Sexual dimorphism of oligodendrocytes is mediated by differential regulation of signaling pathways†
This paper is dedicated to Steve Pfeiffer, who contributed so much to our understanding of oligodendrocyte development. This research was submitted in partial fulfillment for the PhD dissertation of M.S.
Abstract
Sexual dimorphism of white matter has not been considered important, the assumption being that sex hormones are not essential for glial development. We recently showed exogenous hormones in vivo differentially regulate in male and female rodents the life span of oligodendrocytes (Olgs) and amount of myelin (Cerghet et al. [2006] J. Neurosci. 26:1439–1447). To determine which hormones regulate male and female Olg development, we prepared enriched Olg cultures grown in serum-free medium with estrogen (E2), progesterone (P2), and dihydrotestosterone (DHT) or their combinations. P2 significantly increased the number of Olgs in both sexes, but more so in females; E2 had minor effects on Olg numbers; and DHT reduced Olgs numbers in both sexes, but more so in females. Combinations of hormones affected Olg numbers differently from single hormones. The change in Olg numbers was due to changes not in proliferation but rather in survival. P2 increased pAKT by many-fold, but MAPK levels were unchanged, indicating that activation of the Akt pathway by P2 is sufficient to regulate Olg differentiation. DHT reduced pAkt in both sexes but differentially increased pMAPK in males and decreased it in females. Stressing Olgs reveals that both sexes are protected by P2, but females are slightly better protected than males. Females always showed greater differences than males regarding changes in Olg numbers and in signaling molecules. Given the greater fluctuation of neurosteroids in women than in men and the higher incidence of multiple sclerosis (MS) in women, these sexually dimorphic differences may contribute to differences in male and female MS lesions. © 2008 Wiley-Liss, Inc.
Sexual dimorphism has been demonstrated in gray matter, particularly the hypothalamus (Mong and McCarthy, 1999) and hippocampus (Means and Dent, 1991; Zaidel et al., 1994). While studying neurons, researchers noted differences in the numbers of myelinated and unmyelinated axons between sexes (Mack et al., 1995). Studies have shown an increase in the number and density of myelinated axons in the splenium of male rat corpus callosum compared with that of females (Kim et al., 1996). Observations from Skoff's laboratory (Ghandour and Skoff, 1988; Knapp et al., 1990) hint that sexual dimorphism also exists between the number of Olgs and myelin protein expression. More recently, we demonstrated that Olg density and myelin basic proteins are greater in males compared with females at all ages (Cerghet et al., 2006). Our studies also showed greater proliferation and Olg death in females, indicating that Olgs in females have a shorter life span and a higher turnover rate compared with males. Interestingly, the profile of Olgs in castrated male mice gradually matched the profile of females, indicating that exogenous steroid hormones, presumably testosterone, regulated Olg number. However, the CNS also synthesizes neurosteroids from cholesterol, suggesting an interplay between exogenously and endogenously produced steroids (Stoffel-Wagner, 2001; Schumacher et al., 2004).
Very few studies have examined the direct effect of these neuroactive steroids on glia vs. indirect effects caused by neuronally mediated activation of glia. Testosterone enhanced the excitotoxic damage caused by treating Olgs with AMPA or kainic acid (Caruso et al., 2004), whereas 17β-estradiol protects Olgs from cell death (Takao et al., 2004). E2 differentially activated the mitogen-activated protein kinase (MAPK) pathway in male and female astrocytes, and male astrocytes exhibited less death than females (Zhang et al., 2002). These studies clearly indicate that sex hormones have major influences on glial number, proliferation, and morphology. Activation of signaling cascades by sex hormones in neurons has been examined in numerous studies (Behl, 2002) but is poorly understood for glia. More importantly, the question of whether hormones differentially activate signaling pathways in Olgs that lead to functional differences of male and female Olgs has not been investigated. These neurosteroids function via genomic (De Nicola et al., 2003; Patchev et al., 2004) or rapid nongenomic mechanisms (Beyer and Hutchison, 1997) that utilize various signaling pathways (Bhat and Zhang, 1996; Azcoitia et al., 1999). We focused on the protein kinase B (Akt), MAPK, and mammalian target of rapamycin (mTOR) pathways, because these pathways are activated by hormones in most cells and are present in Olgs (Flores et al., 2000; Keshamouni et al., 2002).
Our studies show striking differences between males and females in regard to Olg survival and signaling pathways in response to hormones, confirming our in vivo studies showing that females show greater changes than males in regard to proliferation and death. From a practical perspective, they demonstrate that sex is a critical variable to be considered in planning and evaluating in vivo and in vitro studies of Olg development.
MATERIALS AND METHODS
Two- to three-day-old B6CBA male and female mice (Jackson Laboratories, Bar Harbor, ME) were used for all cell culture experiments. They were sexed based on the presence or absence of a blackened zone in males and females, respectively, between genitalia and base of the tail. Male mice also have an obvious greater anogenital distance compared with females (Vandenbergh and Huggett, 1995).
Enriched Olg Cultures
Primary glial cultures were prepared via routine laboratory procedures (Knapp et al., 1987) from male and female mouse brains that were separately grown in 100-mm Petri dishes (two brains per dish) that were coated with 100 μg/ml polylysine (Sigma, St. Louis, MO). They were grown in DMEM (Gibco-Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS; Gibco) and 1% antibiotic and antimycotic drugs (Gibco-Invitrogen) for 9–11 days, at which time the cells became confluent. Then, the upper layer of cells containing mostly Olg precursors and Olgs was dislodged by gently blowing the cells with media using a 10-cc syringe attached to a 21-gauge needle. The cells were collected with the media and then plated onto another uncoated 100-mm Petri dish to allow microglia and astrocytes to adhere to the dish. After 30 min, floating cells, which are mostly Olgs precursors and Olgs, were collected with a 5-cc pipette into a 15-cc centrifuge tube, spun down, and counted using a hemocytometer; then, equal numbers of cells (about 7,000 cells/coverslip) from male and female Olgs were plated onto 20 μg/ml polylysine-coated 12-mm coverslips. From one Petri dish of primary cultures, we obtain enough Olg progenitors/Olgs for 24 coverslips. These cultures contain more than 95% Olgs (determined by double immunostaining for A007 and glial fibrillary acidic protein) between 3 and 5 days, the time frame used for the studies. Because phenol red is believed to have estrogenic properties, phenol red-free medium (Gibco-Invitrogen) was used for all experiments. Olg cultures for all experiments were grown in DMEM without phenol red or serum but supplemented with selenium (10 nM), transferrin (50 μg/ml), insulin (5 μg/ml; all from Sigma, St. Louis, MO), and ABAM (Gibco-Invitrogen). After 1 day, cultures were treated with 1, 2.5, 5, 10, 50, 100, and 500 nm concentrations of E2, P2, or DHT (Sigma); the number of cells in the coverslips was counted after 3 days of treatment with the hormones (see diagram in Fig. 1), by immunostaining with A007, a marker for postmitotic immature (A007+/PLP–) and mature Olgs (A007+/PLP+) (Bansal et al., 1992).
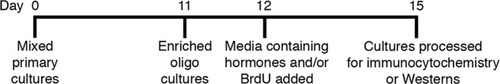
Diagram illustrates timeline for preparation of enriched Olg cultures and hormonal treatment.
Immunocytochemistry
Olgs were immunostained live with the A007 marker and then fixed with 70% ethanol. Expression was visualized with a donkey anti-mouse IgM Texas red secondary (1:500; Gibco-Invitrogen). Nuclei were stained with bisbenzamide (1:1,000 dilution). Only Olgs with healthy, dispersed nuclear chromatin were counted along the two maximum diameters of the coverslip. Olgs with clumped chromatin or absent nuclei (a frequent occurrence) were not counted.
For cell proliferation studies, bromodeoxyuridine (BrdU; 10 nM) was added with hormones to coverslips, and cells were immunostained live for A007 at 2, 24, or 72 hr as described above; they were processed for BrdU immunostaining (Yang and Skoff, 1997; Saluja et al., 2001) using a monoclonal IgG (1:100; Beckton Dickinson, Franklin Lakes, NJ) and visualized with an anti-mouse Alexa green secondary (1:500; Gibco-Invitrogen). For caspase immunocytochemistry, a polyclonal antibody against cleaved caspase-3 (1:100; Cell Signaling Technology, Beverly, MA) was visualized with an anti-rabbit Alexa green secondary antibody (1:1,000; Gibco-Invitrogen).
Microscopy
A007+ Olgs were counted along the two longest diameters of the coverslip under a ×20 objective and ×10 eyepiece with a Leica DM IRB phase fluorescent microscope. The number of Olgs counted using this method covers 70% of the area of the coverslip. Results are expressed as a percentage to the male control using the formula: percentage of cells = (number of cells in treated group/number of cells in male control) × 100.
To determine generation of new Olgs, both BrdU+/A007+ and BrdU–/A007+ cells were counted along the two maximum diameters of the coverslip under a ×40 objective and ×10 eyepiece. Results were expressed as percentage of proliferating Olgs = (number of BrdU+ and A007+ cells/total number of A007+ cells) × 100. The total number of A007+ cells along with cleaved caspase-3+ cells was counted and the percentage of dying cells calculated using the formula given above. Because large numbers of A007+/cleaved caspase-3+ cells are located within the boundaries of the two maximum diameters and it was necessary to use a ×40 objective to visualize chromatin clearly, 20 random fields were counted for each treatment group, and the experiment was repeated twice.
Western Blotting
Enriched Olg cultures were prepared from primary glial cultures as described above but were replated in polylysine-coated T25 flasks. Hormones were added after 1 day in culture. Three days after treatment with the hormones, protein was extracted from cells by homogenization in a lysis buffer [50 mM Tris, pH 7.4, 1 mM dithiothreitol (DDT), and 0.1 mM EDTA] with protease inhibitors (Zhang et al., 2004) and denatured by heating to 95°C for 10 min. Protein concentration was measured by using an Eppendorf biophotometer (Eppendorf, Westbury, NY). One hundred micrograms of protein was loaded into a 10% polyacrylamide gel, followed by electroblotting onto a PVDF transfer membrane. Blots were incubated with pAkt, Akt, pMAPK, MAPK, pmTOR, or mTOR, all at 1:1,000 dilution except for GSK3β (Cell Signaling), followed by incubation with anti-mouse or anti-rabbit antibodies conjugated to horseradish peroxidase (1:5,000; Jackson Laboratories) and were developed with the Chemicon chemiluminescence kit (Chemicon, Tamecula, CA). All blots were stripped and reprobed for β-actin (1:5,000; Sigma), and optical density analysis was performed in Image Quant (Molecular Dynamics, Sunnyvale, CA).
Inhibition of PI3 Kinase
Fifty micromolar LY 294002 (Cell Signaling Technology) was added to enriched Olg cultures 1 hr before adding the hormones and throughout the duration of hormonal treatment (4 days). After 4 days, coverslips were immunostained for A007, and the number of Olgs was counted along the two longest diameters of the coverslip. Results were expressed as a ratio to male control.
Statistical Analysis
One-way ANOVA was used for Figures 3-6 and Student's t-test for Figures 7-11 and 14.
RESULTS
Density of Olgs in Control Cultures
In virtually every experiment (Figs. 3-5, 12, 13), the number of Olgs derived from females was approximately 10–15% greater than those derived from males 4 days after preparing the enriched cultures. The number of Olg progenitors (mitotic) and Olgs (postmitotic) plated onto coverslips was the same for both sexes and was carefully controlled (see Discussion for explanations).
Effects of Hormones on the Density of Olgs
We quantified the number of Olgs in enriched cultures that were treated for 3 days with different concentrations of P2, E2, or DHT (Figs. 2, 3). Three days after hormonal treatment, the number of Olgs counted per coverslip ranged from about 125 to 320 (see Materials and Methods), providing adequate numbers of cells for reproducible cell counts and statistics. P2 increased the number of Olgs for both males and females at 2.5 and 5 nM concentrations (Fig. 3A). With 2.5 nM P2, the numbers of Olgs significantly increased by about 40–50% in males and 90–100% in females compared with untreated male controls. At this concentration, the increase in Olgs in females compared with males in the group was significant. At 5 nM P2, Olgs increased more than twofold in both males and females, with females having 20% more Olgs than males in the group. At 10 nM and higher P2, the number of Olgs returned to control or lower numbers.
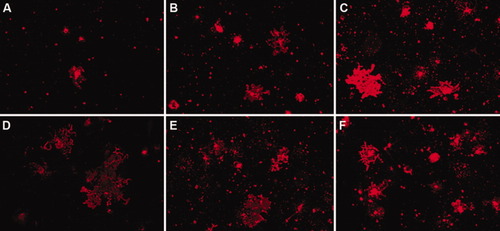
A007 immunostaining of enriched Olg cultures from controls (A,D) or after treatment with 2.5 nM (B,E) or 5 nM (C,F) P2 from either males (A–C) or females (D–F). Mature Olgs elaborate large membrane sheets that resemble unfurled myelin sheaths. Differences in Olg numbers between males and females are not noticeably different in these representative low-magnification pictures.
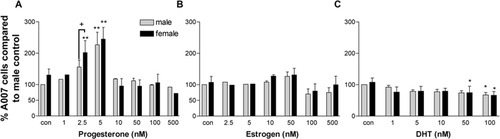
Quantification of the A007+ cells in cultures treated with different concentrations of P2 (A), E2 (B), or DHT (C) derived from males and females. P2 at 2.5 nM shows increases in the number of Olgs in females, and 5 nM P2 shows increases in both males and females. DHT shows a modest reduction in the number of Olgs. The data are expressed as a percentage change compared with male control (male control is set at 100%). The data are an average of two or three experiments, and in each experiment two or three coverslips were counted for each treatment condition. Values represent mean and SEM. *P < 0.05, **P < 0.01 compared with male control. †P < 0.05 between males and females within same treatment group.
In contrast to P2, E2 had little effect on the numbers of Olgs at doses below 50 nM in both sexes but appear to be toxic above 50 nm (Fig. 3B). Although E2 had little effect on numbers of A007+ cells, we observed clusters of 10–100 cells that resemble oligospheres (Vitry et al., 1999). The number and size of these clusters in cultures dramatically increased when treated with 50 nm E2 in females, indicating that E2 may differentially regulate proliferation of neural precursors between sexes (data not shown).
The male hormone DHT reduced the numbers of A007+ Olgs by 10–35% at all concentrations tested compared with the controls in both sexes. This effect, however, is more pronounced in DHT-treated females compared with female controls (Fig. 3C). Because T4 may be converted to E2, obfuscating interpretation of data, we used DHT that is not converted to E2, permitting evaluation of its direct effects on Olgs.
Effect of Hormonal Combinations on Olg Density in Males and Females
Because P2 synergizes with E2 to regulate the ratio of proliferation to apoptosis (Seeger et al., 2005) by altering the levels of P2 receptor immunoreactivity in the brain (Dufourny et al., 1997), we examined the effects of hormonal combinations in our culture system. In contrast to the administration of P2 concentrations that led to Olg increases, the same concentrations of P2 (2.5 and 5 nM) combined with 10 and 50 nM E2 caused a reduction in the number of Olgs in both sexes. The effect was generally greater in females, and 5 nM P2 and 10 nM E2 produced a highly significant decrease in Olg number in females. Lowering the concentration of E2 to 1 and 5 nM with 2.5 and 5 nM P2 caused a slight increase in Olg numbers (Fig. 4A). These results demonstrate that regulation of Olg numbers is exquisitely sensitive to exogenous hormonal concentrations. When DHT was added together with P2, the positive effects of P2 were abolished and, instead, Olg density was reduced 25–30% when treated with 10 nM DHT and 5 nM P2. However, P2 reduces the negative effect of 1 nM DHT concentration in both sexes, although this was not statistically significant (Fig. 4B).
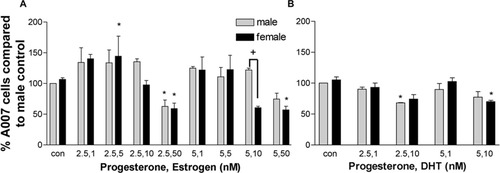
Percentage difference in the number of A007+ cells in cultures treated with different combinations of P2, E2 (A) and P2, DHT (B). Data are expressed as a percentage compared with the male control. Data are an average of two or three experiments, and in each experiment two or three coverslips were counted for each treatment condition. *P < 0.05 compared with male controls. †P < 0.01 between males and females within same treatment group.
Regulation of Proliferation and Cell Death of Olgs by Hormones
The increases or decreases in Olg numbers in response to hormonal treatment could be due to an alteration in proliferation and/or cell death. To determine influence of neuroactive steroids on proliferation, cultures were treated with hormones in the presence of BrdU. Coverslips were immunostained at 2, 24, or 72 hr after treatment for BrdU and A007. Results are expressed as a percentage to the total number of Olgs. With P2 treatment, the percentage of BrdU+ Olgs at any time point was the same as that in controls (Fig. 5A,B). The number of Olgs that incorporated BrdU increased from 5–10% at 2 hr to about 30–40% at 24 hr in both P2 and controls. The low percentage of BrdU+/A007+ cells at 2 hr is predicted because cells are in the S or G2 phase, and A007+ cells are mainly postmitotic (Knapp and Skoff, 1991). The number of BrdU+ Olgs remained the same from 24 to 72 hr in both sexes, indicating that few A007– Olgs proliferated and differentiated into A007+ Olgs in this 48-hr interval. With 5 nM P2 treatment, the total number of Olgs significantly increased at 72 hr compared with the controls, implying that P2 increases the survival of differentiated Olgs (Fig. 5B).
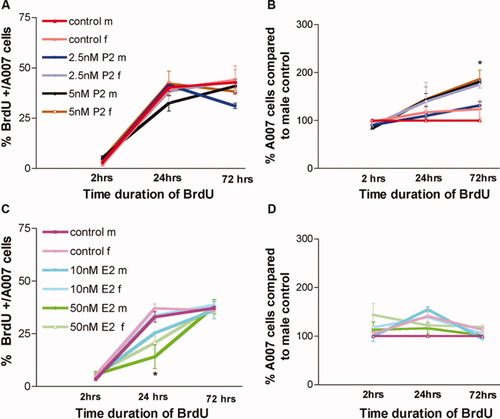
Percentage of BrdU+/A007+ cells and total A007+ cells in enriched Olg cultures treated with different concentrations of P2 (A,C) and E2 (C,D) after 2, 24, and 72 hr of hormonal treatment. Data are the average from three coverslips at each time point from three experiments. *P < 0.05.
With E2, there was a significant reduction in the number of BrdU+ Olgs in response to treatment with 50 nM E2 at 24 hr in both males and females compared with controls. This difference was not noticed at 2 hr and was abolished at 72 hr, indicating that E2 at this concentration might delay the differentiation of Olgs, because we counted only the cells that have proliferated and differentiated at each time point. No significant changes were noted in the total number of Olgs with E2 at any time point tested (Fig. 5C,D). This data set matches the data showing that E2 has little effect on the total Olg numbers (Fig. 3).
Because neuroactive steroids tested did not alter BrdU incorporation, we next looked at differences in cell death in response to treatment with hormones. With 2.5 and 5 nM P2, the number of cleaved caspase-3+ cells in both sexes was reduced by 10–15% compared with controls. This finding indicates that the increase in the density of Olgs in response to P2 is due mainly to its effect on survival of Olgs (Fig. 6A). In contrast to P2, E2 produced no significant changes in Olg death, except for a mild reduction in the number of dying Olgs in females with 10 nM E2 (Fig. 6B). DHT treatment increased the number of dying Olgs by about 15–30% at all concentrations in both males and females. Moreover, the response with 10 nM DHT was significantly higher in the females compared with males (Fig. 6C). The increases or decreases in the numbers of dying Olgs are inversely proportional to the decreases and increases in Olg numbers, respectively.
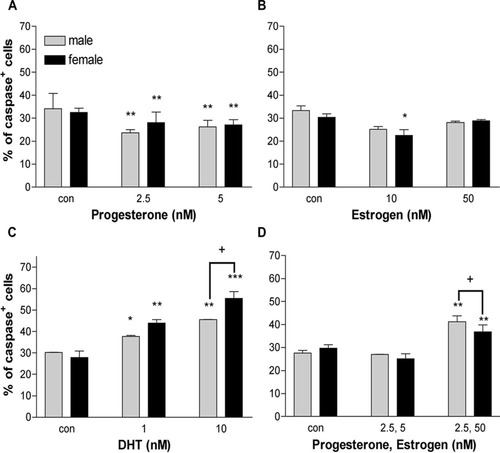
Percentage of dying cells in enriched Olg cultures treated with different concentrations of P2 (A), E2 (B), DHT (C), or combinations of P2 with E2 (D). Cleaved caspase-3+ cells are expressed as a percentage of total A007+ cells. Data are an average of two experiments with cells counted from 20 fields for each treatment group in an experiment. *P < 0.5, **P < 0.01, ***P < 0.001 compared with male control. †P < 0.001 between males and females within the group.
Although P2 by itself reduced the number of caspase-3+ Olgs, E2 and P2 in combination increased Olg cell death at concentrations that did not have any effect when added alone (Fig. 6D). Reducing the concentrations of E2 abolished this effect, correlating well with the total cell numbers.
Regulation of Akt and MAPK Pathways in Olgs by Hormones
Because Akt and MAPK are the two pathways most frequently studied in relation to survival and proliferation, we examined the activation of these pathways in our culture system in response to P2, E2, and DHT. Enriched Olg cultures were plated in 25-cc flasks and treated with P2 (2.5, 5 nM), E2 (10, 50 nM), or DHT (10 nM), concentrations that had the maximum effect on Olg density. After 3 days of hormonal treatment, protein was extracted from the cells, and Western blot analysis was performed for the amount of phosphorylated and total proteins in the Akt and MAPK pathway. P2 treatment activated the Akt pathway that correlated with the effects of P2 on Olg density. Olgs derived from males had a twofold and four- to fivefold increase in the amount of pAkt with 2.5 and 5 nM P2, respectively. The amount of activated Akt was always higher in females compared with males even in the controls, similar to the changes seen in Olg density in response to P2 treatment (Fig. 7A). No significant changes were seen in the total amount of Akt in response to P2 treatment (data not shown). No significant changes were observed in the total or pMAPK (pMAPK42/44) in response to P2 treatment either in males or in females, indicating that pMAPKs are not required for regulating Olg numbers in response to P2 (Fig. 7B–D).
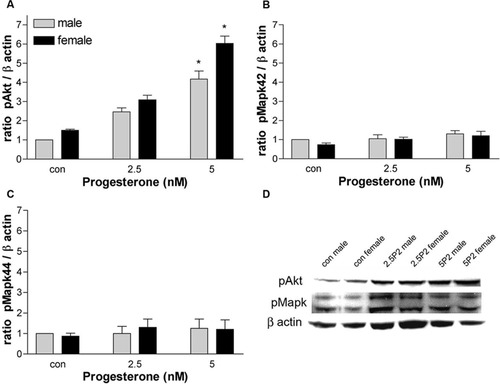
Relative densities of pAkt (A) and pMAPK42/44 (B,C) in enriched Olg cultures treated with different concentrations of P2. The ratio of relative density of pAkt or pMAPK to β-actin was calculated. Values are the mean and SEM of three different samples, with each sample collected from cultures made from six to eight brains. *P < 0.05 compared with male control. D: Western blot of pAkt, pMAPK, and β-actin in control and P2-treated cultures.
In contrast to P2, treatment with 10 or 50 nM E2 in males did not show significant increases in pAkt; however, it caused a significant up-regulation of pAkt in females compared with males given the same amount of P2 (Fig. 8A). Interestingly, this same concentration led to a 10–20% increase in the number of Olgs in females. No significant changes were observed in the MAPK pathway in response to E2 treatment either in the activated form or with the total protein (Fig. 8B,C).
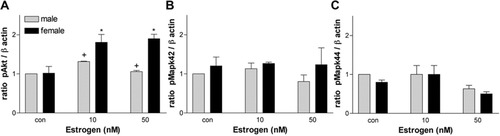
Relative densities of pAkt (A) and pMAPK42/44 (B,C) in enriched Olg cultures treated with different concentrations of E2 expressed as a ratio to β-actin. *P < 0.05 compared with male control. †P < 0.05 comparing males to females.
Although P2 and E2 treatment caused an up-regulation of pAkt, DHT treatment, not surprisingly, significantly reduced the amount of pAkt, especially in females (Fig. 9A). Surprisingly, DHT differentially affected the MAPK pathway in males and females. It caused an increase in amount of pMAPK42/44 in treated males compared with untreated males. DHT led to a decrease in the pMAPK42/44 in treated females compared with untreated females given 10 nM DHT (Fig. 9B,C).
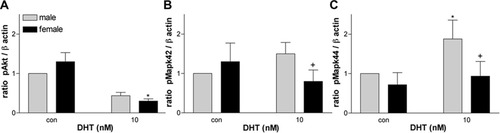
Relative densities of pAkt (A) and pMAPK42/44 (B,C) of Olg cultures treated with different concentrations of DHT expressed as a ratio to β-actin. *P < 0.05 compared with male control. †P < 0.05 between males and females of the same treatment group.
The combinations of P2 and E2 or P2 and DHT produced modest but not significant changes in pAkt that match the changes in Olg numbers. This reconfirms the above-mentioned data showing that activation of pAkt is sufficient in itself to regulate cell numbers (Fig. 10).
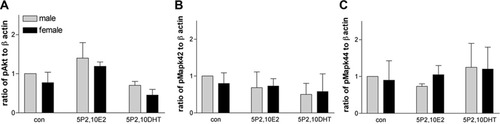
Relative densities of pAkt (A) and pMAPK42/44 (B,C) in Olg cultures treated with different combinations of P2 with E2.
Regulation of Signaling Proteins Downstream of Akt by P2 and E2
Because major changes were noted in the Akt pathway in response to P2 and DHT, we examined regulation of signaling proteins downstream of Akt. Because Akt regulates cell survival by blocking GSK3β and/or by the activation of mTOR pathway via regulation of transcription, we looked at both GSK3β and pmTOR in response to P2 and E2.
Five nanomolar P2 treatment caused an increase in the amount of pmTOR in both sexes, indicating that the Akt/mTOR pathway contributes to the changes seen in Olg density in response to P2. Surprisingly, pmTOR increases many -fold with 10 and 50 nM E2 treatment in both males and females, but moreso in females compared with males (Fig. 11). Because Olg numbers were only modestly increased with E2, the up-regulation of mTOR suggests that it has other functions besides regulating Olg numbers. No changes were seen in total GSK3β levels in response to either E2 or P2 (data not shown), implying that the effects seen on Olgs with the addition of P2 may be due to the activation of mTOR pathway via the activation of Akt.
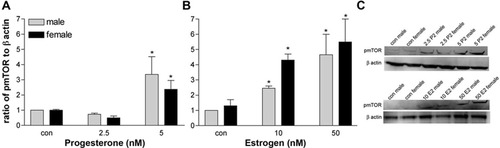
Quantitative analysis of relative densities of pmTOR in enriched Olg cultures treated with different concentrations of P2 (A) and E2 (B) expressed as a ratio to β-actin. *P < 0.05 compared with male control. C: Western blot of pmTOR and β-actin for P2 and E2.
Inhibition of PI3 Kinase Reverses the Effects of P2
To confirm that the changes observed in Olg numbers in response to P2 activate the pAkt pathway, we blocked the activation of Akt with the PI3 kinase inhibitor (LY294002). Blockade of the phosphorylation of Akt reversed the effects of 2.5 nM P2 on Olg numbers. The combination of 5 nM P2 and the inhibitor did not totally reverse Olg numbers. However, 5 nM P2 and the inhibitor caused a significant reduction in the Olg numbers compared with numbers of Olgs without the inhibitor in P2-treated cultures (Fig. 12). The inhibitor by itself did not seem to have any significant effect on the control cultures either in males or in females. Western blot analysis of Olg cultures treated with 5 nM P2 along with 50 μM of the PI3 kinase inhibitor LY294002 showed a reduction in the amount of pAkt less than control levels (data not shown).
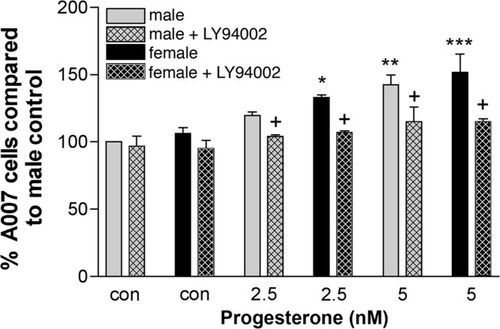
Quantification of the A007+ cells in cultures derived from males and females treated with different concentrations of P2 plus the PI3 kinase inhibitor LY 294002. The effects of P2 on enriched Olg cultures are abolished by the addition of the inhibitor. Data are expressed as a percentage compared with male control. Data are an average of two or three experiments, and in each experiment two or three coverslips were counted for each treatment condition. One-way ANOVA was used to compare each group with the male control. *P < 0.05, **P < 0.01, ***P < 0.001 compared with male control. Two-tailed t-test assuming equal variance was used to compare each group treated with or without the inhibitor. †P < 0.05 within a treatment group with or without the inhibitor.
Effects of Growth Factor Withdrawal on Olgs
Our results definitively show differential responses of male and female Olgs to neuroactive steroids in terms of cell numbers and activation of signaling pathways. To relate this information to in vivo injury models such as MS or TBI, we began to investigate whether male and female Olgs respond differentially to stress. A simple experiment is to withdraw insulin, because it causes cell death in Olg cultures and in vivo (Ye et al., 1995; Shankar et al., 2006). Olg cultures were grown for 3 days, and the insulin was withdrawn from the culture media for 24 hr. After 24 hr of insulin withdrawal, the numbers of Olgs were counted. The number of Olgs was significantly reduced by about 30% in the males; Olgs were reduced by 20%, but not significantly, in females. Addition of 5 nM P2 restored normal levels of Olgs in males; in females, the number of Olgs increased by 20% in comparison with untreated females (Fig. 13). As predicted (Fig. 14), pAkt levels in both sexes decreased in insulin's absence, matching the decrease in Olg numbers. However, the addition of P2 to the insulin-deprived cells led to a modest pAkt increase, but this was still below predicted normal levels. pMAPK42 levels were essentially the same in both sexes in response to insulin withdrawal and remained low even with P2 (Fig. 14A,B). pMAPK44 levels in insulin-deprived cells were lower compared with controls, which agrees with the DHT data showing decreases in both pMAPK44 and Olg numbers. However, the addition of P2 did not increase pMAPK44, as might be predicted based on the increase in Olg numbers. These data suggest that regulation and modulation of other signaling pathways are activated in the absence of insulin.
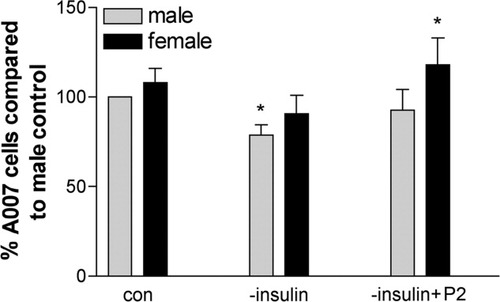
Percentage of A007+ cells in cultures derived from males and females deprived of insulin and supplemented with P2. *P < 0.05 compared with male control.
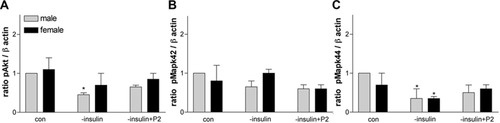
Relative densities of pAkt (A) and pMAPK42/44 (B,C) of Olg cultures deprived of insulin and treated with 5 nM P2 and expressed as a ratio to β-actin. *P < 0.05 compared with male control.
DISCUSSION
Effects of Neuroactive Steroids on Olgs
Our in vivo studies showed that the life span and proliferation of Olgs are partially regulated by castration (Cerghet et al., 2006). To determine which neuroactive steroids contribute to this phenotypic change, enriched Olg cultures were treated with different concentrations of P2, E2, or DHT for 3 days. After 3 days, the vast majority of Olg progenitors became postmitotic (Fig. 5) and had differentiated into MBP+ cells. We routinely observed in these older, control cultures that there was always 10–15% more Olgs on coverslips derived from females vs. males, even though we carefully plated the same number of Olgs. A similar sexually dimorphic difference in the number of Olgs was attributed to serum (Marin-Husstege et al., 2004). However, our culture system is devoid of serum and growth factors except for insulin, suggesting that preexisting conditions contribute to the sexual dimorphism. At the time of plating (2–3 days postnatal), glial progenitors have been exposed to the pre- and neonatal surge of exogenous androgens and, possibly, estrogens (Mack et al., 1996; Bimonte et al., 2000). Another intriguing possibility is that genetically determined factors predispose Olgs to generate these sexually dimorphic differences. Numerous studies now show sexually dimorphic differences in different cell types before exposure to hormones. Muscle stem cells derived from females have higher regenerative capacity than those derived from males even before exposure to hormones (Deasy et al., 2007). Male preimplantation embryos, including human embryos, develop faster than female preimplantation embryos (Avery et al., 1992; Dumoulin et al., 2005). Whatever the causative factors for Olg sexual dimorphism, we found that cultured female Olgs differ from males Olgs in many different parameters whether neurosteroids are absent or present.
Olgs are exquisitely sensitive to concentrations of neurosteroids, females much more so than males. Progesterone, at low concentrations, significantly increased the number of Olgs in females by as much as 50% compared with males. Progesterone, at increasingly higher concentrations, reduced Olg numbers compared with controls, with Olgs derived from females showing greater sensitivity. Although the differences in Olg numbers between males and females may seem minor, the data are based on a short, 3-day survival. If such differences play out in vivo, the sexually dimorphic regulation of Olg numbers might be quite dynamic. The concentrations of neurosteroids we used were based on previous in vitro Olg studies (e.g., Caruso et al., 2004; Marin-Husstege et al., 2004) and our preliminary studies in which we tested a wide range of concentrations. It is difficult to relate the concentrations of P2 that are effective in culture with in vivo sera and brain levels. P2 concentration in mouse sera depends on the phase of estrous cycle. In one mouse study (Ganguly et al., 2007), P2 ranged from 30 to 68 nM depending on the cycle stage, and, in another study (Anupriwan et al., 2008), P2 was greater than 100 nM during pseudopregnancy but decreased to undetectable levels after ovulation. In rat brain, P2 levels appear to be four times greater than in serum, presumably as a result of local steroidogenic synthesis (Schumacher et al., 2004). Because P2 levels fluctuate in female brains and are undoubtedly different from those in male brains, P2 is one hormone that likely contributes to the sexual dimorphism of Olg numbers in mouse brains. In vivo, of course, the brain is exposed to different neurosteroids that may negate or facilitate regulation of Olg numbers. We show that combinations of neurosteroids also affected cultured Olg numbers, and this finding strongly suggests that neurosteroid combinations have an in vivo effect.
We found that P2 is sufficient to maintain Olgs in a state of differentiation (Fig. 6), but it is also protective, because the addition of P2 when added to culture media lacking insulin protects Olgs from death (Fig. 13). Progesterone improves remyelination in a toxin-induced model of demyelination and was more effective in females than in males (Li et al., 2006; Schumacher et al., 2007). Because P2 accelerates neuronal differentiation, it is unclear whether it directly or indirectly affects Olgs.
In contrast to P2, DHT modestly reduced Olg numbers at lower concentrations and highly (20–35%) reduced Olg numbers at higher concentrations. This finding is in agreement with another study (Caruso et al., 2004) in which T4 was toxic to Olgs and enhanced the excitotoxic damage caused by treating them with AMPA or kainic acid. In vivo, its effects are poorly understood, insofar as DHT either protects or damages neurons, depending on whether it acts via a membrane receptor or an intracellular receptor, either through the MAPK or the Akt pathway (Gatson and Singh, 2007).
E2 had little effect on Olg numbers of both sexes at low concentrations; at higher concentrations, it led to a modest reduction in Olg numbers. This finding may seem surprising, because E2 plays a major regulatory role in proliferation of many cell types. Our failure to demonstrate an effect of E2 on Olgs most likely is due to the late stage of differentiation of Olg progenitors when E2 was applied. Our enriched Olg cultures contain clusters of cells lightly attached to the bed layer. The number and size of the clusters increased two- to threefold in response to E2, with females showing a greater increase than males (data not shown). Many cells in the clusters were NG2+ and nestin+, strongly suggesting that E2 affects early-stage Olg progenitors and neural precursors, and this finding confirms that E2 is biologically active in our system.
Effects of Hormonal Combinations
Surprisingly, the combination of P2 and E2, at concentrations at which P2 produced increases in Olg numbers, led to decreases in Olg numbers. However, the combination of P2 and E2 at low concentrations, at which P2 had no effect upon Olg numbers, led to increases in Olg numbers. The combination of P2 with low DHT concentrations led to a modest, although not significant, reduction in cell death compared with DHT alone. Clearly, P2 synergizes with E2 and DHT to regulate Olg differentiation and death. This set of experiments shows 1) the exquisite sensitivity of Olgs to concentrations of neurosteroids and 2) that these neurosteroids act in concert. We still cannot paint a clear picture of how neurosteroid combinations affect Olg differentiaton; other neuronal studies that used hormonal combinations to evaluate their neuroprotective effects have also yielded variable results that depended on the age of the animal, dose of hormones, and treatment before or after injury (Bramlett, 2005).
Proliferation and Death of Olgs
The changes in Olg numbers caused by P2 and DHT are due to regulation of cell death. None of the hormones altered the rate of incorporation of BrdU into A007–/+ cells 2 hr after incubation. This finding indicates these hormones do not stimulate A007– Olg progenitors and A007+ postmitotic Olgs to reenter S phase in greater numbers than controls. E2, however, delayed the exit of Olg precursors from the cell cycle in response to mitogen withdrawal, in agreement with a previous study using a different paradigm (Marin-Husstege et al., 2004). It is unlikely that these E2-stimulated Olgs underwent another round of cell division, because total numbers of A007+ Olgs did not increase above control levels after the 3-day treatment.
Hormonal regulation of Olg death by hormones is evident in that the numbers of caspase-3+ cells change inversely to the change in Olg numbers. The percentage changes in numbers of caspase-3+ cells do not directly match the percentage changes in numbers of Olgs, but this is predictable because dying cells are caspase-3+ for a short time.
Olgs derived from females responded more vigorously than those from males with respect to Olg generation and death in response to hormones. These differences may be viewed as protective or deleterious. In a preliminary study, the withdrawal of insulin for 24 hr without P2 decreased Olg numbers 25% in both sexes, but, with the addition of P2, the numbers of male Olgs returned to their control levels, whereas the numbers of female Olgs increased above their control values. This preliminary study demonstrates the protective effect of P2 when Olgs are stressed, and it suggests that P2 has other protective roles in female Olgs.
Signaling Pathways Activated by Hormones
Predictably, P2 up-regulated pAkt as much as sixfold in both sexes, with females usually having more pAkt than males. Increases in pAkt generally correlate with increases in Olg numbers in a 2.5:1 ratio: a fivefold increase in pAkt corresponds to a twofold increase in Olgs. Surprisingly, P2 did not alter MAPK levels in either sex, indicating that activation of MAPKs above the normal levels is not required for Olg differentiation/maintenance in our system. Akt activation, in turn, phosphorylates either mTOR or GSK3β, which affects proliferation or survival of cells (Hay, 2005; Chong et al., 2007). As expected, P2 treatment increased pmTOR, indicating that mTOR activation is a likely modulator of Olg differentiation. Total GSK3β, predictably, was unaffected. Activation of Akt by P2, blocked by the PI3 kinase inhibitor LY294002, significantly decreased Olg density. pAkt levels, in the presence of P2 and inhibitor, were less than control levels, but total Akt was unchanged, indicating that P2 specifically activates the Akt pathway via phosphorylation (Flores et al., 2000; Pang et al., 2007).
E2 did not lead to changes in pMAPK pathway, even though it activates this pathway in neurons and plays a major role in cell proliferation/cell death (Hayashi et al., 2007; Numakawa et al., 2007). The lack of an effect on MAPKs may be due to the fact that E2 had little effect on Olg numbers; more likely, MAPKs are activated in Olgs when cell death pathways are activated, as shown by DHT.
DHT led to a reduction of pAkt in both sexes, correlating with the reduction in the number of Olgs. In contrast to P2 and E2, with which pMAPKs were unchanged, DHT altered pMAPKs in a sexually dimorphic manner. DHT caused an increase in activation of MAPKs in males but a reduction in females, suggesting that MAPKs function to protect Olgs from death. Activation of MAPKs as well as complete suppression of the MAPK pathway has been described in cell death in Olg precursors (Horiuchi et al., 2006). MAPKs 42/44 are activated in excitotoxic Olg death induced by glutamate (Rosin et al., 2004) and in proinflammatory processes mediating neurodegenerative diseases (Nikodemova et al., 2006). Because downstream targets of MAPK are sensitive to the duration of MAPK activation (Marshall, 1995; Cook et al., 1999), timing and duration of MAPK activation may dictate the extent to which Olg's die. Our studies suggest that MAPKs become activated when apoptosis comes into play, but whether this ameliorates or exacerbates Olg death is still unclear.
Although combinations of these hormones did not show significant changes in either activated or total amount of signaling proteins, certain trends emerged. The combination of P2 and DHT led to a reduction in pAkt levels and an increase in pMAPK44 levels similar to those seen with the treatment of DHT alone. The combination of 5 nM P2 with 10 nM E2 led to an increase in Olg numbers in males and a decrease in females, with no changes in MAPK pathways. We predicted that MAPKs should increase in females, inversely corresponding to the decrease in female cell numbers. These studies show the complexity of interactions between Akt and MAPK pathways, and they also imply that additional signaling mechanisms are activated.
These neurosteroids activate signaling pathways by binding to classical nuclear receptors and to membrane receptors associated with caveolae in plasma membranes; the latter is responsible for signaling through Akt (Honda et al., 2000) and MAPK pathways (Migliaccio et al., 1996; Singer et al. 1999). We and others have shown that these neurosteroid receptors are present in Olgs, but whether genomic or nongenomic pathways are activated is unclear. Indeed, P2, E2, and DHT are further metabolized to act via their own hormone receptors and also other receptors. For example, P2 is metabolized to 5α-dihydroxyprogesterone that acts via the progesterone receptor (PR) and to 3α,5α-tetrahydroprogesterone or allopregnanalone that exerts its effect via the GABA receptor. Although these P2 metabolites increased myelination via the GABA receptors, the effect was completely abolished in PR knockout mice, indicating that PR receptors are essential for P2 and its metabolic action (Ghoumari et al., 2003). Estrogen metabolites also act via ER-dependent and -independent mechanisms (Liu and Bachmann, 1998).
These sexually dimorphic differences described here may also be directly relevant to differences in female and male MS lesions. Men have more destructive CNS lesions than women (Weatherby et al., 2000). Moreover, diffuse axonal loss, even in normal-appearing white matter, is more prevalent in men with MS (Miller and Leary, 2007). In one MS study, serum levels of sex hormones, especially T4, are different compared with controls (Tomassini et al., 2005); in another study, high E2 and low P2 levels correlated with increased numbers of gadolinium-enhancing lesions (Bansil et al., 1999). Treatment with T4 in males with MS improved the cognitive functions, but no changes were noticed in the number of gadolinium-enhanced lesions (Sicotte et al., 2007). Interpretation of these studies is complicated because the differential effects of T4 on MS lesions may be due to its conversion to E2 or DHT. The aforesaid studies should not be construed to imply that levels of sex hormones are the root cause of MS, but they play exacerbating or ameliorating roles. Certainly, sexually dimorphic differences of the immune system account for some of these CNS differences, but further consideration must be given to intrinsic CNS sexually dimorphic glial differences and how they may contribute to differences in MS lesions.
Acknowledgements
The authors thank Drs. M.S. Ghandour and D. Goebel for assistance in oligodendrocyte culture preparation and densitometric imaging.




