The p75 neurotrophin receptor has nonapoptotic antineurotrophic actions in the basal forebrain
Abstract
Because of controversy about the role of the p75 neurotrophin receptor (p75NTR) in the cholinergic basal forebrain (CBF), we investigated this region in p75NTR third exon knockout mice that were congenic with 129/Sv controls. They express a shortened intracellular form of p75NTR, permitting detection of p75NTR-expressing cells. We performed separate counts of choline acetyltransferase (ChAT)-expressing and p75NTR-expressing neurons. In agreement with past reports, the number of ChAT-immunoreactive neurons in knockout mice was greater than in wild-type mice, and this was evident in each of the main anatomical divisions of the CBF. In contrast, the number of p75NTR-immunoreactive neurons did not differ between genotypes. The biggest increase in ChAT neurons (27%) was in the horizontal limb of the diagonal band of Broca (HDB), in which region the number of p75NTR-positive neurons was unchanged. Double staining revealed that some neurons in wild-type mice expressed p75NTR but not ChAT. In the knockout mice, all p75NTR-expressing neurons expressed ChAT. The increase in cholinergic neurons, therefore, was at least partially attributable to a higher proportion of ChAT immunoreactivity within the population of p75NTR-expressing neurons. Cholinergic neurons were also larger in knockout mice than in controls. In the hippocampal CA1 region, knockout mice had a greater number of cholinergic fibers. There was a 77% increase in hippocampal ChAT activity in knockout mice and a 38% increase in heterozygotes. The data do not support an apoptotic role but indicate a broad antineurotrophic role of p75NTR in the cholinergic basal forebrain. © 2011 Wiley Periodicals, Inc.
The expression of the p75 neurotrophin receptor (p75NTR) in the healthy, mature brain is most prominent in the cholinergic neurons of the basal forebrain complex. Outside of this region, expression occurs at a low level and is present in a small number of scattered neurons at several sites, including the hippocampus (Barrett et al.,2005). Despite its localization at the hub of the cholinergic neuromodulatory system, we know surprisingly little about the role of p75NTR in the brain. In the peripheral nervous system, p75NTR has prosurvival actions during embryogenesis (Davies et al.,1993), whereas proapoptotic actions have been shown in postnatal sensory neurons (Barrett and Bartlett,1994; Cheema et al.,1996) and sympathetic neurons (Bamji et al.,1998). The factors that enable p75NTR to switch between two opposite roles, prosurvival and apoptotic signalling, have not been identified with certainty.
The question of whether p75NTR plays a role in regulating survival and apoptosis in cholinergic forebrain neurons is unresolved. In mice with a truncating insertion in the third exon of the p75NTR gene (hereinafter referred to as p75NTR knockout mice), the number of choline acetyltransferase (ChAT)-positive neurons in the basal forebrain has been reported to undergo various degrees of increase, in most cases statistically significant (Abdou et al.,1997; Yeo et al.,1997a; Greferath et al.,2000; Krol et al.,2000; Naumann et al.,2002). More recently, the analysis of a different p75NTR knockout mouse, in which a truncating insertion was made in exon 4, showed an increase in the number of septal cholinergic neurons (Naumann et al.,2002). These findings suggest that p75NTR does, in fact, cause apoptosis in these neurons. We will return to this issue in the Discussion.
Two of the p75NTR knockout studies additionally reported features of cholinergic neuronal hypertrophy (Yeo et al.,1997a; Greferath et al.,2000). There was also increased ChAT activity in cortical target fields (Yeo et al.,1997a). These findings suggest that p75NTR inhibits cholinergic neuronal growth and neurotransmitter production. They imply that p75NTR has antineurotrophic actions in the basal forebrain that are more extensive than simply induction of apoptosis. The proapoptotic role of p75NTR has been intensively studied in recent years, but little is known about other, negative effects of p75NTR on cell size and neurotransmitter production.
To shed further light on p75NTR function, the present study analyzed the p75NTR knockout phenotype on a truly congenic background. Repeated back-crossing produced a mouse substrain that differed from 129/Sv controls only at the p75NTR locus; all other loci were identical, or as nearly identical as is possible among individual mice of a tightly maintained inbred strain. The study was expanded to include heterozygous (p75NTR+/–) mice, which were also congenic with the 129/Sv strain. We performed separate counts of ChAT-positive and p75NTR-positive neurons in the basal forebrain. We were able to do the latter, even in knockout mice, because the third exon insertion allows low-level expression of a truncated receptor lacking the extracellular domain. We measured the size of cholinergic neurons in the basal forebrain and measured ChAT activity in the hippocampus. We also investigated cholinergic innervation of the hippocampus, by counting acetylcholinesterase (AChE) staining neurites in various layers of the hippocampus. Our findings were consistent with p75NTR exerting a number of antineurotrophic actions in the basal forebrain but neither supported or excluded a role in the promotion of apoptosis.
MATERIALS AND METHODS
Immunohistology
Four-month-old male mice were used for morphometric studies of the basal forebrain and hippocampus. Immediately after euthanasia, transcardial perfusion with 10 ml phosphate-buffered saline (PBS) was performed, followed by perfusion with 10 ml Zamboni's fixative (2% paraformaldehyde, 15% picric acid in 0.1 M phosphate buffer, pH 7.3). Brains were then removed and postfixed overnight in the same fixative, followed by 24 hr of incubation in 20% sucrose in PBS, then 30% sucrose in PBS. Brains were then stored frozen in cryoprotectant until we were ready to section them. Frozen sections were obtained using a freezing microtome. The entire basal forebrain was sectioned into 50-μm coronal sections. Starting with the most rostral section, successive sections were used for p75NTR immunofluorescence, ChAT immunofluorescence, and AChE histochemistry, and so on in a recurring pattern. In addition, six extra mice (two of each genotype) were used specifically to obtain sections for double staining with p75NTR and ChAT.
Free-floating sections were washed in PBS, then incubated in p75NTR antiserum (Promega, Madison, WI) or ChAT antiserum (Chemicon, Temecula, CA). The specificity of the p75NTR antiserum has been demonstrated in several studies (Khursigara et al.,1999, 2001). The immunohistological specificity of the ChAT antibody has been demonstrated (Eckenstein and Sofroniew,1983) and verified in a large number of subsequent studies. These antisera were from rabbit and were used at 1:400, and 1:500, respectively, diluted in 3% normal goat serum (NGS) in PBS. For double staining, sections were incubated simultaneously in a mouse monoclonal anti-ChAT antibody (Chemicon) and the polyclonal anti-p75NTR antibody. Incubations in primary antisera were carried out for 24–48 hr at 4°C. Sections were washed in PBS and then incubated with the appropriate secondary antibodies at room temperature for 60 min. The secondary antibodies incorporated one of the fluorescent labels Alexa 488 (green) or Alexa 594 (red), and were from Invitrogen (Carlsbad, CA). They were used at a dilution of 1:400 in PBS. After final rinsing in PBS, sections were mounted onto gelatinized slides, air dried, and coverslipped using fluorescent mounting media (Dako, Carpinteria, CA).
Acetylcholinesterase Staining
Acetylcholinesterase staining was performed as described in the literature (Beck et al.,2002). Free-floating sections (in a 24-well plate) were washed in 0.1 M acetate buffer (pH 6), then incubated on a shaker in 250 μl preincubation solution containing 5 mM sodium citrate, 3 mM cupric sulfate, and 0.5 mM potassium ferricyanide. After 30 min, another aliquot (250 μl) of preincubation solution supplemented with 4.84 mM acetylthiocholine (Sigma, St. Louis, MO) and 0.4 mM ethopropazine (Sigma) was added to each well. The 24-well plate was packed on top of crushed ice and microwaved for 90 sec. The solution was then removed, and the sections were washed in 0.05 M Tris buffer (pH 7.6), followed by 0.1 M acetate buffer. The reaction product was visualized by the addition of diaminobenzidine (DAB), hydrogen peroxide, and nickel sulfate (DAB kit; Vector Laboratories, Burlingame, CA). The sections were again washed in 0.1 M acetate buffer, then mounted onto gelatinized slides. They were air dried overnight, washed and dehydrated in graded ethanol series, cleared in histolene, and coverslipped with DePex mounting media (Gurr BDH Chemicals, Poole, United Kingdom) for imaging.
Neuronal Counts
Separate neuronal counts were made on the basis of the ChAT and p75NTR-stained sections. In each case, every third section throughout the entire basal forebrain was available and was counted in a blinded fashion with regard to genotype. Thus, counts were performed only when an uninterrupted series of section was obtained. The number of brains examined in this way was six for each of the wild-type and knockout mice and seven in the case of heterozygous mice. For consistency, all sections were counted by one observer (J.T.). Each section was checked by a separate observer (U.G.), and, in cases of disagreement, the sections were studied carefully by the second observer and an additional observer (G.L.B.) to ensure an accurate count. The medial septum (MS) and vertical limb of the diagonal band (VDB) were counted together, because there is no unambiguous border between the two regions. The combined MS/VDB region consisted of all immunoreactive neurons rostral or dorsal to the crossing of the anterior commissures, with the exception of ventrally located neurons situated rostral to the crossing of the anterior commissures. The latter group was included in counts of the horizontal limb of the diagonal band (HDB). This part of the HDB is contiguous with the VDB in coronal sections, and we followed the coronal section maps in the fifth edition of the Paxinos and Watson (2005) stereotaxic guide to identify the border between them.
Since the HDB and NBM can be distinguished with only a very small number of neurons falling into the ambiguous junctional region, we have counted them separately in this study. The majority of immunoreactive HDB neurons were caudal or ventral to the crossing of the anterior commissures, with a minority falling rostral to this crossing, as described in the previous paragraph. Particular care was taken in counting ChAT-immunoreactive neurons, to exclude striatal cholinergic neurons, which, though mostly smaller and clearly separated anatomically from the NBM neurons, were sometimes in close proximity to them. To facilitate counting and to allow checking by second and third observers, all immunofluorescent sections were first captured digitally with a Nikon C1 confocal microscope (Coherent Scientific, Adelaide, Australia) at ×100 magnification. After counting immunoreactive neurons in all sections, the numbers of ChAT-and p75NTR-positive neurons were calculated using Abercrombie's correction for cell size. The use of a ×10 objective gave sufficient depth of field to enable us to identify all immunoreactive cells within each section. The strength of p75NTR staining in knockout mice was easily sufficient to obtain consistent cell counts.
Neuronal Size
Neuronal size was assessed by measuring the cross-sectional area of ChAT-immunoreactive neurons in the same sections that were used for neuronal counts. Under ×40 magnification with an oil-immersion objective and Nikon C1 microscope, ChAT-positive cells were digitally photographed and the images imported into Adobe Photoshop (Adobe, San Jose, CA). The final magnification when visualized on the computer screen was ×800. Individual ChAT-positive cells were accurately outlined by using the Photoshop Lasso tool. The histogram tool was then used to measure the number of pixels contained within the outlined cell. The actual cross-sectional area of each cell was then calculated by comparing its pixel value with that of an imaginary box of 250 μm2. A random subset of neurons (25 neurons in the VDB and 50 neurons in each of the MS, HDB, and NBM) was measured in each animal. Average values for each of the four areas were thereby obtained for each mouse. These values were obtained from all mice and used to calculate the means and standard errors of the mean for each of the three genotype populations.
Quantification of the Cholinergic Innervation of the CA1 Region of Hippocampus
AChE histochemistry was used as a marker of cholinergic innervation of the hippocampus. AChE-positive fibers were assessed in the stratum oriens, pyramidal cell layer, and stratum radiatum of the dorsal CA1 region (Bregma–2 to–2.18). The CA1 region was viewed with a Zeiss Axioplan microscope (Zeiss, Oberkochen, Germany), and stacks of images (z-layers) were taken through the entire 50-μm section at a step size of 1 μm with an Axiocam digital camera (Zeiss) using the Plan-Apochromat ×40 oil-immersion objective. For each animal, two serial 50-μm sections were photographed and two different z-layers (i.e., one in the vicinity of each of the top and bottom edges, where the staining was strongest and therefore clearest) were evaluated for each section. The micrographs were analyzed on the computer screen at a magnification of ×1,750. A sampling line of length 100 μm was drawn and randomly placed over each layer at a random angle to the pyramidal stripe of between 0° and 15°. The numbers of AChE positive fibers crossing the line was counted in each layer. The resulting values of the two z-layers were averaged, and two sections were analyzed from each mouse. AChE histochemistry was performed on six male mice of each of the wild-type and knockout strains, and five in the case of the heterozygous strain.
ChAT Assay
The hippocampus was dissected from unperfused brain, weighed, and homogenized in 5 volumes of homogenization buffer, which contained the protease inhibitors phenylmethylsulfonyl fluoride (PMSF), pepstatin, aprotinin, and leupeptin (all from Sigma). Twenty microliters of homogenate was added to the same volume of incubation buffer containing choline, physostigmine (Sigma), and 14C-acetylcholine (Amersham, Buckinghamshire, United Kingdom). This mixture was incubated for 60 min at 37°C. After addition of acetonitrile and kalignost (Ajax Chemicals) and transfer to toluene and water, acetylcholine partitioned into the organic phase, whereas unreacted acetyl-CoA remained in the aqueous layer. Beta counts were performed on the organic layer and used to calculate hippocampal ChAT activity in terms of picomoles of acetylcholine formed per milligram tissue. As in all other aspects of this study, only male mice were used for ChAT assays. Six mice were used for each of the wild-type and knockout strains and 10 for the heterozygous strain.
Statistical Analysis
Analysis of neuronal counts employed a mixed-type two-way ANOVA in which the between-group variable was genotype and the within-group variable was cell type (i.e., ChAT-positive or p75-positive). The genotype variable contained three elements (p75NTR+/+, p75NTR+/−, and p75NTR−/−). Two-way ANOVAs were performed on each of the MS/VDB, HDB, and NBM cell populations.
Analyses of cell size, number of cholinergic fibers, and ChAT assays were all based on one-way ANOVA. They were conducted by using the general linear model component in Minitab software. Comparisons between pairs of genotypes were made by Tukey's post hoc comparisons.
RESULTS
Development and Confirmation of a Congenic 129/Sv p75NTR−/− Strain
The p75NTR knockout mouse used in our studies was originally developed on a mixed 129/Sv and balb/C background (Lee et al.,1992). In-house breeding and back-crossing led to the development of the mice used for our previous study (Greferath et al.,2000), which shared approximately 95% genetic identity with inbred 129/Sv mice. For the present study, we sought to develop p75NTR–/– mice that were congenic with the 129/Sv strain. The p75NTR knockout mice (as used in the previous study) were therefore back-crossed with 129/Sv mice for a further 12 generations, giving a calculated genetic similarity of 99.98% or more. This was more than sufficient to satisfy the criterion for creation of a congenic strain. To screen the genetic background, a random assortment of simple sequence length polymorphisms (SSLPs) was chosen from among those known to exhibit clear differences between the 129/Sv and the balb/C strains (Dietrich et al.,1994; Matouk et al.,1996). Among 33 SSLPs tested, all were homozygous for the 129/Sv genotype; i.e., no balb/c SSLPs were detected. Furthermore, there was no contamination by any other strain.
Numbers of ChAT-Immunoreactive and p75NTR-Immunoreactive Neurons
Neurons were counted in 4-month-old male mice. To assist in differentiating between effects on neuronal numbers and neuronal phenotype, the total numbers of p75NTR-positive and ChAT-positive neurons were each assessed, using adjacent sections. Although the immunofluorescent signal strength of p75NTR in knockout mice was not as strong as in wild-type and heterozygous mice, p75NTR-immunopositive cells were clearly identifiable in p75NTR–/– mice and appeared to follow the same anatomical distribution as was observed in wild-type and heterozygous mice. The MS and VDB were counted as a single entity, because it is difficult in practice to demarcate these two regions consistently. Neuronal numbers were calculated after counting immunoreactive neurons in bilateral coronal sections and applying Abercrombie's rule to correct for bias, as described in Materials and Methods.
MS/VDB
Two-way ANOVA revealed a significant main effect of genotype on cell number (F2,16 = 4.45, P < 0.05). There was no significant effect of neuronal phenotype, but there was a significant interaction between genotype and cell phenotype (F2,16 = 3.98, P < 0.05). The number of ChAT-positive neurons in the MS/VDB of p75NTR knockout mice (3,820 ± 244) was significantly higher than the number in wild-type mice (3,240 ± 290, P < 0.05; Fig. 1). The number of ChAT-positive neurons in heterozygous mice was approximately midway between these two values. The numbers of p75NTR-positive neurons, by contrast, were constant across all three genotypes. In p75NTR knockout and heterozygous mice, the numbers of p75NTR-positive neurons were in good agreement with the numbers of ChAT-positive neurons, as expected. In wild-type mice, the number of p75NTR-positive neurons (3,655 ± 276) was greater than the number of ChAT-positive neurons by 415, but the difference was not statistically significant.
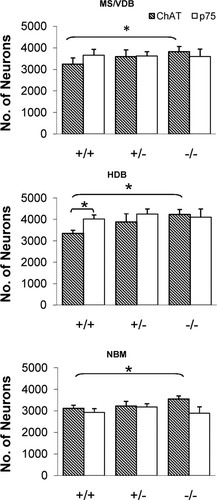
Neuronal counts in the basal forebrain of male 4-month-old mice. The number of ChAT-immunoreactive neurons (hatched) is shown alongside the number of p75NTR-immunoreactive neurons (white) in the combined medial septum and vertical limb of the diagonal band (MS/VDB; top), horizontal limb of the diagonal band (HDB; middle), and basal nucleus of Meynert (NBM; bottom). In each of the three anatomical compartments, there were significantly more ChAT-positive neurons in–/–mice than in +/+ mice (two-way ANOVA, Tukey's post hoc test, P < 0.05). In the HDB, +/+ mice had more neurons positive for p75NTR than for ChAT (P < 0.05). Values shown are mean ± SEM. N = 7 (+/–), 6 (–/–), and 6 (+/+).
HDB
In the HDB, there was a significant main effect of genotype on cell number (F2,16 = 5.81, P < 0.05) and a significant main effect of neuronal phenotype (F1,16 = 5.06, P < 0.05). There was also a significant interaction between genotype and cell phenotype (F2,16 = 4.75, P < 0.05). The intergenotype difference in number of ChAT-positive neurons in the HDB was marked; p75NTR knockout mice had 27% more ChAT-positive neurons (4,239 ± 228) than wild-type mice (3,349 ± 144, P < 0.05), and heterozygotes had an intermediate number (3,888 ± 380). In contrast, the number of p75NTR-positive neurons was unchanged across the three genotypes. In wild-type mice, the number of p75NTR-positive neurons (4,014 ± 204) was significantly greater than the number of ChAT-positive neurons (P < 0.05), consistent with the significant main effect of neuronal phenotype. Clearly, a substantial number of HDB neurons must have been positive for p75NTR but not for ChAT. Heterozygous mice also had a larger number of p75NTR-positive neurons (4,256 ± 240) than ChAT-positive neurons in the HDB, but this difference was not statistically significant. In knockout mice, the number of p75NTR-positive neurons in the HDB (4,099 ± 398) was quite close to the number of ChAT-positive neurons, and there was no significant difference between them.
NBM
In the NBM, as in the other two regions, there was a significant main effect of genotype on cell number (F2,16 = 4.86, P < 0.05). However, there was no effect of neuronal phenotype and no significant interaction between genotype and neuronal phenotype. The number of ChAT-positive neurons in the NBM was significantly higher in p75NTR knockout mice (3,548 ± 150) than in wild-type mice (3,120 ± 144, P < 0.05), and there was an intermediate number in heterozygotes. The number of p75NTR-positive neurons did not differ significantly among the three genotypes. In wild-type mice, the number of ChAT-positive neurons did not differ significantly from the number of p75NTR-positive neurons (2,930 ± 175). In p75NTR heterozygous mice, the numbers of ChAT-positive and p75NTR-positive neurons in the NBM (3,230 ± 212 and 3,175 ± 162, respectively) were nearly identical. In p75NTR knockout mice, ChAT-positive neurons were in excess of p75NTR-positive neurons, of which there were 2,888 ± 304 (P < 0.05). However, this did not register as significantly different by two-way ANOVA.
Double staining
The excess of p75NTR-positive over ChAT-positive neurons in the MS/VDB and HDB of wild-type mice was borne out by examining double-stained basal forebrain sections. Although most of the immunolabeled neurons in these regions were positive for both p75NTR and ChAT, a sizable minority of p75NTR-positive neurons was negative for ChAT (Fig. 2). These single-labeled neurons appeared to be scattered more or less randomly among the double-immunolabeled neurons. However, a small region of the rostromedial HDB, close to the junction with the VDB, consisted mainly of p75NTR-positive neurons that were ChAT negative (Fig. 2). The presence of this relatively concentrated cluster of p75NTR-positive ChAT-negative neurons in this region has been described previously (Batchelor et al.,1989). Interestingly, the corresponding VDB/HDB border region in heterozygous and knockout mice contained neurons positive for both markers (Fig. 2). In general, there were fewer p75NTR-positive ChAT-negative neurons in the MS/VDB and HDB of heterozygous mice and none in p75NTR knockout mice
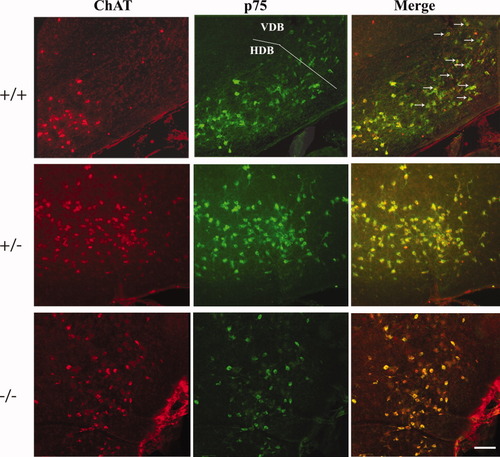
Fluorescent imaging of p75NTR-and ChAT-immunopositive neurons in the HDB of wild-type (p75+/+), heterozygous (p75+/–), and homozygous p75NTR mutant mice (p75–/–). The coronal sections were taken near the rostral pole of the HDB, where it adjoins the VDB, and a small part of VDB appears at the top of the sections. The Alexa 594-coupled secondary antibody for ChAT produces red fluorescence, and the Alexa 488-coupled secondary antibody for p75NTR produces green fluorescence. In the merged picture, double-stained neurons are yellow, and p75NTR+ ChAT– neurons appear green (arrows). In wild-type mice, p75NTR+ ChAT– neurons were abundant in the rostromedial part of the HDB, as shown. In the corresponding sections in p75NTR mutant and heterozygous mice, the p75NTR-expressing neurons were all positive for ChAT. The red neurons in the upper part of the frame are ChAT+ p75NTR– striatal interneurons. Scale bar = 120 μm.
The situation in the NBM was slightly more complex. The NBM of wild-type mice contained significant numbers of neurons that were positive only for ChAT or only for p75NTR (Fig. 3). The existence in the NBM of a subpopulation of ChAT-positive neurons that does not express p75NTR is well known; these neurons innervate the basolateral and some other nuclei of the amygdala (Henderson and Evans,1991; Hecker and Mesulam,1994; Nickerson Poulin et al.,2006). The abundance of the ChAT-positive, p75NTR-negative neuronal subpopulation appeared to be the same in heterozygous and knockout mice as in wild-type mice.
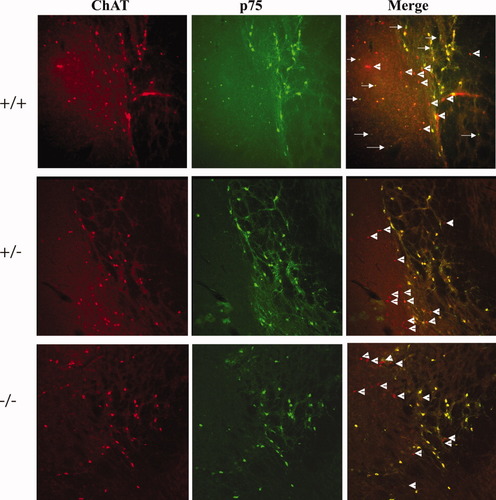
Imaging of p75NTR-and ChAT-immunopositive neurons in the NBM. The NBM contained numerous ChAT+ p75NTR– neurons, which appear red in the merged images (arrowheads), and were equally abundant in all three genotypes in coronal sections. In the merged images, p75NTR+ ChAT– neurons appear green and were most abundant in wild-type mice (arrows). Similar neurons were encountered rarely in heterozygote NBM, and were not found in knockout NBM.
The NBM of wild-type mice, as with other parts of the CBF, contained a sizeable minority of p75NTR-positive ChAT-negative neurons (Fig. 3). The absence of such neurons in the knockout NBM, combined with the presence of the NBM-specific ChAT-positive, p75NTR-negative subpopulation, accounts for the higher number of ChAT-positive than p75NTR-positive neurons counted in the knockout NBM as noted above.
Cholinergic Neuronal Size
Neuronal size was assessed by measuring cross-sectional areas of ChAT-immunoreactive neurons in coronal sections. These measurements were carried out in the same 4-month-old mice that were used for cell counts. Neurons in the MS, VDB, HDB, and NBM were assessed as separate groups.
In all four regions, the average size of cholinergic neurons was greater in p75NTR knockout than in wild-type mice, although the difference did not always reach statistical significance. In the MS, there were no significant size differences between any of the genotypes (Fig. 4). In the VDB, p75NTR knockout neurons (347 ± 15 μm2) were significantly larger than heterozygous neurons (297 ± 9 μm2, P < 0.05). In the HDB, cholinergic neurons in p75NTR knockout mice (299 ± 16 μm2) were significantly larger those of wild-type mice (251 ± 11 μm2, P < 0.05) and heterozygous mice (258 ± 14 μm2, P < 0.05). In the NBM, there were no significant size differences among genotypes.
Cholinergic Processes in the Hippocampus
We assessed the concentration of cholinergic fibers in the proximity of the pyramidal cell layer, near their innervation targets. Within the CA1 region, most cholinergic afferents are distributed in the stratum oriens, where they synapse with the basal dendritic tree of the pyramidal neurons (Amaral and Witter,1995). The cell bodies of pyramidal neurons also receive cholinergic synapses, provided mostly by distinctively fine, delicate fibers within the pyramidal layer (Nyakas et al.,1987). Cholinergic fibers are also present within the stratum radiatum and stratum moleculare, making contact with the apical dendrites and interneurons. We used an AChE staining technique to visualize these fibers and quantified them by counting the number of positively stained fibers that crossed a standard 100-μm line in each layer. In the stratum oriens, there were significantly more crossings in p75NTR–/– mice (23.5 ± 1.8) than in p75NTR+/+ mice (16.3 ± 1.4, P < 0.05, ANOVA; Fig. 5). The number of crossings in p75NTR+/– mice was 19.4 ± 0.7, and this was significantly greater than that in p75NTR+/+ mice (P < 0.05, ANOVA) but not significantly less than that in p75NTR–/– mice. A similar pattern was seen in the pyramidal cell layer, where the number of crossings was greater in p75NTR–/– mice (29 ± 1.8) than in p75NTR+/+ mice (23 ± 1.8, P < 0.05, ANOVA). There were 26.4 ± 1.5 crossings in p75NTR+/– mice, a value that was intermediate between, but not significantly different from, the values for the other two genotypes. In the stratum radiatum, there were 24.6 ± 1.6 crossings in p75NTR–/– mice and 17.4 ± 1.2 in p75NTR+/+ mice (P < 0.05, ANOVA). There were 21.1 ± 2 crossings in p75NTR+/– mice, a value that did not differ significantly from the values of other two genotypes.
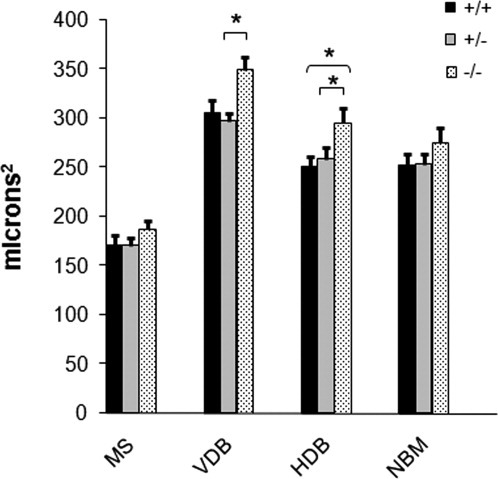
Differences in the size of cholinergic neurons. In the VDB, p75NTR–/– neurons were significantly larger than p75NTR+/– neurons but did not differ significantly from wild-type neurons. The size difference between wild-type and heterozygote in the VDB was not significant. In the HDB, the neurons of p75NTR–/– mice were significantly larger than those of heterozygotes and wild-type mice. In the MS and NBM, neuronal sizes did not differ between any of the genotypes. Data were taken from seven p75NTR+/– mice, six p75NTR–/– mice, and six p75NTR+/+ mice, the same mice used for neuronal counts. In each mouse, 25 neurons in the VDB and 50 neurons in each of the MS, HDB, and NBM were measured. The mean value was computed for each mouse (for each of the four regions), and this was treated as one data point for statistical purposes. Values shown are mean ± SEM. *P < 0.05 (ANOVA).
Hippocampal ChAT Activity
Hippocampal ChAT activity was examined in 4-month-old mice, an age similar to that of the mice used for morphometric studies. Hippocampal ChAT activity (expressed as picomoles of acetylcholine per minute per milligram protein) was 226 ± 18 in homozygous knockout mice compared with 170 ± 12 in heterozygous mice and 128 ± 22 in wild-type mice (Fig. 6; n = 6, 10, 6, respectively). ChAT activity in homozygous knockout mice was therefore 77% higher than in wild-type mice (P < 0.05, ANOVA) and 33% higher than in heterozygotes (P < 0.05).
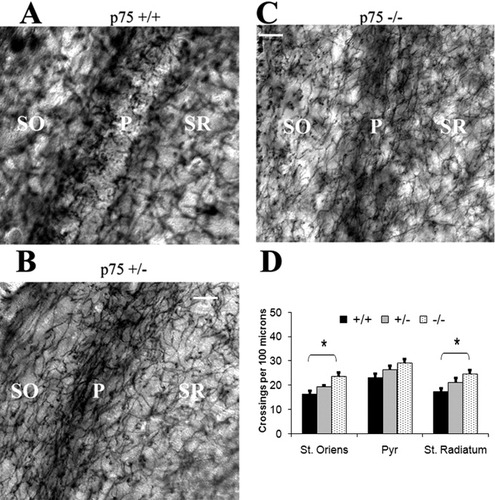
Increased number of acetylcholinesterase-positive axons in the CA1 region of the hippocampus in p75NTR–/– mice. A: In wild type, there were few positive fibers in the middle of the pyramidal cell layer. B,C: In p75NTR+/– and p75NTR–/– mice, AChE-positive fibers were apparent across the whole width of the pyramidal layer. The number of immunopositive fibers appeared to be higher in all layers compared with the wild-type. SO, stratum oriens; Pyr, pyramidal cell layer; SR, stratum radiatum. D: Analysis revealed that sections from p75NTR–/– mice had more positive fibers than did wild-type mice in stratum oriens, the pyramidal cell layer, and the stratum radiatum. Results are mean ± SEM. N (number of mice used) = 6 (p75NTR+/+), 5 (p75NTR+/–), and 6 (p75NTR–/–). Fifty neurons from each of the MS, HDB, and NBM and 25 neurons from the VDB were measured in each mouse. *P < 0.05. Scale bars = 40 μm.
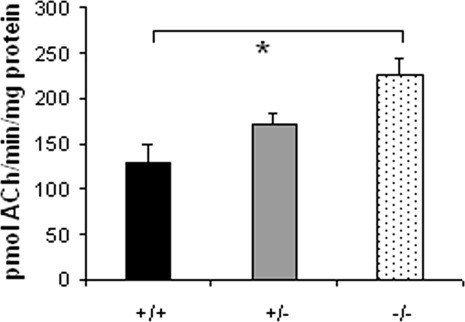
Hippocampal ChAT activity was higher in p75NTR–/– mice compared with wild-type and heterozygous mice at 4 months of age. The asterisks indicate significant differences compared with p75NTR–/– mice (P < 0.05, ANOVA). Error bars = SEM. The number N of mice in each of the wild-type, p75NTR+/–, and p75NTR–/– groups was 6, 10, and 6, respectively.
DISCUSSION
The present results, showing an increased number of ChAT-positive neurons in the basal forebrain of p75NTR knockout mice, are in accordance with several earlier studies that have reported similar increases (Yeo et al.,1997b; Krol et al.,2000; Naumann et al.,2002). Because of p75NTR's well-known apoptotic role, many investigators believe that the increase reflects survival of cholinergic neurons that would normally have undergone p75NTR-dependent apoptosis. An alternative possibility is that the number of neurons is unchanged, but a greater proportion of them expresses ChAT. It is also possible that both factors contribute to the increase. By undertaking separate counts of the p75NTR-immunopositive and ChAT-immunopositive neurons in the main subdivisions of the basal forebrain, and performing double staining, we have been able to cast some light on this issue. We were able to identify reliably p75NTR-expressing neurons in knockout mice, as a result of the expression of an intracellular fragment of p75. This fragment is reliably identified in magnocellular neurons that normally express p75NTR, as confirmed by the observation that essentially all ChAT-positive neurons in the MS, VDB, and HDB of knockout mice were positive for this fragment. The expression of this fragment, though convenient for the present article, means that the term knockout applies only to the full-length version of p75NTR. In the NBM, a subpopulation of cholinergic neurons was absolutely negative for p75NTR and is discussed further below.
In contrast to the increase in ChAT-positive neurons in knockout mice, the number of p75NTR-positive neurons in the basal forebrain was constant across the three genotypes. Even in the HDB, where the increase in ChAT-positive neurons was most pronounced, there was no difference between wild-type, heterozygote, and knockout in the number of p75NTR-positive neurons. The most likely explanation for the increase in ChAT-positive neurons, therefore, is that a higher proportion of p75NTR-positive neurons expressed ChAT compared with wild-type mice.
Double staining of the basal forebrain in wild-type mice revealed the presence of p75NTR-positive neurons that were negative for ChAT. These neurons were most evident in the VDB and HDB, but similar neurons were also apparent in the NBM, and to a lesser extent in the MS. Such neurons were encountered less frequently in heterozygotes and not at all in knockout mice. The occurrence of significant numbers of p75NTR-positive ChAT-negative neurons in the basal forebrain in wild-type rats has been described previously by several investigators (Batchelor et al.,1989; Dreyfus et al.,1989; Pioro and Cuello,1990). In our study, p75NTR-positive ChAT-negative neurons were usually intermingled with double-stained neurons, but they were particularly numerous in the rostromedial HDB, adjoining the VDB. The cluster of p75NTR-positive ChAT-negative neurons at this site has been described previously (Batchelor et al.,1989), although the authors of that report considered the location to be part of the VDB rather than HDB.
The presence of p75NTR-positive ChAT-negative neurons in the basal forebrain of wild-type but not knockout mice helps to explain the greater numbers of p75NTR-positive than of ChAT-positive neurons in wild-type mice. It is also consistent with the view that, in the knockout mice, a higher proportion of existing p75NTR-positive neurons expresses ChAT.
The excess of p75NTR-positive neurons compared with ChAT-positive neurons in wild-type mice, although clearly apparent in the MS/VDB and HDB, was not present in the NBM; there was no significant difference between numbers of p75NTR-positive neurons and ChAT-positive neurons in the wild-type NBM. In addition to p75NTR-positive ChAT-negative neurons and doubly positive neurons, the wild-type NBM contained considerable numbers of ChAT-positive p75NTR-negative neurons. The abundance of ChAT-positive p75NTR-negative neurons, as assessed qualitatively in double-stained sections, was the same in heterozygous and knockout NBM as in the wild-type NBM. Cholinergic neurons in the NBM that do not express p75NTR have been described in a number of earlier studies; they are known to innervate specific nuclei in the amygdala, namely, the basolateral nucleus and the nucleus of the lateral olfactory tract (Henderson and Evans,1991; Hecker and Mesulam,1994; Nickerson Poulin et al.,2006). Their presence accounted for the overall surplus of ChAT-positive relative to p75NTR-positive neurons in the knockout NBM. In wild-type NBM, both ChAT-positive p75NTR-negative and p75NTR-positive ChAT-negative neurons were present and tended to cancel each other out. In knockout mice, however, the only single-labeled neurons in the NBM were ChAT-positive p75NTR-negative neurons.
The present findings do not support the view that p75NTR causes apoptosis in cholinergic basal forebrain neurons. As we have noted, the number of p75NTR-expressing neurons in the basal forebrain was no different between p75NTR mutant and wild-type mice. The proportion of these neurons that coexpressed ChAT underwent an increase in p75NTR mutant mice. Although expression of p75NTR and expression of ChAT in the basal forebrain are often considered synonymous phenomena, it is well documented that a small proportion of neurons that express p75NTR is negative for ChAT (Batchelor et al.,1989; Dreyfus et al.,1989; Pioro and Cuello,1990). It is not clear whether these are just weakly ChAT-expressing neurons or neurons of a completely different neurotransmitter phenotype. It has been suggested that some p75NTR neurons may be GABAergic (Dreyfus et al.,1989), although most GABAergic basal forebrain neurons are clearly negative for p75NTR. Whatever the neurotransmitter phenotype of p75NTR-positive ChAT-negative neurons in wild-type basal forebrain, the equivalent neurons in p75NTR mutant mice appear to express ChAT strongly. This is in accordance with the 77% increase in hippocampal ChAT level, which suggests that increased ChAT expression may be a general phenomenon in p75NTR-positive neurons of p75NTR mutant mice.
Our findings do not disprove that p75NTR causes apoptosis in the basal forebrain. It is possible that p75NTR has pro-and antiapoptotic actions at different developmental stages, as it does in sensory neurons (Barrett and Bartlett,1994), and that these balance out. Neverthless, it remains clear that a higher prevalence of ChAT expression among p75NTR-positive cells accounts for some of the increased number of cholinergic neurons in p75NTR mutant mice.
The p75NTR-deficient phenotype in the basal forebrain also included enlargement of cholinergic cell somata, an increase in the number of cholinergic axonal fibers in the hippocampus, and an increased ChAT activity in the hippocampus. The increase in cell soma size was greatest in the VDB and HDB, but the same trend was evident in the MS and NBM. The increased number of cholinergic fibers in the hippocampus is consistent with the increase in number of ChAT-positive neurons in the MS/VDB and the hypertrophied cell somata of these neurons.
The effects of p75NTR deficiency seen in this study were strikingly similar to the well-known effects of chronic nerve growth factor (NGF) treatment on cholinergic forebrain neurons. NGF delivered intracranially has been shown to increase cholinergic neuronal size (Fischer et al.,1987; Gage et al.,1988), to stimulate ChAT synthesis (Hagg et al.,1989; Williams,1991), and to enhance cholinergic innervation of the hippocampus (Hagg et al.,1990; Phillips et al.,2004). The simplest interpretation is that p75NTR antagonizes the trophic effects of NGF, including control of neuronal size and neurotransmitter synthesis, in the basal forebrain. Thus, p75NTR may be said to have antineurotrophic actions in the basal forebrain. Induction of apoptosis is, of course, antineurotrophic, but the present results cast doubt on the apoptotic role of p75NTR in the basal forebrain.
The mechanisms by which p75NTR regulates neuronal growth and neurotransmitter synthesis are unknown. Indeed, the mechanisms behind any of the actions of p75NTR are far from resolved, and the canonical p75NTR signal transduction pathway, if there is one, remains to be established. It has often been noted that p75NTR is promiscuous, in that it interacts with a diverse range of signalling systems (Barker,2004; Gentry et al.,2004). Conceivably, the signalling pathways used by p75NTR to downregulate cell growth and neurotransmitter production in cholinergic forebrain neurons may overlap the pathways by which, in different cell types and circumstances, p75NTR induces apoptosis. A different, intriguing possibility was suggested by Kawaja and coworkers (Krol et al.,2000); they showed that p75NTR is necessary for retrograde transport of NGF from axonal terminals and that it thereby removes NGF from the site of TrkA signalling. Regardless of the mechanism, the emerging evidence is that p75NTR exerts an antineurotrophic role in the basal forebrain.
The present data provide a new way of looking at trophic control of the forebrain cholinergic system, with NGF and p75NTR driving the system in opposite directions. Just as NGF deficiency produces basal forebrain cholinergic atrophy (Chen et al.,1997), p75NTR deficiency produces hypertrophy. It has long been appreciated that production of NGF is needed to sustain the cholinergic system throughout life. The cholinergic system undergoes well-characterized deficits with aging, and, experimentally, NGF has been powerful in reversing these. Pronounced cholinergic deficits also occur in some neurodegenerative diseases, particularly Alzheimer's disease, motivating investigators to try to devise ways to supply NGF or its mimetics to the brain. The present work suggests that therapies aimed at downregulating or blocking p75NTR may provide a valuable new approach to treating or preventing these cholinergic deficits.
Acknowledgements
We thank Dr. U. Grunert of the National Vision Research Institute of Melbourne University for access to the Zeiss Axioplan microscope and for help with the imaging of z-series.




