylate (Grilli et al.,1996; Mohanakumar et al.,2000; Vartiainen et al.,2003). The mechanisms underlying neuroprotection by aspirin or salicylate may well be beyond antiplatelet action through the inhibition of cyclooxygenase and subsequent prostaglandin production (Vane,1971). Several studies have indicated that millimolar concentrations of aspirin or salicylate decrease cell adhesion, oxygen free radicals, superoxide generation, activity of inducible nitric oxide synthase, and plasma membrane viscosity (Philips et al.,1988; Abramson et al.,1990; Umeki,1990). In addition, millimolar concentrations of aspirin or salicylate modulate specific neuronal receptors/ion channels, including the voltage-gated sodium channel, voltage-gated potassium channel, voltage-gated calcium channel, γ-aminobutyric acid type A (GABAA) receptor, glycine receptor, and N-methyl-D-aspartate (NMDA) receptor in the central nervous system (Akada et al.,2003; Guitton et al.,2003; Peng et al.,2003; Liu and Li,2004; Xu et al.,2005; Liu et al.,2007; Lu et al.,2009). For acid-sensing ion channels (ASICs), the study by Voilley et al. (2001) has indicated that ASIC currents mediated by homomeric ASIC3 channels or heteromeric ASIC3-containing channels are inhibited by both aspirin and salicylate. Overall, multiple action sites are responsible for the neuroprotective effects of aspirin and salicylate in the central nervous system.
ASICs, present throughout the nervous system and other tissues, are activated by a decrease in extracellular pH (Waldmann and Lazdunski,1998; Krishtal,2003). In the brain, tissue acidosis, which may activate ASICs, is a common feature of brain ischemia, epileptic seizure, tumor, and Parkinson's disease (Chesler and Kaila,1992; Reeh and Steen,1996; Helmlinger et al.,1997). Acidosis-induced neuronal injury occurs in these pathological processes. Cumulative evidence has demonstrated the roles of ASICs in these neurological diseases (Berdiev et al.,2003; Sluka et al.,2009), and ASIC-mediated acidotoxicity has been accepted as the unique pathway for neuronal injury (Xiong et al.,2004; Gao et al.,2005). Several extracellular molecules that modulate ASIC function have been identified, and targeting ASICs in the brain has proved its potential therapeutic value (Xu and Xiong,2007). For instance, inhibition of ASIC activities has been fully elucidated to be neuroprotective against neuronal injury in ischemic stroke models (Xiong et al.,2004; Gao et al.,2005; Pignataro et al.,2007; Li et al.,2010).
Aspirin and salicylate have been shown to inhibit ASIC current mediated by the ASIC3 channel in the peripheral sensory neuron (Voilley et al.,2001). However, the study by Dorofeeva et al. (2008) has shown that 0.5 mM aspirin and salicylate has no significant effect on ASIC current in the hippocampal neuron. We therefore employed whole-cell patch-clamp recordings to examine the effects of millimolar concentrations of salicylate and aspirin on ASICs in the cultured cortical neuron of the rat. Moreover, we carried out cell viability assessments to explore whether aspirin and salicylate prevent acid-induced neuronal injury via inhibition of ASIC activities.
MATERIALS AND METHODS
Animal care and handling were in accordance with the policies of the Ethics Committee of Anhui Provincial Hospital Affiliated to Anhui Medical University. Every effort was made to minimize the number of animals used and their suffering.
Primary Cultured Cerebral Cortical Neurons
The cerebral cortical neurons of the rat were isolated by a standard enzyme treatment protocol (Wang et al.,2007). Briefly, timed-pregnant (embryonic day 16) and postnatal day 1 Sprague-Dawley rats were sacrificed by cervical dislocation following anesthesia with halothane. The cortical tissues were removed rapidly and incubated with 0.125% trypsin-EDTA for 10 min at 37°C. Tissues were then triturated with fire-polished glass pipettes, and the dissociated cells were plated (1–2 × 105 cell/ml) on poly-D-lysine (Sigma, St. Louis, MO)-coated coverglasses in Dulbecco's modified eagle medium (DMEM; Gibco, Invitrogen, Grand Island, NY) with L-glutamine (Gibco) plus 10% fetal bovine serum (Gibco) and 10% F-12 nutrient mixture (Gibco). After 1 day, the medium was changed to Neurobasal Medium (1.5 ml; Gibco) supplemented with 2% B-27 (Gibco) and replaced every 3–4 days. Treatment with 5-fluoro-5′-deoxyuridine (20 μg/ml; Sigma) on the fourth day after plating was used to block cell division of non-neuronal cells, which helped to stabilize the cell population. The culture was maintained at 37°C in a 5% CO2 humidified atmosphere. Neurons were used for electrophysiological recordings 7–10 days after plating. We did not observe any difference in the biophysical and pharmacological properties of ASICs in the cultured cerebral cortical neurons from either 16-day-old embryonic or neonatal rats.
Electrophysiology
The whole-cell patch-clamp recordings were performed in voltage-clamp or current-clamp mode. Patch pipettes were pulled from glass capillaries with an outer diameter of 1.5 mm (Narishige, Tokyo, Japan) on a two-stage puller (PP-830; Narishige). The resistance between the recording electrode filled with pipette solution and the reference electrode was 4–6 MΩ. Membrane currents were measured using a patch-clamp amplifier (Axopatch 200B; Molecular Devices, Sunnyvale, CA), filtered at 1 kHz, sampled and analyzed using a DigiData 1320A interface (Molecular Devices) and a computer with the pCLAMP system (Molecular Devices). The series resistance, estimated from optical cancellation of the capacity transient, was 10–30 MΩ, and in most experiments 70–90% series resistance was compensated. Under voltage-clamp conditions, the neurons tested were voltage-clamped at –50 mV throughout the experiments, except when the current-voltage (I-V) relationships for ASIC currents were examined. All experiments were carried out at room temperature (23–25°C).
Solutions and Chemicals
The ionic composition of the standard extracellular solution contained (mM): 150 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, buffered to various pH values with either 10 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES; pH 6.0–7.4) or 10 mM 2-(N-morpholino)-ethanesulfonic acid (MES; pH < 6.0). The osmolarity of all extracellular solutions was adjusted to 325–330 mOsm with sucrose and Micro-Osmometer (model 3300; Advanced Instruments, Norwood, MA). The patch pipette solution for whole-cell patch-clamp recording contained (mM): 120 KCl, 30 NaCl, 0.5 CaCl2, 1 MgCl2, 5 EGTA, 2 MgATP, and 10 HEPES; pH was adjusted to 7.2 with Tris base. The osmolarity of the pipette solution was adjusted to 280–300 mOsm with sucrose and Micro-Osmometer (Advanced Instruments). The extracellular pH was adjusted to different values by addition of 1 N NaOH or 1 N HCl and was routinely checked before and during experiments. When the I-V relationships for ASIC currents were examined, 300 nM tetrodotoxin (TTX) and 100 μM CdCl2 were added to the extracellular solutions, and KCl was replaced with equimolar CsCl in the pipette solution. Cd2+ (100 μM) had no significant impact on ASIC currents. For measurement of acidosis-induced depolarization in current-clamp experiments, 5 μM nimodipine, 10 μM 5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine (MK-801), and 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were added to the bath solution to prevent potential activation of voltage-gated Ca2+ channels or glutamate receptors. The extracellular bath solution also contained 300 nM TTX and 100 μM CdCl2, and KCl in the pipette solution was replaced with equimolar CsCl. Changes in the content of extracellular solution and pipette solution were kept iso-osmotic.
The TTX was purchased from Hebei Fisheries Research Institute of China, the ASIC1a antagonist psalmotoxin-1 (PcTX-1; Escoubas et al.,2000) was purchased from the Peptide Institute of Japan, the ASIC3 antagonist APETx2 (Diochot et al.,2004) was purchased from Wuhan More Biotechnology, and the other chemicals were purchased from Sigma (St. Louis, MO). Sodium salicylate and amiloride were first dissolved to 100 mM in the standard extracellular solution and then diluted to the desired concentrations just before use. Aspirin (3 mM) was soluble in the standard extracellular solution at room temperature and was dissolved just before use. Both PcTX-1 and APETx2 were dissolved to 1 mM in distilled water and stored at –20°C. They were diluted to the desired concentrations at room temperature just before use. Drugs were applied using a rapid application technique termed the Y-tube method as previously described (Wang et al.,2006).
Data Analysis
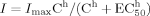 (1)
(1)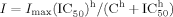 (2)
(2)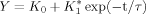 (3)
(3)All data are shown as mean ± SEM. Statistical comparison was carried out using Student's t-test for two groups and one-way analysis of variance (ANOVA) for multiple comparisons. Statistically significant differences were assessed as P < 0.05 or P < 0.01. P and n represent the values of significance and the number of neurons, respectively.
LDH Measurement
At 7–10 days in culture, the cultured cortical neurons were randomly divided into several groups for treatment. MK801 (10 μM), CNQX (20 μM), and nimodipine (5 μM) were included in extracellular solutions in all groups to eliminate potential activations of glutamate receptors and voltage-gated Ca2+ channels. Cells were rinsed three times with standard extracellular solution and subjected to either extracellular pH 7.4 or pH 6.0 solution for 1 hr. These extracellular bath solutions contained different treatment agents (3 mM salicylate, 3 mM aspirin, or 100 μM amiloride). After 1 hr of treatment, cells were rinsed with standard extracellular solution and incubated in Neurobasal Medium (Gibco) at 37°C for 24 hr. Then, lactate dehydrogenase (LDH) release was measured in culture medium using the CytoTox-ONE Homogeneous Membrane Integrity Assay Kit (Promega, Madison, WI). Medium (100 μl) was transferred from a culture well to a 96-well plate and mixed with 100 μl reaction solution provided with the kit. Optical density was measured at 560 nm 10 min later, utilizing a microplate reader (FLX800T; BioTek Instruments, Winooski, VT). Background absorbance was subtracted. The maximal releasable LDH was obtained in each well by 15 min of incubation with 1% Triton X-100 at the end of each experiment. The released LDH is expressed as percentage of total LDH.
Propidium Iodide Staining and Cell Injury Counts
Non-viable cells were assessed by propidium iodide (PI) staining. Cultures were rinsed three times with standard extracellular solution and incubated with PI (10 mg/ml; Sigma) for 20 min, then rinsed with standard extracellular solution. Images were obtained shortly after staining by light and fluorescent microscopy (BX61; Olympus, Tokyo, Japan) with a ×20 objective.
Percentage of non-viable cells was calculated as the number of cells labeled by PI enumerated under fluorescent microscopy divided by the total counted cells in the same field enumerated under light microscopy. The number of counted cells was determined by light or fluorescent microscopic examination at ×10 magnification. Five randomly selected fields were counted and averaged per culture.
RESULTS
Acid-Induced Transient Currents in the Cultured Cerebral Cortical Neuron of the Rat Were Mediated by ASICs
The cultured cerebral cortical neuron of the rat had an average resting membrane potential of –53.6 ± 1.7 mV (n = 12). We employed extracellular acidic solutions to 106 cultured cerebral cortical neurons at a holding potential (VH) of –50 mV and recorded transient, rapidly activating and desensitizing inward currents in 95.3% of the neurons (101/106) tested (Supp. Info. Fig. 1A). The threshold for activation of the acid-induced transient currents occurred at about pH 6.8, and the EC50 value and Hill coefficient for the currents were 6.2 ± 0.5 and 1.0 ± 0.3, respectively (n = 4–6). We employed extracellular pH 6.0 solution in subsequent experiments, because this concentration is around the EC50. In all pH 6.0-induced transient inward currents, 92.1% of the transient currents almost completely desensitized (typeI current), 5.9% of the transient currents were followed by slowly desensitizing currents (type II current), and the remaining 2% of pH 6.0-induced currents were transient activating currents followed by sustained currents (type III current; Supp. Info. Fig. 1B). The ratio of the sustained current to the transient current was 47.2 ± 0.7% (type III current, n = 2). We focused on typeI current in subsequent experiments, because this type of current was most abundant in the neurons that we examined in this study. When the VH of the tested neuron was changed from –60 mV to +20 mV, a liner I-V curve was obtained (Fig. 2C). The reversal potential of the transient currents was 44.3 ± 4.1 mV (n = 6), close to the theoretical Na+ equilibrium potential (41.2 mV), suggesting that these channels are mainly permeable to Na+. The τ value for type I current at pH 6.0 was 746.6 ± 0.3 msec (n = 6). Additionally, amiloride, the known ASIC antagonist, reversibly reduced the amplitude of the transient current induced extracellular pH 6.0 solution in a concentration-dependent manner, with an IC50 value of 18.4 ± 1.4 μM and a Hill coefficient of 1.1 ± 0.1 (n = 5; Supp. Info. Fig. 1C). In all the three types of pH 6.0-induced currents, the transient activating components were sensitive to 100 μM amiloride, whereas the slower activating components were insensitive to amoliride (data not shown). Taken together, these data demonstrate that extracellular acidic solutions activate ASICs in the native cerebral cortical neuron of the rat.
We also examined the effects of PcTX-1, the specific antagonist for homomeric ASIC1a channel, and APETx2, the specific antagonist for ASIC3-containing channel, on ASICs in the cortical neuron. PcTX-1 (20 nM) exerted an inhibitory effect in 85.7% of the neurons tested and reduced the amplitude of the transient current induced by pH 6.0 to 43.3% ± 4.5% of the control (n = 6; Supp. Info. Fig. 1Db), and the APETx2 (3 μM) exerted an inhibitory effect in 71.4% of the neurons tested and reduced the amplitude of the transient current to 52.3% ± 3.2% of the control (n = 5; Supp. Info. Fig. 1Da). The sustained component of the ASIC current was not affected by these antagonists (data not shown).
The cerebral cortical neuron of the rat has been shown to express another type of acid-sensitive current, the current mediated by transient receptor potential vanilloid receptor subtype1 (TRPV1; Kauer and Gibson,2009). For eight neurons tested, we did not detect any current induced by 10 μM capsaicin, the selective agonist for TRPV1. Nevertheless, in these same neurons, ASIC currents induced by extracellular pH 6.0 solutions were recorded (data not shown).
Inhibitory Effects of Salicylate and Aspirin on the ASIC Current in the Cultured Cortical Neuron of the Rat
Sodium salicylate did not induce any detectable current when it was applied alone at various concentrations. Pretreatment of the cortical neuron with salicylate for 15 sec, followed by simultaneous application of extracellular pH 6.0 solution and the same concentration of salicylate, rapidly and reversibly inhibited the amplitude of ASIC currents induced by extracellular pH 6.0 solution. As shown in Figure 1A, 300 μM salicylate exerted a slight, but not statistically significant, inhibition of ASIC currents (n = 5; P > 0.05). When the concentration of salicylate reached 1 mM, the drug exerted astatistically significant inhibition of ASIC currents (n= 6; P < 0.01). Within the range of 300 μM to 30 mM, salicylate inhibited ASIC currents in a dose-dependent manner, with an apparent IC50 value of 4.6 ± 0.4 mM and a Hill coefficient of 0.4 ± 0.2 (n = 5–9; Fig. 1B). The inhibitory effect of salicylate on the ASIC current was fully reversed within 3 min. Prolonging the pretreatment duration from 15 to 180 sec did not significantly affect the salicylate inhibition of the ASIC currents (n = 5; P > 0.05, ANOVA), confirming a rapid action of salicylate on ASICs. We employed 3mM salicylate in most of subsequent experiments, becasuse this concentration is around the apparent IC50. Salicylate did not significantly affect the desensitization rate of ASIC currents induced by pH 6.0 (without salicylate τ = 746.6 ± 0.3 msec, and with salicylate τ =836.5 ± 0.4 msec; n = 6; P > 0.05).
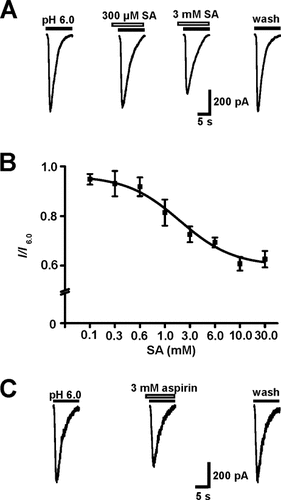
Effects of salicylate and aspirin on ASIC currents in the cultured cortical neuron of the rat. A: Representative traces showing that salicylate (SA) rapidly and reversibly inhibited the amplitude of ASIC currents. The neurons were preperfused with salicylate for 15 sec before simultaneous application with extracellular pH 6.0 solution. Note that 300 μM salicylate exerted a slight, not statistically significant inhibition of ASIC currents (n = 5; P > 0.05). B: The dose-response curve for inhibition of the amplitude of ASIC currents by salicylate. All responses were normalized to I6.0 in the absence of salicylate. Each point represents the average response of five to nine neurons. In this and subsequent figures, the horizontal bars indicate the drug application duration, and the vertical bars show the mean ± SEM; the VH was –50 mV, unless otherwise stated. C: Representative traces showing that 3 mM aspirin rapidly and reversibly inhibited the amplitude of ASIC currents.
We also tested the effect of aspirin on ASIC currents in cultured cortical neurons of the rat. Aspirin at a concentration of 3 mM rapidly and reversibly reduced the ASIC peak currents to 83.7% ± 5.6% of the control (n = 5; Fig. 1C). The inhibitory effect of aspirin on the ASIC current fully reversed within 3 min.
Voltage-Independent Modulation of ASIC Current by Salicylate
To explore whether the effect of salicylate on ASICs is influenced by alternation in membrane potentials, we changed the VH from –60 mV to +20 mV, then recorded ASIC currents in the absence or presence 3 mM salicylate (Fig. 2A). We found that the inhibitory effect of 3 mM salicylate on ASICs was not significantly affected by change in membrane potential (n = 6; P > 0.05, ANOVA; Fig. 2B). The liner I-V relationship curve also suggests a voltage-independent effect of salicylate on ASICs (Fig. 2C). In the presence of 3 mM salicylate, the reversal potential was 42.5 ± 3.7 mV, which is close to the theoretical Na+ equilibrium potential (41.2 mV) and the reversal potential for ASICs in the absence of salicylate (n = 6).
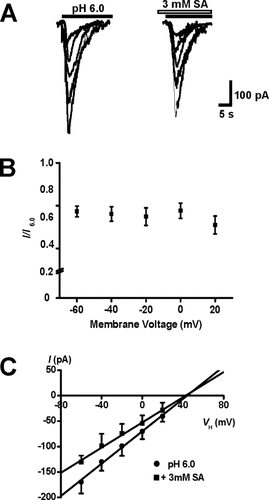
Effects of salicylate on ASIC currents were independent of changes in membrane voltage. A: Representative traces of ASIC currents elicited by extracellular pH 6.0 solution in the absence or presence of 3 mM salicylate in a single cortical neuron voltage-clamped at various VH values (from –60 to +20 mV). B: Scatter graph showing the relative inhibition of the peak currents recorded at various VH values in the presence of 3 mM salicylate. Salicylate inhibition of ASIC currents did not depend on VH change (n = 5; P > 0.05, ANOVA). C: The I-V relationship curve for the ASIC current elicited by extracellular pH 6.0 solution in the absence or presence of 3 mM salicylate. The reversal potentials were both close to the theoretical Na+ equilibrium potential. Each point was the average response of six neurons.
Comparison of Salicylate Effects on ASICs in Different Drug Application Modes
To further examine the mechanism of salicylate's action on ASICs, we employed three different modes of salicylate application. In the coapplication protocol (protocol a), extracellular salicylate and pH 6.0 solution were coapplied to the neurons; in the sequential application protocol (protocol b), ASICs were activated by pH 6.0 solution alone, following 15 sec of preperfusion of neurons with salicylate; in the pretreatment protocol (protocol c), ASICs were pretreated with salicylate for 15 sec and then activated by pH 6.0 solution plus the same concentration of salicylate. As shown in Figure 3A, all three sequences of salicylate (3 mM) application produced inhibitory effects on the amplitude of ASIC currents. Salicylate decreased the amplitude of ASIC currents by 15.2% ± 2.7% (protocol a), 14.8% ± 3.7% (protocol b), and 27.2% ± 2.9% (protocol c) of the corresponding controls, respectively (n = 5; Fig. 3B). Most notably, the maximal inhibition was present during protocol c (P < 0.01, ANOVA).
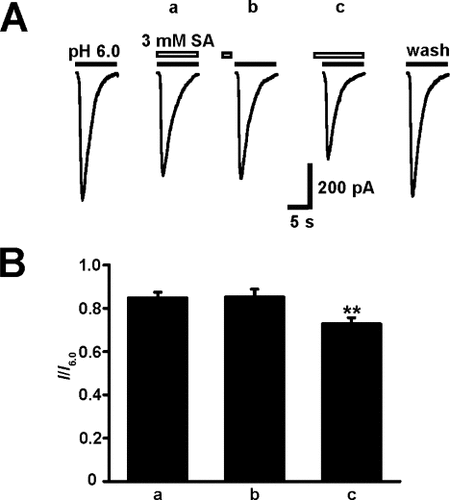
Distinctive effects on ASIC currents in three modes of salicylate application. A: Representative traces illustrating that 3 mM salicylate modulated ASIC currents in three modes of drug application (a–c). Coapplication of extracellular salicylate and pH 6.0 solution (a), preperfusion of neuron with salicylate for 15 sec followed by pH 6.0 solution alone (b), preperfusion of neuron with salicylate for 15 sec followed by pH 6.0 solution plus the same concentration of salicylate (c). B: Statistical results showing the inhibition of the amplitude of ASIC currents by 3 mM salicylate in the three application modes indicated in A. All responses were normalized to I6.0. Each graph was the average response of five neurons. ∗︁∗︁P < 0.01, ANOVA.
Salicylate Reduced the Acidosis-Induced Membrane Depolarization
The ASICs in the cortical neuron mediate acidosis-induced membrane depolarization (Chu et al.,2004), and salicylate affects neuronal excitation (Gong et al.,2008). Therefore, we tested whether salicylate affects acidosis-induced membrane depolarization in the cortical neuron. As shown in Figure 4A, an acidic solution at pH 6.5, a moderate drop in extracellular pH, elicited a small ASIC current in voltage-clamp mode and correspondingly, in the same neuron, induced a increased membrane depolarization (change in membrane potential of 25.6 ± 1.8 mV, n = 10), which was reversibly blocked by 100 μM amiloride (change in membrane potential of 15.2 ± 1.3 mV) in current-clamp mode with no current injection. This acidosis-induced change in membrane depolarization was reversibly reduced by 3 mM salicylate to 21.9 ± 1.8 mV. The effect of salicylate (3 mM) on the membrane depolarization induced by pH 6.5 solution was statistically inhibitory (P < 0.01, paired t-test) and fully reversed within 3 min.
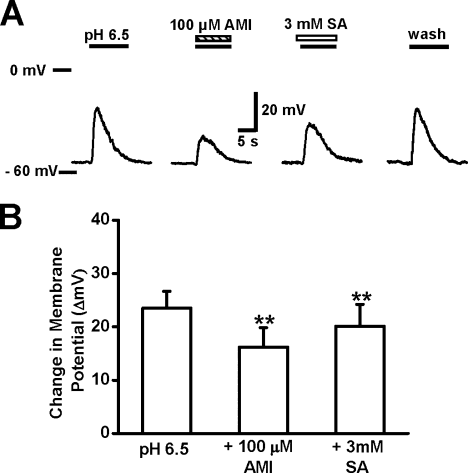
Salicylate reduced acidosis-induced membrane depolarization in the cultured cortical neuron. A: Representative traces showing the membrane depolarization induced by extracellular acidic solution at pH 6.5. This depolarization was reversibly inhibited by 100 μM amiloride (AMI) and by 3 mM salicylate. Ticks on the left side of the representative traces indicate the 0 mV level (upper tick) and the −60 mV level (lower tick). B: Statistical data showing that acidosis-induced changes in membrane potential (ΔVm) were reduced by 100 μM amiloride or 3 mM salicylate. ∗︁∗︁P < 0.01, paired t-test. The membrane potential was recorded in current-clamp mode. Each column was the average of five neurons.
Attenuation of Acidosis-Induced Neuronal Injury by Salicylate and Aspirin
The measurement of LDH release in cultured cortical neuron has been demonstrated to assess acidosis-induced neuronal injury (Xiong et al.,2004). Compared with the neurons treated with extracellular pH 7.4 solution, 1 hr of acid incubation of extracellular pH 6.0 solution induced a significant increase in LDH release. Twenty-four hours after the treatment, 26.5% ± 3.4% of maximal LDH release was detected in the acid-treatment group (n = 12 wells). Addition of 100 μM amiloride during the 1-hr acid incubation decreased LDH release to 18.8% ± 2.2% (n = 12 wells), whereas addition of amiloride in extracellular pH 7.4 solution did not induce significant increase in LDH release (n = 9 wells; P > 0.05; data not shown), suggesting that ASICs are responsible for the acidosis-induced neuronal injury. Extracellular pH 7.4 or pH 6.0 solution plus 3 mM salicylate was used to incubate the cultured cortical neurons for 1 hr. LDH was measured 24 hr after the incubation. As shown in Figure 5A, treatment of the cells with extracellular pH 6.0 solution containing 3 mM salicylate induced a statistically significant reduction in LDH release compared with the cells treated with extracellular pH 6.0 solution alone (n = 12 wells; P < 0.05). Similarly, aspirin (3 mM) significantly reduced LDH release induced by incubation with extracellular pH 6.0 solution (n = 12 wells; P < 0.05). Neither salicylate nor aspirin in extracellular pH 7.4 solution induced significant increase in LDH release (n = 12 wells; P > 0.05; data not shown). Consistently with the LDH assay, 1 hr acid treatment combined with salicylate or aspirin produced a reduction in the number of PI-positive cells compared with the treatment with acidic solution alone (n= 6 wells; P < 0.01, ANOVA; Fig. 5B,C).
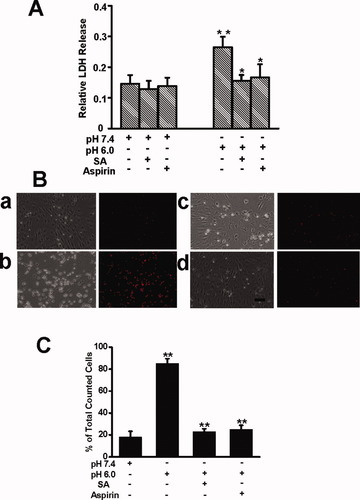
Salicylate and aspirin attenuated acidosis-induced neuronal injury. A: Summary column graph showing that the released LDH in culture medium significantly increased 24 hr after 1 hr incubation of extracellular pH 6.0 standard solution. Salicylate and aspirin attenuated acidosis-induced LDH release, respectively. Each column was the average of twelve wells. ∗︁∗︁P < 0.01 between control (pH 7.4) and pH 6.0 groups, and ∗︁P < 0.05 between pH 6.0 and pH 6.0 + salicylate (3 mM) groups or between pH 6.0 and pH 6.0 + aspirin (3 mM) groups. B: PI staining of nuclei of dead neurons. The representative light images (left) and fluorescent images (right) were obtained 24 hr after 1 hr incubation of pH 7.4 (a), pH 6.0 (b), pH 6.0 + salicylate (c), pH 6.0 + aspirin (d) respectively. Scale bar = 20 μm. C: Summary column graph showing the percentage of PI-positive cells among total counted cells in extracellular pH 7.4 solutions, extracellular pH 6.0 solutions, extracellular pH 6.0 solutions containing 3 mM salicylate, and extracellular pH 6.0 solutions containing 3 mM aspirin respectively. Each column was the average of six wells. ∗︁∗︁P < 0.01 between control (pH 7.4) and pH 6.0 groups, between pH 6.0 and pH 6.0 + salicylate (3 mM) groups, and between pH 6.0 and pH 6.0 + aspirin (3 mM) groups, respectively (ANOVA). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary. com.]
DISCUSSION
Inhibitory Effects of Millimolar Salicylate and Aspirin on ASICs in the Brain
In the cultured cerebral cortical neuron of the rat, we recorded acid-induced transient inward currents with an activation threshold of around pH 6.8. The linear I-V relationship curve suggests reversal potential was close to the Na+ equilibrium potential. Moreover, these transient currents were reversibly blocked by amiloride, the known ASIC antagonist. These typical electrophysiological and pharmacological properties are consistent with those described in previous studies on ASICs in the cerebral cortical neuron (Varming,1999; Chu et al.,2004; Xiong et al.,2004), we therefore attribute these currents to ASICs. We also found that these ASICs were activated with an EC50 of 6.2 ± 0.5 and a Hill coefficient of 1.0 ± 0.3, and the majority of the ASICs desensitized rapidly with the τ value of 746.6 ± 0.3 msec at pH 6.0. These properties closely match those of heteromeric ASICs that contain ASIC3 subtype in the sensory neuron or in the heterologous expression system (Waldmann et al.,1997; Sutherland et al.,2001; Xie et al.,2002; Hesselager et al.,2004; Tan et al.,2007; Hattori et al.,2009). Furthermore, the inhibition of ASIC current by APETx2, which specifically inhibits the ASIC3-containing channel (Diochot et al.,2004), confirms the functional expression of ASIC3 subtype in the cortical neuron. Additionally, PcTX-1 at a concentration of 20 nM or APETx2 at a concentration of 3 μM that almost completely inhibits the currents mediated by homomeric ASIC1a or ASIC3 (Escoubas et al.,2000; Diochot et al.,2004), respectively, could produce only partial inhibition on ASIC currents, suggesting that ASIC currents in the cortical neuron are mediated by mixtures of different ASIC subtypes.
In this study, we found rapid and reversible inhibition on ASIC current by salicylate and aspirin using whole-cell patch-clamp recordings. Moreover, salicylate reduced acidosis-induced, ASIC-mediated membrane depolarization. These data suggest that ASICs in the cortex are molecular targets of high-dose salicylate and aspirin.
The study by Voilley et al. (2001) has shown that 0.5 mM aspirin and salicylate exerts inhibitory effects on ASICs in the peripheral neuron of the rat. However, the study by Dorofeeva et al. (2008) showed that 0.5 mM aspirin and salicylate has no significant effect on the ASIC current in cultured hippocampal neurons. In this study, we found that high doses of salicylate and aspirin exerted partial inhibition on ASICs in the cultured cerebral cortical neuron. We address the difference as follows: 1) the concentration of salicylate and aspirin used in the study by Dorofeeva et al. was 0.5 mM (Dorofeeva et al.,2008), whereas in this study the concentration that produced significant inhibition was above 1 mM; 2) the regions we examined in this study are the cortex, for which no reports have described the effects of salicylate and aspirin on ASICs to date. In addition to ASIC1a, ASIC2a, and ASIC2b, our recent study has indicated that ASIC3 is present in the cortex of the rat (Biagini et al.,2001; Alvarez de la Rosa et al.,2003; Meng et al.,2009). Moreover, as discussed above, ASIC currents in this region are mediated by multiple ASIC subtypes, including ASIC3. It is conceivable that the specific expression pattern of ASIC subtypes in the cortex may account for the effects exerted by salicylate and aspirin on ASICs compared with those in dorsal root ganglion or hippocampus. Overall, the inhibitory effects of salicylate and aspirin on ASICs in the cortex of the rat may be attributed to the application of high concentrations of these drugs to a region that expresses specific ASIC subtypes. Bec ause functional ASICs in the native cerebral cortical neuron are mixtures of different ASIC subtypes, it is reasonable that, even at high concentrations, salicylate and aspirin could not completely inhibit the ASIC currents in this region.
We observed partial inhibition of acidosis-induced membrane deplorization by amiloride at 100 μM, a concentration that almost completely inhibits ASIC current. This phenomenon may result from incomplete blockade of some acid-sensitive ion channels, such as TRPV1 or background K+ channels TASK-1 or TASK-3, which have been demonstrated to affect acidosis-induced membrane depolarization (Kauer and Gibson,2009; Enyedi and Czirjak,2010; Nilius and Owsianik,2011). Although we employed chemical agents to block most receptors and ion channels that affect the membrane excitability, we could not completely rule out the contributions of activation or inhibition of these acid-sensitive ion channels to the membrane depolarization.
Possible Mechanisms Underlying the Inhibition on ASICs by Salicylate
In this study, salicylate produced an immediate and reversible inhibition on ASICs (Fig. 1A), and prolonging the pretreatment duration from 15 to 180 sec did not significantly affect the salicylate inhibitory effect, suggesting that salicylate directly modulates ASIC activity via site(s) on the exterior portion of this channel. The liner I-V relationship indicates that the effect of salicylate on ASICs is not modified by change in membrane potentials (Fig. 2C), suggesting that salicylate's action sites are not within the channel pore, where it would experience the membrane electric field. Additionally, salicylate inhibited ASIC currents during both the coapplication protocol and the sequential application protocol (Fig. 3), suggesting that this drug modulates ASICs in both resting and open states. Multiple action sites could explain this phenomenon. We speculate that salicylate affects ASIC activity via multiple action sites in the exterior portion of the channel.
Functional Implications
High-dose aspirin or its main metabolite salicylate is used to treat patients with inflammatory diseases. In these patients, the serum concentration of salicylate can reach up to several millimoles (Insel,1996), and the concentration of this drug in the cerebrospinal fluid of the rat can reach one-third of that in the serum (Jastreboff et al.,1986). Therefore, the concentration of salicylate and aspirin used in this study may be achieved in clinical medication.
Aspirin and salicylate are widely used to relieve pain, treat chronic inflammatory diseases, and prevent the recurrent ischemic stroke. Recent studies have demonstrated the involvement of these drugs in the treatment of neurodegenerative disease (Esposito et al.,2007). Correspondingly, cumulative evidence has suggested the involvement of ASICs in the diseases mentioned above (Sluka et al.,2009). This study showed that ASICs in the cortex are targets of millimolar concentrations of salicylate and aspirin. Given that modulation of neuronal receptors/ion channels is one of the multiple pathways responsible for the effects of salicylate and aspirin, our findings may contribute to a better understanding of the complicated therapeutic mechanisms of these drugs in a broad spectrum of diseases.
Low-dose aspirin is routinely used in the prevention of ischemic stroke (van der Worp and van Gijn,2007). Nevertheless, increasing evidence obtained from studies in cellular and animal ischemic models has suggested a neuroprotective role of high-dose salicylate or aspirin in brain ischemia. This study showed that millimolar concentration of salicylate reduced acidosis-induced membrane excitability and attenuated the acidosis-induced neuronal injury in the cortical neuron of the rat. We attributed the acidosis-induced neuronal injury to activation of ASIC, because we used blocker cocktail to prevent the potential activations of voltage-gated Ca2+ channels and glutamate receptors (Xiong et al.,2004; Sherwood and Askwith,2009) and used a moderate solution at pH 6.0 to activate ASICs during the assay for acidosis-induced neuronal injury. Blocking ASICs in brain ischemia plays a protective role in acidosis-induced, ASIC-mediated neuronal injury (Xiong et al.,2004; Gao et al.,2005; Li et al.,2010), so we speculate that high-dose salicylate and aspirin could be used as neuroprotective agents in the treatment of ischemic stroke.
In conclusion, this study shows that ASICs in rat cortical neurons are molecular targets of millimolar concentrations of salicylate and aspirin. These drugs inhibit ASIC currents and reduce acidosis-induced membrane depolarization. Moreover, these drugs attenuate acidosis-induced, ASIC-mediated neuronal injury. These findings may help in understanding the therapeutic mechanisms of these drugs in pain, Parkinson's disease, and ischemic stroke. Moreover, this study provides new evidence on salicylate and aspirin as neuroprotective agents in the treatment of ischemic stroke.
Acknowledgements
We are grateful to Drs. Lin Chen and Jiang-Ning Zhou for technical assistance.




