Epigenetic control of somatostatin and cortistatin expression by β amyloid peptide
Abstract
β Amyloid, present in senile plaques, has been related largely to neuronal loss in the brain of patients with Alzheimer's disease. However, how neurons respond to β amyloid insults is still poorly understood. Here we show that β amyloid increases somatostatin and cortistatin gene expression mainly through an increase in histone 3 lysine 4 methylation (H3K4me3), a modification associated with transcriptional activation. Somatostatin and cortistatin partially decreased β amyloid toxicity in primary cortical neurons in culture. Thus we suggest that neurons respond to β amyloid insults by releasing somatostatin and cortistatin, which will act as a protective agent against β amyloid toxicity. Our results suggest a relevant function for both neuropeptides against β amyloid toxicity, providing new insights into Alzheimer's disease. © 2011 Wiley Periodicals, Inc.
β Amyloid, the main component of the senile plaques found in the brains of Alzheimer's disease patients (Masters et al.,1985), shows a toxic effect on neuronal cells. However, little is known about how neurons respond to β amyloid insults. Recently, it was shown that β amyloid increases somatostatin expression in cultured cortical neurons (Geci et al.,2007). Somatostatin is expressed in the central nervous system, and changes in the levels of somatostatin and some of its cellular receptors have been reported in neurodegenerative disorders such as Alzheimer's disease (Epelbaum et al.,2009; Gahete et al.,2010).
In addition to somatostatin, a strongly related neuropeptide has been identified and named cortistatin after its predominant expression in GABAergic cortical neurons (de Lecea,2008). Despite cortistatin binding in vitro to the five somatostatin receptors, with similar affinity to that of somatostatin, cortistatin shows some functional differences (de Lecea and Castano,2006; de Lecea,2008). In this work, we have analyzed the effect of β amyloid on cortistatin expression in cortical neurons and have studied the mechanism underlying the increase of somatostatin expression in the presence of β amyloid. Our results indicate an epigenetic control of the expression of both somatostatin and cortistatin, by β amyloid peptide and that somatostatin could act as a protective factor for β amyloid toxicity. The epigenetic control of somatostatin and cortistatin expression is through the methylation at H3K4 associated with the promoter of somatostatin and cortistatin. It is well known that a modification in this lysine is related to an open state of the chromatin and correlates with an increase in gene expression (Justin et al.,2010). Our data suggest an important role for somatostatin and cortistatin in neuroprotection, with potential implications for the pathology of Alzheimer's disease.
MATERIALS AND METHODS
Primary Cortical Cultures and Treatment
Cortical tissue was dissected from E18 C57BL6 mouse embryos. Cells were dissociated with papain (Worthington Biochemical, Lakewood, NJ; Banker and Cowan,1977), and the neurons were plated on poly-L-lysine (100 μg/ml) plus laminin (10 μg/ml)-coated plastic dishes (1 × 106 cells/P35) or coated coverslips (1 × 105 cells/24 well). The cultures were grown in Neurobasal medium (Gibco, Carlsbad, CA) supplemented with 10% (v/v) horse serum, 2 mM glutamine, 1 mM pyruvate, 100 U/ml penicillin, and 100 U/ml streptomycin. The cells were cultured in a humidified atmosphere of 5% CO2/95% air at 37°C. Every 2–3 days, half of the medium was replaced adding 5 μM arabinofuranosylcytosine (Ara-C; Calbiochem, San Diego, CA) to prevent glial proliferation. After 8 days in vitro, the medium was changed to Neurobasal medium, and different compounds were added at 37°C for 24 hr (N = 3 per condition). Treatments were as follows: 5–40 μM β amyloid (Aβ 25–35; Polypeptides Group, Strasbourg, France), 20–40 μM somatostatin (somatostatin 1–14; Sigma-Aldrich, St. Louis, MO), 20–40 μM somatostatin + 40 μM β amyloid, 10 μM cortistatin (cortistatin 1–14; Neomps, Strasbourg, France), or 10 μM cortistatin + 40 μM β amyloid. Control cells were treated with the same volume of vehicle. Then, RNA extraction, viability assay, protein extraction, or chromatin immunoprecipitation assay (Chip) was performed as described below. The stock of β amyloid was resuspended at 1 mM in Neurobasal medium, and one aliquot was visualized in an electron microscope (model JEM1010).
RT-PCR Analysis
Total RNA purification and DNase treatment were performed using an RNA Miniprep Kit (Stratagene, La Jolla, CA). Then, a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE) was used to check RNA concentration and quality. RNA was reverse transcribed using the first-strand cDNA synthesis kit (AMV; Roche, Basel, Switzerland). Each PCR was carried out in triplicate as follows: 1 μl of the template, 5 μl of SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), and 150 nM of the primers (Table I).
| Gene primers | Sequence | |
|---|---|---|
| RT-PCR | β-Actin (F) | GGCGCTTTTGACTCAGGATT |
| β-Actin (R) | GGGATGTTTGCTCCAACCAA | |
| CST (F) | ACAGTGTGCAGGAAGCCACC | |
| CST (R) | CAAGGAAAGTCAGAAGGCCG | |
| SST (F) | GGAAACAGGAACTGGCCAAG | |
| SST (R) | GGCATCATTCTCTGTCTGGTTG | |
| Chip control genes | MmChipGapdh (F) | ACCGAAGAACAACGAGGAGA |
| MmChipGapdh (R) | GAGGAGTCCTTGGAGTGTGC | |
| MmChipRPL38 (F) | TGATGAACGCATCCTTTCTG | |
| MmChipRPL38 (R) | AGCGATGAGAACCGAAGAGA | |
| MmChipMyod1 (F) | CACGACTGCTTTCTTCACCA | |
| MmChipMyod1 (R) | ACAAAGGTTCTGTGGGTTGG | |
| MmChiptdrd1 (F) | CCTCCGGGACACCTTTCTA | |
| MmChipTdrd1 (R) | GAGCTGCTCTGATTGATCACC | |
| Chip-tested genes | MmChipCST (F) | CACAGGAAAAGTCCCGAGTC |
| MmChipCST (R) | ACCTACACGCCTCTCCACAC | |
| MmChipSST (F) | ATTTTGCGAGGCTAATGGTG | |
| MmChipSST (R) | TATGGAGCTCTCCACGGTCT |
Thermocycling conditions were 10 min at 95°C, then 50 cycles of 15 sec at 95°C and 1 min at 60°C. Finally, to determine reaction specificity, a dissociation curve was obtained raising the temperature from 60°C to 95°C. Fluorescent signal was acquired by the ABI Prism 7900HT Sequence Detection System (Applied Biosystems), and the mean threshold cycle (Ct) values plus standard deviations were normalized using β-actin data. No-template reactions were used as negative controls, and reverse transcriptase minus template reactions were performed to assess genomic DNA contamination.
Western Blot Analysis
Untreated or treated cells were homogenized in ice-cold extraction buffer composed of 20 mM HEPES, pH 7.4, 100 mM NaCl, 20 mM NaF, 1% Triton X-100, 1 mM sodium orthovanadate, 5 mM EDTA, and protease inhibitors (2 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μg/ml pepstatin). The samples were centrifuged at 15,000g for 20 min at 4°C. The resulting supernatant was collected, and protein content was determined by Bradford assay. Thirteen micrograms of total protein was run on 10% SDS-polycrylamide gel and transferred to a nitrocellulose membrane (Schleicher & Schuel, Keene, NH). After blocking for 30 min with a solution containing 0.05% (vol/vol) Tween 20 and 5% (wt/vol) nonfat dry milk in PBS, the following primary monoclonal antibodies were added for 1 hr: anti-GSK3β (1/1,000 in blocking solution; BD Transduction, Franklin, Lakes, NJ), anti-p-serine 21/9-GSK3α/β (1/5,000; Cell Signaling, Boston, MA), anti-p-tyrosine 216/279-GSK3α/β (1/200; Invitrogen, Carlsbad, CA), and anti-β tubulin (1/1,000, Sigma-Aldrich, St. Louis, MO). Then, the membrane was incubated with an HRP-linked secondary goat anti-mouse antibody (1 hr, 1/2,000; Dako, Glostrup, Denmark). To visualize the immunodetection, Western Lightning reagents (Perkin Elmer Life Sciences, Boston, MA) were used, and densitometric quantification was performed in the Quantity One program (Bio-Rad, Hercules, CA).
Chip Assay
Chip assay has been performed on extracts from control cells and treated cells (40 μM β amyloid; 24 hr) using the low cell Chip kit (catalog No. Kch-maglow-016; Diagenode). The cross-link and the sonication of all the samples were done at the same time in order to reduce the technical background. To carry out the Chip, 1 mg DNA was used for each histone mark (H3K4me3 as active mark; histone H3 trimethylated at lysine 9 H3K9me3 and histone H3 trimethylated at lysine 27 H3K27me3 as inactive marks). Primers used in quantitative PCR are detailed in Table I.
Viability Assay
Toxicity was assayed by using the Live/Dead viability/cytotoxicity kit (Invitrogen). After staining, the cells were visualized on a Leica fluorescence microscope, and images were acquired using the associated software at ×10 magnification in randomly selected fields (N = 5 fields per coverslip; N = 3 coverslips per condition). Toxicity was defined in each image as the percentage of live cells vs. the total number of cells.
Statistical Analysis
Data are represented as mean ± SEM. Statistical analyses were performed via ANOVA, followed by a Bonferroni post hoc test for the results shown in Figure 1A,B and Student's t-test for the other figures.
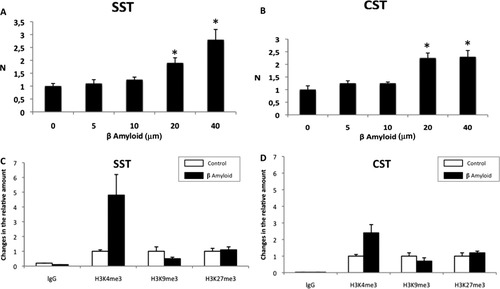
β Amyloid regulates somatostatin and cortistatin expression. A,B: Real-time Q-PCR analysis of somatostatin (A; SST) and cortistatin (B; CST) genes in primary cortical neurons incubated in the presence of 5, 10, 20, and 40 μM β amyloid for 24 hr. Note the upregulation in the expression of somatostatin and cortistatin. β-Actin was used as an internal control. Results are shown as mean ± SEM (N = 3). *P < 0.05 in comparison with controls. C,D: Quantification of the relative amount of H3K4me3, H3K9me3, and H3K27me3 associated with somatostatin (C; SST) and cortistatin (D; CST) promoters. β Amyloid treatment in cortical neurons (40 μM; 24 hr) increases the active H3K4me3 histone mark on the promoters of somatostatin and cortistatin genes. A slight decrease in inactive H3K9me3 histone mark was observed in both genes, and no significant differences were found in the methylation of H3K27 residue. Fig. 2
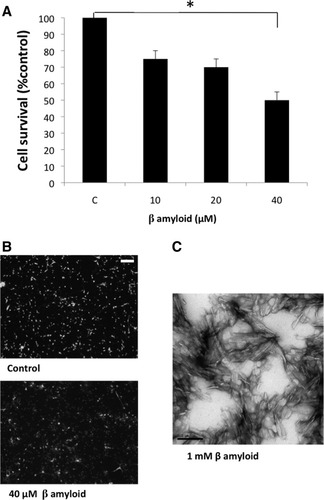
β Amyloid induces neuronal death. A: Quantification of cell survival in neurons incubated in the presence of different doses of β amyloid (10, 20, 40 μM; 24 hr). Viability assay was performed using the Live/Dead kit containing calcein AM (live cells) and propidium iodide (dead cells). Cell survival was significantly decreased in the presence of 40 μM β amyloid. Data are shown as means ± SEM (N = 3). *P < 0.05 in comparison with controls. B: Representative fluorescent micrographs of the viability assay from control and treated cells (40 μM β amyloid; 24 hr). Calcein AM (green fluorescence) and propidium iodide (red fluorescence) detect live cells and dead cells, respectively. C: Electron micrography of 1 mM β amyloid in Neurobasal medium. Scale bars = 100 μm in B; 0,2 μm in C.
RESULTS
β Amyloid Increases Somatostatin and Cortistatin Expression
It has previously been shown that β amyloid peptide induces somatostatin expression in primary cortical neurons obtained from rat embryos (Geci et al.,2007). To determine the effect of β amyloid in our model, we incubated murine cortical neurons in the presence of 5 μM, 10 μM, 20 μM, and 40 μM β amyloid for 24 hr. Then, quantitative real-time PCR measurements of somatostatin were performed using the SYBR Green reagent. In agreement with the Geci et al. findings, our experiments indicated that β amyloid treatments induce a gradual increase (concentration dependent) in somatostatin mRNA. Figure 1A shows that incubations with 20 μM or 40 μM β amyloid increase significantly somatostatin expression twofold and nearly threefold, respectively.
Because cortistatin belongs to the somatostatin family and the effect of β amyloid on cortistatin expression had not been previously studied, we analyzed cortistatin mRNA in cortical neurons treated with different concentrations of β amyloid (5–40 μM; 24 hr). We found that cortistatin expression was increased twofold in the presence of 20 μM β amyloid, reaching a plateau at this concentration (Fig. 1B). Thus, β amyloid peptide also promotes cortistatin expression, albeit at a lower rate than somatostatin expression at 40 μM β amyloid.
To determine how β amyloid increases somatostatin and cortistatin expression and to analyze whether somatostatin and cortistatin are under epigenetic regulation, we used chromatin isolated from cortical neurons grown in the presence of 40 μM β amyloid to perform immunoprecipitation with antibodies directed against modified histones, followed by PCR amplification with primers that recognized somatostatin and cortistatin promoters. As shown in Figure 1C,D, the Chip technique revealed that β amyloid peptide increases H3K4me3 histone mark on the promoters of cortistatin and somatostatin genes (2.5 and 4.9 times, respectively; Supporting Information Table IA). This mark is related to an open state of the chromatin and usually correlates with an increase in gene expression (Justin et al.,2010).
Changes in two repressive marks (H3K9me3 and H3K27me3) associated with gene silencing were also studied for both somatostatin and cortistatin promoters. In this case, a slight decrease in H3K9me3 was observed in both genes (0.7 for cortistatin gene and 0.6 times for somatostatin gene), but no significant differences were found in the methylation of H3K27 residue (Fig. 1C,D, Supporting Information Table I). Thus, our results suggest that β amyloid increases somatostatin and cortistatin gene expression mainly through an increase in lysine methylation of the H3K4 residue, a modification associated with transcriptional activation. Interestingly, enhanced somatostatin expression in comparison with cortistatin correlates with the observed H3K4me3 enrichment. However, we cannot confirm whether this increase takes place in a specific neuron population.
Similar Chip assays were also performed using optimized quantitative PCR primers to amplify different gene promoters used as PCR controls. Indeed, glyceraldehyde 3-phosphate dehydrogenase gene (GAPDH) and 60S ribosomal protein L38 gene (RPL38) were chosen among housekeeping genes actively transcribed (positive control), together with myogenic differentiation 1 gene (Myod 1) and Tudor domain containing 1 gene (Tdrd1) involved in muscle cell differentiation and in spermatogenesis, respectively, which were tested as inactive genes (negative control) in cortical neurons. Consistently, GAPDH and RPL38 promoters were associated with increased H3K4 methylation level and reduced H3K9 and H3K27 methylation level, and the opposite was found for Myod1 and Tdrd1 genes (Supporting Information Table IB, Supporting Information Fig. 1). Under control conditions, GADPH and RPL38 promoters are constitutively fully activated. This activation has been associated with the presence of high H3K4 methylation as indicated in Figure 1. These results demonstrate that a complete and reliable analysis of the histone modifications has been performed with the Chip assay.
β Amyloid Induces Neurotoxicity in Primary Cortical Neurons, but Somatostatin and Cortistatin Partially Decrease β Amyloid Toxicity
To determine the effect of β amyloid peptide on neuronal survival, primary cortical neurons were incubated with different doses (10, 20, 40 μM) of β amyloid for 24 hr. Cell viability was assessed using calcein AM and propidium iodide to detect live cells and dead cells, respectively. Our experiments show that β amyloid induces a concentration-dependent death of cortical neurons (Fig. 2A,B). Cell survival was significantly decreased in the presence of 40 μM β amyloid. It is noteworthy that the stock of 1 mM β amyloid in Neurobasal medium was visualized by electron microscopy before being added to the culture. Under these conditions, β amyloid was polymerized as shown in Figure 2C.
According to our results and the previously reported neuroprotective actions of somatostatin (Forloni et al.,1997; Kumar,2008; Kiagiadaki et al.,2010) and cortistatin (Braun et al.,1998), the expression of both neuropeptides in our model could be a protective response to the toxic effect of β amyloid peptide. To determine the neuroprotective action of somatostatin and considering that our cellular model expresses the five somatostatin receptors (Rubio et al.,2008), we have analyzed the effect of 20 μM somatostatin on cortical neurons treated with 40 μM β amyloid peptide. In these experiments, the proportion of cortical neurons in the presence of somatostatin + β amyloid was similar to the control conditions, indicating that somatostatin could partially prevent β amyloid neurotoxicity (Fig. 3). Similar results were found in the presence of 10 μM cortistatin. Thus, these data suggest that somatostatin and cortistatin mediate in vitro a protective action on cortical neurons.
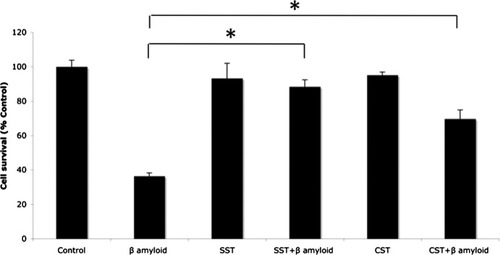
Somatostatin and cortistatin protective action. Somatostatin or cortistatin partially prevent β amyloid toxicity to neuronal cells. Cell survival was determined using the Live/Dead kit in neurons grown for 24 hr in the presence of 40 μM β amyloid, 20 μM somatostatin, 20 μM somatostatin + 40 μM β amyloid, 10 μM cortistatin, or 10 μM cortistatin + 40 μM β amyloid. Data are means ± SEM (N = 3). *P < 0.05.
β Amyloid and Somatostatin Regulate GSK3 Activity
β Amyloid peptide has several cellular receptors that, after their activation, produce different downstream intracellular signals (Forloni et al.,1997; Supnet and Bezprozvanny,2010). One of these intracellular signals is an increase in GSK3 activity (Townsend et al.,2007; Magdesian et al.,2008). In addition, it has been described that not only β amyloid but also GSK3 overexpression causes cell death (Pap and Cooper,1998), and it has therefore been suggested that the toxic effect promoted by β amyloid could occur, at least in part, through GSK3 activation (Townsend et al.,2007). Thus to study further how β amyloid mediates its toxic action and to determine the mechanism underlying the somatostatin and cortistatin protective effect, GSK3 activity was studied under different experimental conditions.
Two isoforms of GSK3 protein named α (51 kDa) and β (48 kDa) have been previously described (Woodgett,1990). A variant for GSK3β with a lower electrophoretic mobility than GSK3β has been described more recently (Soutar et al.,2010). GSK3 activity is regulated by phosphorylation at specific residues. Phosphorylation at Ser9 of GSK3β and the equivalent Ser21 of GSK3a inhibits its activity, whereas phosphorylation at Tyr216 and Tyr279 of GSK3β and GSK3α, respectively, is thought to increase its activity (Hughes et al.,1993; Lochhead et al.,2006).
To analyze whether the effect of β amyloid on neuronal toxicity correlates with an increase in GSK3 activity, Western blots were performed using specific antibodies that recognize P-Ser21/9-GSK3α/β, P-Tyr216/279-GSK3α/β, and GSK3β. Under these conditions, a decrease in serine phosphorylation of GSK3α/β (Ser21/9) was found in cortical neurons incubated in the presence of 40 μM β amyloid (0.5 times for P-Ser21/9-GSK3α/β; Fig. 4). This result indicates that β amyloid peptide activates both kinases in vitro. It should be indicated that no changes were found when total GSK3β (Fig. 4) or phosphotyrosine GSK3 (not shown) was analyzed, indicating that β amyloid regulates specifically phosphoserine GSK3. In contrast to β amyloid, serine phosphorylation of GSK3α/β was not altered in the presence of somatostatin + β amyloid (Fig. 4) or cortistatin + β amyloid (not shown).
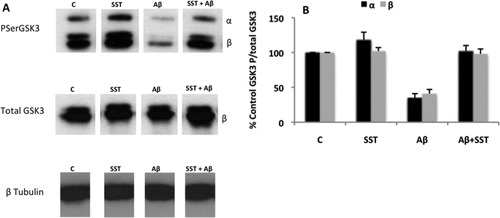
Effect of β amyloid and somatostatin on GSK3 phosphorylation. A: Western blots using specific antibodies against phospho-Ser21/9-GSK3α/β, total GSK3β, and β-tubulin. Protein extracts were obtained from primary cortical neurons incubated in the absence (control; C) or presence of 40 μM β amyloid, 40 μM somatostatin, or 40 μM somatostatin + 40 μM β amyloid for 24 hr. B: Quantitation of GSK3 phosphorylation at Ser21/9 for GSK3α and-β respectively. GSK3 phosphorylation data were normalized with total GSK3β level. β Amyloid induces a decrease in serine phosphorylation of GSK3α/β (Ser21/9); this decrease was reversed in the presence of somatostatin. Results in arbitrary units are mean ± SEM (N = 3).
Similar results were found for total GSK3β (Fig. 4) or phosphotyrosine GSK3 (not shown), under every condition. Thus, somatostatin (or cortistatin) prevents the increase of GSK3 activity induced by β amyloid, suggesting that somatostatin's (or cortistatin's) neuroprotective effect could be mediated through an increase in GSK3 serine phosphorylation.
DISCUSSION
We have found that β amyloid increases the expression of somatostatin and cortistatin in primary cortical neurons. In agreement with Geci et al. (2007), our results indicate that incubations with 20 μM or 40 μM β amyloid increase somatostatin expression in a dose-dependent fashion. Interestingly, our data describe, for the first time, how cortistatin expression was also significantly increased under the same conditions, but with saturation of the effect at 20 μM. Despite the high degree of homology exhibited by somatostatin and cortistatin, the different effects of β amyloid on their mRNA expression suggest that these neuropeptides could play a different role in the pathology of Alzheimer's disease. Also, the different responses to β amyloid on the expression of somatostatin or cortistatin could be the consequence of the presence of different subsets of neurons expressing somatostatin or cortistatin. Indeed, functional differences between somatostatin and cortistatin have been previously reported for the central nervous system, in that somatostatin (in contrast to cortistatin) enhances cortical excitability, increases REM sleep, and causes hypermotility (de Lecea et al.,1996).
Here we show that somatostatin and cortistatin expression is regulated through the increase of the levels of H3K4me3 and a small decrease in H3K9me3 associated with somatostatin and cortistatin gene promoters. Interestingly, the increase of somatostatin expression is greater than that of cortistatin, and it also correlates with a higher level of H3K4me3 for somatostatin compared with cortistatin samples. No increase in the expression of the other genes, tested as negative controls, was found. Our work is the first report describing the epigenetic regulation of somatostatin and cortistatin genes through histone methylation. Histone acetylation and DNA methylation have been previously described for different subtypes of human somatostatin receptors, supporting the idea of the importance of the epigenetic regulation in the whole pathway (Liu et al.,2008; Torrisani et al.,2008).
The epigenetic regulation in brain and in neurodegenerative processes is an expanding field of study. Implications of epigenetic regulation in Huntington's (Sadri-Vakili and Cha,2006), Parkinson's (Jowaed et al.,2010), Alzheimer's (Lee and Ryu,2010) diseases and normal processes of learning (Miller et al.,2010), among others, have been described. In addition, we found that somatostatin or cortistatin partially decreased β amyloid-induced toxicity. However, we cannot indicate that the endogenous release of somatostatin, or cortistatin, is sufficient to exert neuroprotective actions against β amyloid. Our preliminary experiments show that the proportion of GABAergic neurons (detected with anti-GABA antibody) in the presence of β amyloid peptide increases, whereas the proportion of total neurons decreases (detected with anti-NeuN antibody). These results suggest that GABAergic neurons (some of them expressing somatostatin or cortistatin) are more resistant to β amyloid insults. Similar results were found by Geci et al. (2007) when somatostatin cells were analyzed. Somatostatin's neuroprotective action has been previously reported (Forloni et al.,1997; Kumar,2008; Kiagiadaki et al.,2010), and it could be exerted through its binding to somatostatin receptors, which belong to the G-protein-coupled receptor family. In our model, the five somatostatin receptors (sst1–5) have been shown to be expressed, sst2 being the most abundant (Rubio et al.,2008). Cortistatin has also been shown to exert a protective effect against kainate-induced neurotoxicity (Braun et al.,1998). Here we have found a similar behavior of somatostatin or cortistatin in preventing amyloid peptide toxicity.
In addition to somatostatin receptors, growth hormone secretagogue receptor 1a (GHS-R1a; Deghenghi et al.,2001) and MrgX2 (Robas et al.,2003; Nothacker et al.,2005) have been proposed as selective cortistatin receptors. However, the functional significance of both receptors remains unclear. GHS-R1a has not been shown to be activated by cortistatin in vivo (Siehler et al.,2008), and MrgX2 receptor might bind additional peptides in vivo. Moreover, MrgX2 receptor is not expressed in human cerebral cortex and has not been found in rodents (Allia et al.,2005).
The β amyloid treatment also increases GSK3 activity, and this could be related to the epigenetic control of somatostatin and cortistatin genes, although we cannot exclude the possibility of parallel effects. Nevertheless, the possible relation between GSK3 and epigenetic control is not a new idea; GSK3 has been previously related to the epigenetic control of other loci (Popkie et al.,2010), but how are they connected, and their implications, is a new, exciting field that should be extensively investigated in the future.
In the present work, we have shown the relation β amyloid → GSK3 activation → H3K4me3. How GSK3 activation is related to H3K4me3 is still poorly understood. An interesting possiblity for being modified by GSK3 is SET-9, responsible for the methylation of lysine 4 of histone 3, which shows several GSK3 phosphorytable motifs (Lachner and Jenuwein,2002). If GSK3 is phosphorylating SET-9 or other COMPASS-like complex proteins should be investigated further.
GSK3 activation by β amyloid has been shown to promote cell death (Alvarez et al.,1999; Townsend et al.,2007; Magdesian et al.,2008). We also report that β amyloid regulates GSK3 through the phosphorylation of the phosphoserine residues present in GSK3α, (Ser21) and in GSK3β (Ser9) isoforms (Mukai et al.,2002; Soutar et al.,2010). According to our results, the expression of somatostatin or cortistatin could be a protective response to the toxic effect of β amyloid peptide (Forloni et al.,1997) in cortical neurons. Both somatostatin and cortistatin seem to protect neurons against β amyloid toxicity through inactivation of the enzyme GSK3 (by phosphorylation at serine 9 in GSK3β or serine 21 in GSK3α). Similar results were found when corticotropin-releasing hormone was studied in neurons in culture (Bayatti et al.,2003). In this case, the presence of corticotropin-releasing hormone was shown to prevent β amyloid-induced toxicity by inactivation of GSK3.
Finally, it should be indicated that somatostatin expression is regulated during development, decreasing its basal level with aging (Lu et al.,2004) and in Alzheimer's disease (Gahete et al.,2010). Thus, our work is compatible with a higher susceptibility of neurons to β amyloid toxicity in aging or in neurodegenerative disorders such as Alzheimer's disease, by downregulation of somatostatin. The specific function of somatostatin and cortistatin and its epigenetic regulation in these pathologies should be studied further to evaluate its therapeutic potential.




