Disposition of axonal caspr with respect to glial cell membranes: Implications for the process of myelination
Abstract
Neurofascin-155 (NF155) and caspr are transmembrane proteins found at discrete locations early during development of the nervous system. NF155 is present in the oligodendrocyte cell body and processes, whereas caspr is on the axonal surface. In mature nerves, these proteins are clustered at paranodes, flanking the node of Ranvier. To understand how NF155 and caspr become localized to the paranodal regions of myelinated nerves, we have studied their distribution over time in myelinating cultures. Our observations indicate that these two proteins are recruited to the cell surface at the contact zone between axons and oligodendrocytes, where they trans-interact. This association explains the early pattern of caspr distribution, a helical coil that winds around the axon, resembling the turns of the myelin sheath. Caspr, an axonal membrane protein, therefore seems to move in register with the overlying myelinating cell via its interactions with myelin proteins. We suggest that NF155 is the glial cell membrane protein responsible for caspr distribution. The pair act as interacting partners on either side of the axoglial contact area. Most likely, there are other proteins on the axonal surface whose distribution is equally influenced by interaction with the nascent myelin sheath. The fact that caspr follows the movement of the spiraling membrane has a direct affect on the interpretation of the way in which myelin is formed. © 2009 Wiley-Liss, Inc.
As the myelination program is activated, a glial cell establishes close contact with an axon. This activates signaling pathways that lead to profound changes in both cells. On the neuronal side, the most noticeable change is the phosphorylation of neurofilaments in the axon, expanding the spacing between them, and, as a consequence, the axonal caliber increases (de Waegh and Brady, 1991; de Waegh et al., 1992). On the myelinating cell side, contact with the axon signals complex cellular events, including cytoskeletal reorganization and extensive plasma membrane synthesis. The signals that trigger myelination and determine that some axons become myelinated whereas others of the same caliber are only ensheathed by Schwann cells (SCs) have only now begun to be understood. A membrane-bound form of neuregulin-1, expressed by axons, seems to determine that a SC activates its myelination program (Taveggia et al., 2005). Overexpression of neuregulin-1 type III results in a markedly thicker myelin sheath (Michailov et al., 2004).
The details of myelin assembly are still elusive. For both myelinating cell types [oligodendrocytes (OLs) and SCs], myelination demands a concerted effort of synthesis and targeting. Molecules mediating the early axon–glia recognition might later be down-regulated or redistributed so that in mature myelin they may not be abundant or they may no longer be expressed at the axoglial surface. Once myelination is completed, a very close physical contact between the axon and the myelinating cell persists at the paranodes. Therefore, in the mature nerve, the paranodal axoglial junction is the most likely domain at which axon–glia communication takes place. The first proteins identified to be localized specifically to the axoglial junction are caspr/paranodin on the axonal side (Einheber et al., 1997; Menegoz et al., 1997) and NF155 on the glial cell side (Tait et al., 2000). In the mature myelinating glial cell, NF155 clusters in apposition to axonal caspr at the paranodes. These proteins are important in the establishment and stabilization of the axoglial contact and the restriction of sodium channels to the nodal regions (Tait et al., 2000; Bhat et al., 2001; Pedraza et al., 2001).
By silver-enhanced immuno-EM, caspr has been found to distribute along the length of the paranodal septate junction, an electron-dense structure observed at the axoglial junction. This implies that caspr might contribute to the formation of the septa. However, in the rat spinal cord, the septa are not formed until P21, although caspr is already clustered at the junctions by P15 (Marcus et al., 2002). These data indicate that caspr is not a constituent of the transverse bands of the septa, but it may be crucial for the assembly of those structures. Clearly, several other players are involved in the organization of the septa, insofar as transgenic mice lacking caspr (Bhat et al., 2001), contactin (Boyle et al., 2001), NF155 (Sherman et al., 2005), MAG (Marcus et al., 2002), or Gal-C transferase (Dupree et al., 1998) all fail to assemble it properly.
Since Ben Geren's (1954) seminal discovery that PNS myelin is formed by the Schwann cell plasma membrane wrapped around the axon, several theories have been proposed to explain how this membrane wrap evolves into a perfect, almost crystalline structure. The most widely accepted explanation, based on studies of Bunge and collaborators (1989), is that the inner “tongue” of the SC advances around the axon, adding layers of plasma membrane. This “front” of new membrane has been assumed to be homogeneous along the length of the internode. In contrast, the idea that the formative process that generates the myelin sheath is far from being “smooth” was initially articulated 30 years ago. The uneven growth of membrane at different levels of the same internode, in both CNS and PNS (Webster, 1971; Knobler et al., 1976), was clearly demonstrated. However, this hypothesis was never generally accepted.
In this study, we observed how the glial cell first contacts the axon and how it influences the organization of axonal components. We observed NF155-rich, thin processes formed by the myelinating cell at early stages of axon–glial contact. We observed caspr-positive axonal “lines” that are likely to move in register with the leading edges of the thin membrane that the myelinating cell first extends along an axonal segment. We also detected marked focal increases of caspr labeling along axonal segments that were in contact with OL membrane expanses. This recruitment of caspr is mirrored by an increase in NF155 labeling on the OL side. Furthermore, caspr and NF155 codistribute from the time of initial axon–glial contact, until they reach their final distribution at the paranode.
The new observation that caspr, a protein located at the axonal surface, follows the movement of the coiling myelin membrane has led to a revision in our interpretation of the way in which myelin is formed. The close association between caspr and NF155, established at the time when the myelinating cell touches the axon, persists through the completion of myelination, indicating that this early interaction is never broken. This notion challenges the hypothesis that new membrane is added by the progression of the inner mesaxon around the axon, which would naturally require continuous breaking of the axon–glia bond.
MATERIALS AND METHODS
Reagents
Nerve growth factor (NGF) was from Roche (Indianapolis, IN). Matrigel from Collaborative Biomedical Products (Cambridge, MA). Neurobasal, B27, and L15 were from Gibco (Grand Island, NY). Fetal bovine serum (FBS) was from Hyclone (Logan, UT). Alexa-488-conjugated secondary antibodies were from Molecular Probes (Eugene, OR). Cy5- and rodamine X red-conjugated secondary antibodies were from Jackson Immunoresearch (West Grove, PA). Antiperiaxin polyclonal serum (rabbit) and antineurofascin serum were gifts from Drs. Peter Brophy and Diane Sherman, University of Edinburgh, Scotland. The other polyclonals were produced in our laboratory. Neurofilament-200 kD monoclonal was from Sigma (St. Louis, MO), Rip was from the Universityof Iowa Hybridoma Bank, and ZO-1 was from Chemicon (Temecula, CA).
Cell Cultures
Dorsal root ganglion (DRG) cultures were prepared following the procedure that we previously described (Svenningsen et al., 2003). Briefly, 12-mm glass coverslips inserted into four-well dishes and four-well permanox Lab-Tek slide chambers (Nunc) were coated with Matrigel (diluted 1:20 in L15). Spinal cords were removed from embryonic day 16 Sprague-Dawley rat embryos, and the attached DRG were removed and either plated directly onto the Matrigel-coated coverslips or dissociated by trypsin treatment before plating. DRG explants or dissociated DRG were plated in Neurobasal/B27/NGF. The cultures were kept in Nb/B27/NGF to allow endogenous SCs to repopulate the culture. Ascorbic acid (50 mg/ml) was added to the media to trigger myelination. Some cultures were treated with two cycles of antimitotic feeding and reseeded with pure SCs. Primary SCs were prepared from newborn rat sciatic nerves following the method described by Brockes and collaborators (1979) and immediately plated on purified DRG neurons.
Cell Transfection
The expression vector F-GFP (Clontech, Palo Alto, CA), encoding a membrane-bound form of GFP, was used for these experiments. Cultures were fed with myelinating medium (see above) on the day before transfection. Cells were transfected by lipofection (DOTAP; Roche). The expression of F-GFP was monitored in living cells.
Immunocytochemistry
For immunolabeling, cells were washed with PBS, fixed with 4% formaldehyde in PBS for 15 min, rinsed, and permeabilized with 0.1% Triton X-100 for 5 min. Cells were incubated with blocking solution (5% normal goat serum in PBS) for 1 hr, followed by 2 hr of incubation with the primary antibodies diluted in blocking solution, at room temperature. Species-specific fluorochrome-conjugated secondary antibodies diluted 1:400 were used for detection. Confocal microscopy was performed with a Leica TCS 4D or an Olympus X81 confocal scanning microscope. For double or triple staining, data from two or three channels were collected simultaneously. For the two-channel scans, the data from one channel is indicated in green, and data from the other channel is indicated in red. Yellow indicates colocalization. For the three-channel scans, the data from the third channel are represented in blue, and white indicates colocalization.
RESULTS
To have access to the early events of the axon–myelinating cell interaction, we performed our studies in myelinating cultures. These cultures recapitulate all the stages of the myelination program (Eldridge et al., 1989). The axons of the DRG neurons are susceptible to being myelinated by OLs as well as by SCs (Windebank et al., 1985). Therefore, we used the same neuronal type to study the effect of CNS and PNS myelinating cells on the organization of axonal compartments.
Initial Steps in Axoglial Contact and Wrapping
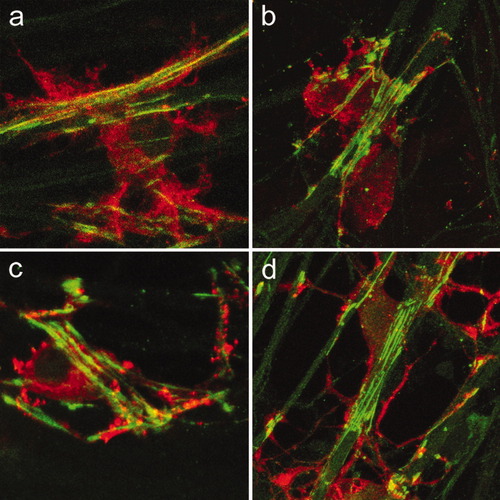
An early sign that a SC is committed to myelinate an axon is the deposition of basal lamina components. Basal lamina formation induces the polarization of the SC (Bunge et al., 1990). At this stage, actin filaments can be readily visualized within a thin SC membrane that spreads along the axon and turns or wraps around it, resembling focal regions where the SC “holds on” to the axon as myelination begins (Fig. 1a–c). Periaxin, a PDZ domain protein postulated to have an essential role in stabilizing the Schwann cell–axon unit in the myelinated fibers of the PNS (Scherer et al., 1995; Gillespie et al., 2000) was found throughout the SC plasma membrane. Because periaxin is the earliest marker for myelin membrane formation in the PNS, this labeling allowed us to observe early events in the investing of an axon by an SC (Fig. 1d–f).
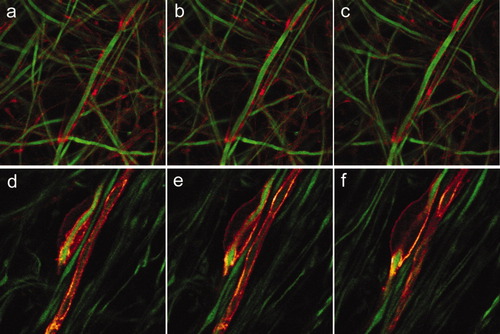
Initial steps in axon–SC contact and wrapping. a–c: Actin filaments (red) in the SC turn or wrap around the axon. a–c: Consecutive optical sections taken 0.5 μm apart. d–f: Periaxin (red) is localized throughout the SC plasma membrane. d–f: Consecutive optical sections. In all panels, axons are labeled with neurofilament-H Ab (green).
Previous studies from our laboratory (Fannon et al., 1995) have demonstrated that E-cadherin (Ecad) mediates adherens junctions in PNS myelin. Cadherins are transmembrane proteins that play important roles in cell adhesion. Ecad, through its cytoplasmic interactions with β- and α-catenins, regulates the actin cytoskeleton. Ecad was found distributed along the areas of contact between axons and SCs during early myelination, suggesting that cadherin-mediated adhesion between SC membranes might facilitate the initial steps of the wrapping process. Clusters of E-cad immunoreactivity appeared along axonal segments that had been invested by an SC (Fig. 2a, green). As myelination proceeded, Ecad labeling was almost continuous along the cytoplasmic loops of SCs (Fig. 2b,c). We also detected ZO-1 at this stage. ZO-1 is first seen along the axonal–SC surface (Fig. 2d). This protein later redistributed to paranodes and cytoplasmic loops (Fig. 2e,f). The distribution of ZO-1 was very similar to that of Ecad, so we investigated whether these two proteins colocalize. Optical sections of a SC double labeled with Ecad and ZO-1 showed their partial colocalization (Fig. 3a,b), particularly at the paranodal loops, where both proteins appear to be involved in stabilizing the loop-to-loop bond. Paranodal loops are held together at least in part by adherens junctions (Fannon et al., 1995). Tight junctions at the paranode may have a dual role of sealing the paranodal compartment and preventing lateral diffusion of transmembrane proteins restricted to the paranode. The presence of tight junctions in myelin has been observed by freeze-fracture studies in the CNS (Dermietzel, 1974; Mugnaini and Schnapp, 1974; Reale et al., 1975; Schnapp et al., 1976) as well as in peripheral myelin (Tetzlaff, 1978). ZO-1 is a component of these tight junctions. In the myelinating cell, tight junctions and adherens junctions are very close to each other, less than 50 nm apart (see drawing in Fig. 3c), so it is difficult to resolve both structures independently at the level of resolution of the light microscope (estimated to be 170 nm).
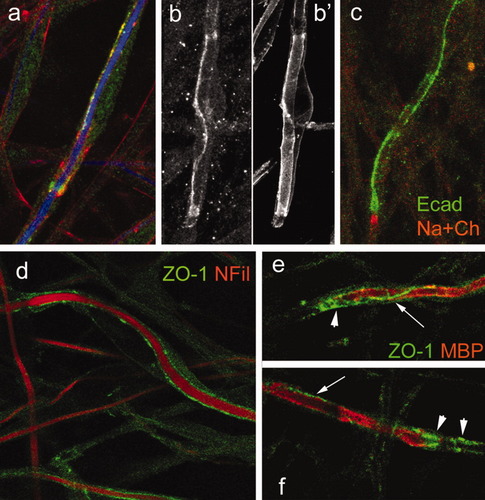
E-cadherin and ZO1 participate in early SC–axon interaction. a: Clusters of E-cad immunoreactivity (green) appear along the axonal segment (NFil, blue) that has been invested by an SC. Actin filaments are shown in red. b: As myelination proceeds, Ecad labeling is almost continuous along the cytoplasmic loops of the SC. The same cell, labeled with periaxin, is shown to highlight the full SC membrane (b′). c: Ecad labeling in more mature myelin is strong and continuous, mainly at the outer and inner mesaxon and paranodal loops (green). A heminode, labeled with Na+ channel antibody, is shown in red. d: ZO-1 (green) is first detected along the axonal–SC surface during early myelination. Axons are labeled with neurofilament-H antibody (NFil, red). e,f: As myelination proceeds, ZO-1 (green) is restricted to paranodes (arrowheads) and outer cytoplasmic loops (arrows). Myelin is labeled with MBP antibody (red).
Adherens junctions and tight junctions at the paranode. a,b: Single optical sections of a paranode and part of the internode double labeled with Ecad (green) and ZO-1 (red). Images in a and b were taken at two different optical levels. c: Drawing of a paranodal region. Adherens junctions (AJ) and tight junctions (TJ) are between paranodal loops. Axoglial junctions (2) are between paranodal loops and the axonal membrane; the boundary between paranodal loops and compact myelin (1) is also marked.
The myelin sheath produced in culture exhibits the same exquisite compartmentalization as in situ, with MBP-rich compact myelin and CNP-filled cytoplasmic channels (Fig. 4A, right panels). Interestingly, when the distribution of these markers was investigated at early stages, we found that MBP and CNP do not overlap, as if they were restricted to separate membrane domains even before myelin compartments are formed (Fig. 4A, left panel). This observation suggested that, when examining the early events of the myelination program using antibodies that recognize some of the myelin-specific proteins, we are not seeing the full membrane extent of SCs. For a complete view of the SC plasma membrane that is formed at early stages, we labeled SCs with membrane-bound forms of GFP (farnesyl-GFP or palmitoyl-GFP). The inner bilayer of the plasma membrane becomes labeled in this way, and the label resists removal by permeabilization. Importantly, cells labeled in this way retain the ability to myelinate. This approach permitted us to study the wrapping process in living cells, as well as after fixation and double labeling with antibodies.
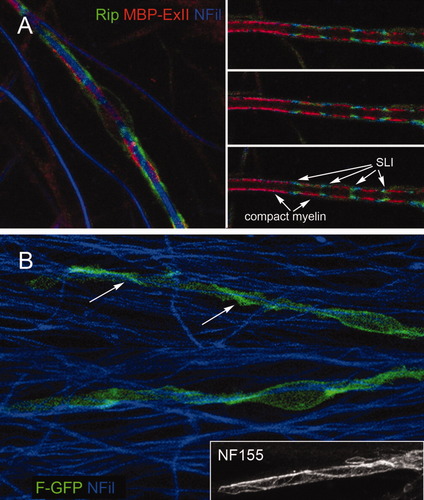
Early stages in the wrapping of axons by SCs. A: An inmature (left) and a mature (right) internode are labeled with Rip antibody (which labels CNP, green), ExII-MBP (red), and NFil (blue). The three panels at right are consecutive optical sections of the mature internode. The alternate pattern of compact myelin and cytoplasmic channels is evident. Schmidt-Lanterman incisures are indicated (SLI). Note that the segmental axonal labeling in the right panels (blue) is an artifact caused by limited antibody penetration through multiple membrane layers. B: Farnesyl-GFP-transfected Schwann cells (green) have extended along axons (blue) in a ribbon-like configuration (arrows). Inset: Three oblique turns of a Schwann cell ribbon labeled with NF155.
When analyzing the pattern of membrane deposition at early stages, we observed farnesyl-GFP-expressing SCs that have engaged axons and have extended their membranes along them in a ribbon-like configuration (Fig. 4B). The actin cytoskeleton is likely involved in the turning of membranes that generates this pattern. The ribbon-like image, characteristic of the early stages of the process, was observed with markers that labeled the whole plasma membrane (F-GFP) as well as with antibodies detecting a glia-specific protein, NF155 (inset in Fig. 4B). This wave of glial membrane growth is mirrored by the distribution of an axonal protein, caspr, strongly suggesting that the myelinating cell influences the organization of membrane components on the axonal surface.
The Myelinating Cell Organizes Membrane Compartments of the Axon
Compelling evidence showing the influence exerted by the myelinating cell on axonal membrane organization comes from observing the distribution of caspr during the initial axoglial interaction. Neurons cultured with OLs exhibit strong caspr accumulation on axonal segments touching OLs-membrane expanses, whereas the adjacent areas of the same axon show only diffuse caspr expression (Fig. 5). The recruitment of caspr seems to be an early response of the DRG neurons to the presence of OLs in the culture, because OLs in premyelinating stages are able to induce it. OLs labeled with Rip, a marker that is expressed earlier than MBP, have already recruited caspr to the contact zone (Fig. 5a,b). Rip labeling permits examination of the whole membrane expanse of the OL. More mature OLs, expressing MBP, also exhibit a strong capacity to recruit caspr (Fig. 5c,d). The accumulation of caspr at the contact zones between axons and glial membrane most likely reflects the redistribution of preexisting molecules on the axonal surface, although local up-regulation of caspr synthesis could not be ruled out.
Axonal contact with oligodendrocyte membrane expanses causes local accumulation of caspr on the axonal surface. Caspr labeling is green. The OL plasma membrane is labeled with Rip (a,b) or with MBP (c,d) antibodies (red).
The Striking Accumulation of Axonal Caspr Along the Axon–Glia Interface Is Mirrored by NF155 on the Glial Surface
DRG neurons cultured with OLs were immunolabeled with caspr and NF155 (Fig. 6). Although there is NF155 all over the OL plasma membrane, there is an enrichment of this protein at the zones where caspr has been recruited, suggesting that NF155 could be the glial protein responsible for recruiting caspr to the contact sites between axons and glia.
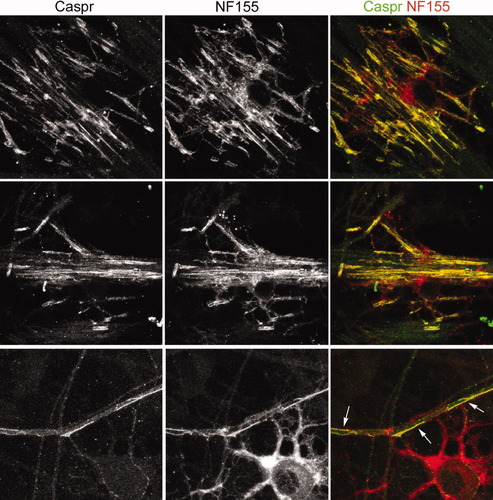
Initial steps in axon–OL contact and wrapping: NF155 accumulates on the OL plasma membrane surface with an identical distribution to caspr on the axonal surface. Two myelinating OLs are shown (upper and middle row). Columns marked caspr and NF155 show the single labeling of these proteins. The panels in the last column show colocalization of axonal caspr (green) and glial NF155 (red). Lower row: Detail of OL processes engaging an axon. Caspr labeling is intense at these contact points (arrows).
It is important to note that premyelinating SCs do not have this effect on axonal caspr. A main difference between myelinating glia of the PNS vs. CNS is that SCs do not express myelin-specific proteins before they have activated its myelination program in response to axonal signals. Conversely, OLs synthesize myelin proteins and target it to membrane expanses even in the absence of axons. Premyelinating SCs do not have NF155 to be recruited to the cell surface in response to axonal contact. Because of this difference, DRG neurons cultured with SCs under nonmyelinating conditions do not recruit caspr to the contact surface. This will happen at a later stage, when SCs invest axons with its plasma membrane and start to synthesize myelin-specific proteins (see Fig. 7).
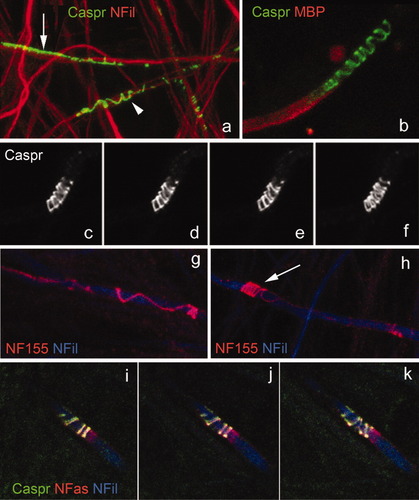
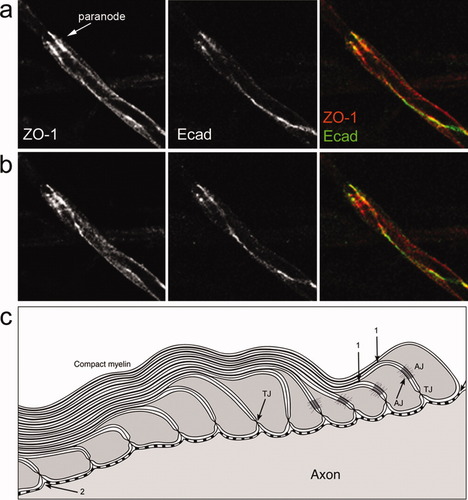
Caspr labeling on the axon reflects the overlying turns of the myelin sheath. a,b: At early stages of the axon–SC interaction, caspr (green) appears as a line on the axonal surface (a, arrow). As myelination proceeds, the caspr line changes into a loose spiral (a, arrowhead, and b). The red labeling in a corresponds to neurofilaments and in b to MBP. c–f: Detail of a paranode immunolabeled with caspr. c–e: Single optical sections taken at different focal planes. f: 3-D reconstruction of the paranode (12 consecutive optical sections were stacked). g,h: NF155 (red) forms the loose coils already described for caspr (g) and later clusters at the paranode (h, arrow). i–k: Optical sections of a paranode labeled for caspr (green) and NF155 (red) reveal that these two proteins move in perfect register with each other. [Note that there are two neurofascin isoforms in the nervous system, a glial one (NF155), which localizes to the paranodes of mature myelin segments; and an axonal one (NF186), which localizes to the node of Ranvier and axons initial segments. By using an antibody that recognizes both isoforms (NFas), we highlighted the node of Ranvier too.]
These observations compelled us to study the distribution of caspr and NF155 throughout the progression of myelination in the axon–SC unit. At the onset of myelin formation, when the axon and the myelinating cell have just established their first contact, caspr appears as a line on the axonal surface, along the area of contact with the myelinating cell (Fig. 7a, arrow). As myelination proceeds, the caspr line of immunoreactivity changes into a loose coil (Fig. 7a, arrowhead, and b). A detail of a paranode immunolabeled with caspr is depicted in Figure 7c–f. The distribution observed here strongly indicates that the axonal protein moves in register with the myelinating cell, because the coils that are labeled with caspr correspond to the leading edges of the growing myelin. A most likely candidate to mediate this interaction between axonal caspr and the myelinating cell is NF155 at the glial cell surface. We found that caspr and NF155 codistribute at all developmental stages. NF155 forms the loose coils already described for caspr and finally clusters at the paranode (Fig. 7g,h). Optical sections of a paranode double labeled for caspr and NF155 show that these two proteins move in perfect register with each other (Fig. 7i–k). Based on their identical distribution at all developmental stages, our data add weight to the conclusion from other studies that NF155 is a binding partner for caspr (Charles et al., 2002), and we further suggest that NF155 is one of the glial molecules responsible for recruiting caspr to the axonal surface. This implicates NF155 and caspr in axoglial recognition and early communication, as well as in the establishment of membrane compartments or domains, where molecules are restricted from freely diffusing in the lateral plane of the axonal membrane (Pedraza et al., 2001).
Caspr Localizes at Interjunctional Zones of the Paranodes
According to the data described above, caspr does not seem to be a major component of septate densities in the axoglial junction as has been postulated (Einheber et al., 1997; Menegoz et al., 1997). Instead, the caspr-contactin-NF155 complex may be a component of intramembranous particles (IMPs) at interjunctional zones of the paranode, structures described in early freeze-fracture electron microscopy studies (Rosenbluth, 1976). In fact, the interjunctional distribution of caspr correlates well with its helical pattern (Fig. 8a), configured by the overlying turns of the paranodal membrane (Fig. 8c). Its interjunctional distribution was demonstrated by immunocytochemistry as a dynamically migrating and compressing axonal coil, due to its restriction during glial membrane expansion and progression around the axon. These observations provide new insights into the assembly mechanism of myelin sheaths, in which the expansion of lamellar glial membranes during myelinogenesis results in the trafficking of axoglial junction proteins to the lateral (paranodal) domains while retaining axon–glia interaction.
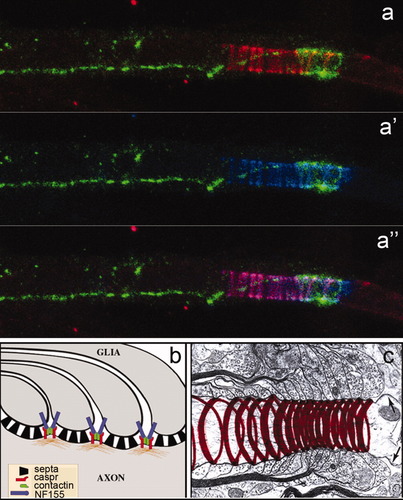
Model in which the axonal protein caspr flanks, but is not a constituent of, axoglial septa. a: A paranode double labeled for caspr (red) and Ecad (green). Bands of Ecad reactivity can be seen between the caspr spirals. This distribution corresponds to the loop-to-loop junctions. Ecad-mediated adherens junctions are more concentrated at the outermost loops. a′: An identical labeling pattern was obtained with NF155 antiserum (blue). a′′: Caspr and NF155 colocalization (purple) agrees with the prediction that NF155, caspr, and contactin form an interacting complex that spans both glial and axonal membranes (b). c: Position of a “mature” caspr spiral flanking a node of Ranvier (arrows).
DISCUSSION
Our data suggest that there is no continuous front of membrane growing and spiraling around the axon at the axon–glial interface but rather an outward flow of membrane expanding laterally from a central compartment, closer to the cell body. The fact that axonal caspr reflects the movement of the wrapping glial membrane has altered our view of the way in which myelin is formed. The close association between caspr and NF155 that is established at the time when the myelinating cell touches the axon persists through the completion of myelination, indicating that their original interaction is never broken and calling into question the proposal that new membrane is added by advancing the inner mesaxon around the axon. However, the turnover of caspr and NF155 during the process of myelination is not well understood. Thus, one cannot exclude the possibility of significant turnover in individual components without disrupting an extended interaction between populations of molecules. Whether the same caspr and NF155 molecules are interacting over the course of the process, or it is a changing pool of molecules that maintain a physical and functional relationship over the course of myelination, the final result would be the same.
EM studies of CNS and PNS myelination showed a variation in the length and number of turns around the axon at different levels along the same internode (Webster, 1971; Remahl and Hildebrand, 1990a, b). These data strongly indicate that membrane advancement occurs at different rates within the internode rather than as a uniform advancement of a cytoplasmic sheet.
By reconstructing serial EM sections of myelinating OLs, Knobler et al. (1976) showed that adjacent membrane segments in the same sheath often expand in opposite directions. Transient microtubule-containing protrusions, arising locally from the lateral edges of the myelin membrane, were found to extend along axons. These were termed “vermicular processes,” and we conclude that these are the same ribbon-like structures that we identify in this work. These membrane segments eventually merge and give way, most likely by cytoskeletal remodeling, to synchronized lamellipodial expansion and elongation along the axon to generate a myelin sheath (Knobler et al., 1976). Mice with reduced levels of stathmin-1, a microtubule-destabilizing protein expressed by neurons and OLs that is targeted to paranodes, exhibit vermicular-like processes beneath myelin sheaths, suggesting that aberrant regulation of cytoskeletal dynamics might cause the persistence of those structures in mature myelin (Southwood et al., 2004). Paranodal markers in this mutant exhibit a less compact pattern than in the wild type, with caspr coils winding into the juxtaparanodal area. Interestingly, as would be predicted from our studies, NF155 still lines up in perfect register with caspr.
When interpreting the ribbon-like structures that we detected in the forming myelin membrane, it is relevant to mention the contribution that the high content of galactosylceramides could have in driving their formation. Galactosylceramides and their sulfated relatives, i.e., sulfatides, are normally enriched in myelin. These sphingolipids are thought to provide structural stability to membranes by way of their strong intermolecular interactions and to impart and maintain the curvature and cylindricalshape of certain membranes (Curatolo and Neuringer, 1986; Maggio et al., 1988). The tendency of naturally occurring galactosylceramides to form helical ribbon-like structures was originally noted in the cells of patients afflicted with globoid cell leukodystrophy (Yunis and Lee, 1970). Transmission EM, freeze-fracture, and atomic force microscopy studies showed that, on cooling, aqueous dispersions of Gal-C and Glu-C form helical ribbons and nanotubular structures (Kulkarni et al., 1999).
Anchoring proteins bridging transmembrane proteins to the actin cytoskeleton could allow the transduction of mechanical forces across the cell membrane, necessary for the growth and expansion of the myelin sheath. This wave of glial membrane growth is mirrored by the distribution of caspr, an axonal protein, demonstrating that the myelinating cell influences the organization of membrane proteins on the axonal surface.
From detailed examination of the caspr distribution, it is clear that the perfect caspr coil that surrounds the axon is in register with the individual loops that constitute the leading edges of the growing myelin membrane (Figs. 7f, 8). This indicates that caspr, an axonal protein, moves in register with the myelinating cell membrane.
Further evidence showing the close interaction between caspr and NF155 comes from studies performed in Shiverer mutant mice (Tait et al., 2000). At a developmental stage in which NF155 has already clustered at the paranode of wild-type mice (where it colocalizes with caspr), it is largely retained in the Shiverer's OL cell body and distributes at ectopic sites along the axon, where it still colocalizes with caspr. Therefore, although these nerves are unable to establish normal axoglial junctions, caspr was not diffusely distributed along the axon but instead was concentrated in discrete patches where it colocalizes with NF155, indicating that caspr and NF155 were still apposed. Furthermore, although the main defect of the shiverer mutant is the lack of compaction of the myelin sheath, because of the absence of MBP, Na+ channels are clustered in irregular, elongated zones, and paranodal axoglial junctions are not formed (Rosenbluth, 1981). It is tempting to speculate that the disruption of the molecular fence that we proposed exists at the compact myelin–paranodal loop boundary (Pedraza et al., 2001) causes the diffusion of NF155, normally concentrated at the paranode, into the defective myelin sheath. As a consequence, caspr is not properly organized at the axonal surface, and, because the axoglial interactions are not properly established, the ion channels are not restrained within their normal compartments.
Studies in neurofascin null mice provided strong evidence for the role of NF155 in organizing the paranode (Sherman et al., 2005). In neurofascin null mice, lacking both NF isoforms, neither paranodal adhesion junctions nor nodal complexes are formed. Transgenic expression of NF155 in the myelinating glia of NF−/– nerves rescues the axoglial adhesion complex by recruiting the axonal proteins caspr and contactin to the paranodes. This data strongly suggest that NF155 orchestrates the assembly of the paranodal structure.
Based on our observations, we propose that caspr may not be a major component of the transverse bands of the axoglial junction but rather may distribute at interjunctional zones that flank the septate densities, corresponding to the “caspr helix” observed during myelinogenesis. This finding suggests that caspr and its binding partner NF155 may function as a signaling complex to trigger the formation of the axoglial septate densities. Actually, the junctional indentations appear to be sites of particle exclusion, bordering particle accumulations at the nonjunctional clefts (Tao-Cheng and Rosenbluth, 1982). Transverse bands are a late-developing structure in the rodent paranode (Tao-Cheng and Rosenbluth, 1983). Examination of paranodal regions in the spinal cord at P15 reveals that paranodal loops are able to adhere to the axolemma before transverse bands spanning the intercellular space appear (Marcus et al., 2002). This suggests that transverse bands function to secure the paranodal axoglial attachments once they have been established but that they are not used during early interactions at the paranode.
We demonstrate here that some molecules involved in the early recognition between axons and glial cells keep their bond along the process of myelination. The accumulation of molecules interacting with each other on either side of the axoglial interface creates a diffusion barrier at this level that restricts large axonal membrane proteins to the heminodes, not allowing them to diffuse back beneath the area that has been already covered by the myelinating cell (Pedraza et al., 2001). Our interpretation of the loose coil evolving into a paranodal cluster is that the coil represents the leading edges of the growing myelin membrane, advancing to cover the axonal segment that an individual cell is “dedicated” to myelinate. Once the length of the future internode has been defined, the growing membranes cannot expand more laterally because of the restriction imposed by the node of Ranvier. As a consequence, the leading edges that are still advancing draw closer to each other, and the loose coil becomes so tight that the individual turns are almost not discernible by light microscopy.
Acknowledgements
The authors acknowledge support from Rio Tinto Alcan, The Molson Foundation, and a Center of Excellence Award (2007) to the MNI from the Government of Canada.




