Cadmium modulates proliferation and differentiation of human neuroblasts
Abstract
Cadmium is an environmental pollutant inducing numerous pathological effects, including neurological disorders and brain diseases. However, little is known about the molecular mechanisms of cadmium in affecting neurons and in inducing neurotoxicity in the development of the human brain. We have recently established, cloned, and propagated in vitro a primary long-term cell culture (FNC-B4) obtained from the human fetal olfactory neuroepithelium. In the present study, we show that different concentrations of cadmium chloride (CdCl2) induced dose-dependent biological effects in FNC-B4 cells. A low concentration (10 μM) of CdCl2 stimulated neuroblast growth, whereas a high concentration (100 μM) inhibited the growth and the viability of neuroblasts inducing morphological and cytoskeletal alterations as well as apoptotic cell death. We also observed that CdCl2 affected, in a dose-dependent manner, the differentiation of FNC-B4 neuroblasts, with increased mRNA and protein levels of differentiation markers and decreased expression levels of neuronal stem markers. Furthermore, differentiated cells co-expressed glial and neuronal markers. We suggest that CdCl2 in FNC-B4 neuroblasts might represent a selective cue by which, in a heterogeneous primary culture, the more differentiated mature cells die, whereas the undifferentiated cells, at the same time glial and neuronal progenitors, are forced to access a state of differentiation. © 2008 Wiley-Liss, Inc.
Human cadmium poisoning results mainly from occupational and environmental exposures linked to heavy metal mining, metallurgy, industrial use, waste incineration, farm fertilizers, cigarette smoking, and mud purification (Bertin and Averbeck, 2006). Exposure to cadmium may result in adverse effects on several tissues and organs (Sudo et al., 1996; Koizumi et al., 1996; Xu et al., 1996; Jones et al., 1997; Waalkes, 2003). Acute intoxication is responsible for injuries to the testes, liver, and lungs (Waalkes, 2003; Waisberg et al., 2003). Chronic exposure leads to obstructive airway diseases, emphysema, end-stage renal failures, diabetic and renal complications, deregulated blood pressure, bone disorders, and immune suppression (Bertin and Averbeck, 2006). Cadmium intoxication is also linked to cancer induction. It is strongly associated with lung cancers and somewhat with cancers of the prostate and kidney (Waalkes, 2003). Some studies indicate the possible occurrence of liver, pancreas, and stomach cancers, but these observations are controversial (Nakamura et al., 2002).
Cadmium affects cell cycle progression, proliferation, differentiation, DNA replication and repair, and apoptotic pathways (El Azzouzi et al., 1994; Figueiredo-Pereira et al., 1998; Galán et al., 2000; Waisberg et al., 2003; Huang et al., 2006; Mao et al., 2007; Blechinger et al., 2007; Cao et al., 2008). In neuronal cells, cadmium induces a multitude of biological effects: on human neuroblastoma NB-1 cells, cadmium stimulates neurite outgrowth (Pramanik et al., 2001); on mouse ganglion cells, it induces degeneration (Habeebu et al., 2001); on rat fetal cortical neurons, cadmium induces apoptosis (Lopez et al., 2003); on human neuroblastoma BE(2)-C cells, it inhibits tyrosine kinase signaling (Monroe and Halvorsen, 2006).
The toxic effects of cadmium in the brain are poorly understood, but it is thought that, during brain development, when the blood–brain barrier is not well established, cadmium may enter the brain and exert its toxic effects (Lafuente and Esquifino, 1999). It has been suggested that several diseases and symptoms such as learning disabilities and hyperactivity in children (Pihl and Parkes, 1977; Marlowe et al., 1985), Parkinson disease (Okuda et al., 1997), and amyotrophic lateral sclerosis (Bar-Sela et al., 2001) as well as some malformations such as spina bifida (Kalter, 1985) and forelimb ectrodactyly (Lee et al., 2006) may be related to cadmium exposure.
The effect of cadmium on neuronal cells and its role in brain development are rather complex and are still debated. To clarify this biological problem, in this study we used the FNC-B4 human neuroblast cell line (Vannelli et al., 1995). This long-term primary cell culture has been established, cloned, and propagated in vitro from human fetal olfactory epithelium. FNC-B4 cells synthesize both neuronal proteins and olfactory markers and respond to odorant stimuli, suggesting their origin from the stem cell compartment that generates mature olfactory receptor neurons. Moreover, this system represents a model of neurogenesis that, simplifying the cellular heterogeneity of the developing nervous system, may help in investigating in vitro pathologic perturbations of the activation, self-renewal, differentiation, and survival of primary neuronal precursors. Thus, FNC-B4 cells represent a human in vitro model that can be useful in providing additional information on the effects of cadmium in neurons.
In the current paper, we demonstrate that cadmium can modulate the growth and differentiation of immature neurons and that these effects are associated with morphological and biochemical alterations of the cells consistent with the induction of a differentiated phenotype. In particular, we evaluate the alterations of the cytoskeletal organization and of cell morphology as well as the expression of several genes and proteins, markers of differentiation or of neuronal pluripotency, before and after exposure to different concentrations of cadmium.
MATERIALS AND METHODS
Cell Culture and Treatments
The primary human neuroblast long-term cell line FNC-B4 was isolated, cloned, and propagated in vitro from human fetal olfactory neuroepithelium, as previously described (Vannelli et al., 1995). These cells express neuronal stem/differentiation markers indicating that they originate from the neuroblastic precursor compartment, which gives rise to mature neurons throughout life. FNC-B4 cells express olfactory-specific markers: olfactory marker protein (OMP; a phylogenetically conserved protein whose function has remained largely elusive); olfactory-type G protein (Golf; which activates the lyase adenylate cyclase that converts ATP in cyclic AMP); olfactory cyclic nucleotide-gated channel (OCNC; a channel activated by binding of cAMP and conducting a depolarizing receptor current that leads to electrical excitation of the neuron); Olf-1 (an olfactory neuron-specific trans-acting factor capable of interacting with some olfactory neuron-specific genes). FNC-B4 are cultured in Coon's modification of F12 medium, supplemented with 10% fetal bovine serum and antibiotics. Materials for cell culture were bought from Invitrogen Co. (Carlsbad, CA). The FNC-B4 cell line grows as a monolayer, is nontumorigenic, and has a normal human kariotype. Cryogenically preserved, early passages of FNC-B4 cells were used in the present study.
FNC-B4 was switched, 24 hr before the treatments, from complete culture medium to Coon's modification of F12 serum-free medium; then, at the beginning of each experiment, the medium was replaced with a Coon's modification of F12 serum-free medium containing 10 and 100 μM cadmium chloride (CdCl2; Sigma-Aldrich, St. Louis, MO). Treatments with and without CdCl2 were carried out for 24 hr.
Phase-Contrast Light Microscopy
For phase-contrast microscopic observation, FNC-B4 cells were cultured on Petri dishes in serum-free medium for 24 hr and then sham exposed or incubated with 10 and 100 μM CdCl2. Cells were then viewed with a Nikon Microphot-FX microscope (Nikon Instruments SpA., Milan, Italy) at the original magnfication of ×40.
Immunohistochemistry and Confocal Laser Scanning Microscopy
FNC-B4 cells were cultured on slides in the appropriate medium and then fixed with 3.7% paraformaldehyde (pH 7.4) for 10 min and permeabilized for 10 min with PBS containing 0.1% Triton X-100 (Sigma-Aldrich). After rinsing in PBS, the slides were incubated with 2% bovine serum albumin for 15 min. Immunostaining was performed using the following primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA): vimentin antibody (V9; dilution 1:100; incubation overnight at 4°C); β-tubulin III antibody (TU-20; dilution 1:100; incubation overnight at 4°C), and glial fibrillary acidic protein (GFAP) antibody (H-50; dilution 1:500; incubation overnight at 4°C). Then, incubations with appropriate secondary conjugated antibodies were performed: A-11001 Alexa Fluor 488 goat anti-mouse IgG conjugated antibody (Invitrogen Co.; dilution 1:200; incubation for 60 min at room temperature) for the detection of vimentin; R6393 rhodamine red goat anti-mouse IgG (H + L; Molecular Probes, Eugene, OR; dilution 1:200; incubation for 60 min at room temperature) for the detection of β-tubulin III; A-11001 Alexa Fluor 488 goat anti-rabbit IgG (H + L; Molecular Probes; dilution 1:200; incubation for 60 min at room temperature) for the detection of GFAP. Cells were then viewed with a Bio-Rad MCR 1024 ES confocal laser scanning microscope (Bio-Rad, Hercules, CA) equipped with a 15-mW Kr-Ar laser for fluorescence measurements and with differential interference contrast optics for transmission images. To minimize spectral bleed-through between the fluorescent channels, the emission of the different fluorochromes was measured by detecting the channels sequentially instead of simultaneously. Fluorescence was collected using a Nikon PlanApo ×40 and ×60 lens objective (Nikon Instruments SpA.), and images were analyzed in ImageJ software.
The percentage of cells positive to β-tubulin III and/or to GFAP was calculated by counting the number of stained cells over the total cells in at least 15 separate fields per slides. Each experiment was performed in triplicate.
Cell Proliferation and Cell Viability
Cell proliferation was evaluated as incorporation of [3H]thymidine (Amersham Bioscences, Little Chalfont, United Kingdom) in duplicating DNA. Cells were seeded in complete medium at a density of 7 × 104 in six-well culture plates; then, control and treated cells, after starvation, were labelled with [3H]thymidine (10 μCi/ml) at 37°C for 24 hr. Cells were then harvested, and incorporated radioactivity was measured by a liquid scintillation counter (Packard Instrument, Groningen, The Netherlands).
Cell viability was evaluated by a trypan blue exclusion test. Five microliters of trypan blue (0.5% in PBS) was added to 40 μl of resuspended cells. Trypan blue-excluding (live) cells were counted in a hemocytometer in three randomly selected fields and averaged.
SDS-PAGE, Western Blotting Analysis, and Immunoblotting
For nestin, β-tubulin III, and full-length and cleaved PARP analysis, cells were homogenized in an ice-cold lysis buffer (50 mM Tris HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton, 0.25% sodium dodecyl sulfate) supplemented with a protease inhibitor cocktail (Sigma- Aldrich) and centrifuged for 15 min at 4°C at 10,000g. The supernatant was collected and protein concentration measured using a Coomassie Bio-Rad protein assay kit. Aliquots containing 20 μg of proteins were diluted in a 4× reducing Laemmli's sample buffer (250 mM Tris-HCl, pH 6.8, 20% glycerol, 8% SDS, 20% 2-mercaptoethanol, 0.008% bromophenol blue) and loaded into 10% SDS-PAGE. Then, proteins were transferred on polyvinylidene difluoride membranes (Hybond-P; Amersham Bioscience, Piscataway, NJ). Membranes were blocked for 1 hr at room temperature in 5% BSA-TTBS buffer (0.1% Tween 20, 20 mM Tris-HCl, 150 mM NaCl, pH 7,5), washed in TTBS, and incubated at 4°C overnight with primary antibodies: anti-β-actin 1:10,000 (Santa Cruz Biotechnology), antinestin 1:1,000 (Chemicon, Temecula, CA), anti-β-tubulin III 1:2,000 (Santa Cruz Biotechnology); and anti-PARP 1:2,000 (Santa Cruz Biotechnology). Primary antibodies were diluted in TTBS and incubations followed by peroxidase-conjugated secondary IgG treatment (Santa Cruz Biotechnology). Finally, the reacted proteins were revealed with an enhanced chemiluminescence system (ECL Plus; Amersham Bioscience).
Quantitative Real-Time RT-PCR
RNA extraction
Total RNA was isolated from samples by RNeasy Micro kit (Qiagen SpA., Milan, Italy), according to the manufacturer's instructions. RNA concentration and purity were checked spectrophotometrically.
cDNA synthesis
RNA was reverse-transcribed using a commercial kit based on the random primers technique (Taqman Reverse Transcription Reagents; Applied Biosystems, Foster City, CA) according to manufacturer's instructions. The reaction was carried out in a final volume of 20 μl. The reaction mix contained buffer 1× MgCl2 5.5 mM, dNTP 2 mM, random hexamers 2.5 μM, RNAse inhibitor 0.4 U/μl, reverse transcriptase 1.25 U/μl. The reaction was performed under the following conditions: 25°C for 10 min, 48°C for 30 min, and 95°C for 2 min.
Real-time PCR
mRNA expression was measured by real-time PCR methods based on the use of Taqman probe. Reagents were purchased from Applied Biosystems, as pre-made kits: Nanog (Hs02387400_g1), CD15 (Hs00275643_ s1), nestin (Hs00707120_s1), CD271 (Hs00609976_m1), CD117 (Hs00174029_m1), GFAP (Hs00157674_m1), β-tubulin III (Hs00801390_s1), and D2r (Hs00241436_m1), D1r (Hs00265245_ s1). In a total volume of 12.5 μl, the PCR mixture contains 6.25 μl of Universal Master Mix (Applied Biosystems), 0.625 μl of the ready-made specific probe and primers mix (Applied Biosystems), and 2.5 μl of cDNA. The thermal cycle conditions were as follows: one hold at 50°C for 2 min, one hold at 95°C for 10 min, and 45 cycles of a two-step amplification protocol (95°C for 15 sec and 60°C for 1 min). All samples were analyzed in triplicate. Analysis of relative gene expression was obtained by using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Statistical Analysis
The results are expressed as mean ± SEM. Comparison between groups was performed by the Mann-Whitney test or by the Wilcoxon test, as appropriate. P < 0.05 was considered statistically significant.
RESULTS
FNC-B4 Cell Morphology
Observation by phase-contrast light microscopy reveals that 10 μM CdCl2-treated cells (Fig. 1C,D) show a bulky body shape and long neuritic processes vs. control, untreated cells (Fig. 1A,B); synaptic bundles are well represented, and manifold intercellular contacts are clearly evident. After 100 μM CdCl2 exposure, cell morphology appears significantly modified (Fig. 1E,F); cells are stretched and thinner even though neurites are still present and normal in length, and there are numerous synaptic bundles.
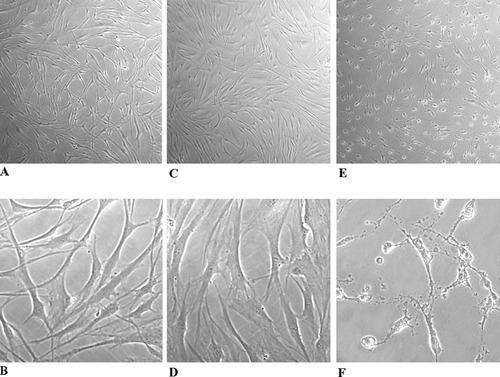
FNC-B4 cell morphology before and after stimulation with CdCl2. A,B: Control, untreated cells at different magnifications. Cells show the typical neuronal morphology, with neuritic processes and manifold intercellular contacts. C,D: 10 μM CdCl2-treated neuroblasts. Cell shape and synaptic network appear unaffected by the treatment in comparison with control neuroblasts. E,F: 100 μM CdCl2-treated neuroblasts. The exposure induces changes of the polyedric cell shape so that FNC-B4 neurons acquired a spindle-shaped morphology; cytoplasmic processes are well represented. Phase-contrast light microscopy. Upper panels: total magnification ×40. Lower panels: total magnification ×400.
Cytoskeleton and Vimentin Network Organization
Analysis by confocal laser scanning microscopy shows that exposure of FNC-B4 human neuroblasts to a high concentration of CdCl2 results in significant cytoskeletal network modifications. In fact, after 10 μM CdCl2 exposure, vimentin appears unaffected in its distribution and organization (Fig. 2B) in comparison with untreated cells (Fig. 2A); both in 10 μM CdCl2-treated and in control cells, vimentin is evident and uniformly distributed in the cell bodies and in the neurites. Instead, after 100 μM CdCl2 exposure (Fig. 2C), vimentin appears accumulated and aggregated in neuronal cell bodies and axons, giving FNC-B4 cells a deeply modified cytoskeletal phenotype. Both control and treated cells (with low and high concentrations of CdCl2) show branched neurites and several synaptic contacts, considered hallmarks of neuronal plasticity and of differentiation (Cowen and Gavazzi, 1998).
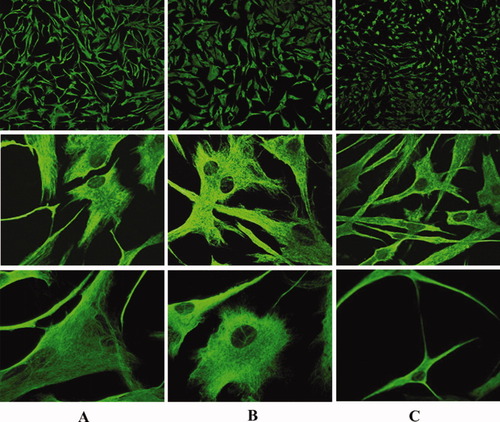
FNC-B4 cytoskeletal modifications before and after stimulation with CdCl2. A: Control, untreated cells at different magnifications. Vimentin, revealed by appropriate primary and fluorescent secondary antibodies, appears in green. Fluorescence appears uniformly distributed in the cell bodies and outgrowths. B: 10 μM CdCl2-treated cells. CdCl2 treatment does not significantly affect vimentin distribution and architecture. C: 100 μM CdCl2-treated cells. Vimentin appears denser and closely packed in the cell body and in the outgrowths in comparison with control and with 10 μM CdCl2-treated cells. Immunohistochemistry and confocal laser scanning microscopy. Upper panels: total magnification ×40; middle panels: ×400; lower panels: ×600.
Cell Proliferation and Cell Viability
To investigate the role of CdCl2 in cell proliferation, we measured the [3H]thymidine incorporation in control and treated cells. Neuroblasts treated with 10 μM CdCl2 have a statistically significant increase of [3H]thymidine incorporation in comparison with control, untreated cells (Table I). In contrast, 100 μM CdCl2-treated FNC-B4 cells show a significant decrease in the incorporated radioactivity in comparison with both control and 10 μM CdCl2-treated cells (Table I).
| Treatments | Cell proliferation |
|---|---|
| 10 μM CdCl2 | 119 ± 6 |
| 100 μM CdCl2 | 23 ± 7 |
- * Results are expressed as percentage increase over the control value (100%); each value of n = 3 experiments represents the mean ± SEM.
Cell viability, measured by trypan blue exclusion assay, is not significantly affected by 10 μM CdCl2 treatment in comparison with control, untreated cells. When cells were exposed to 100 μM CdCl2, the number of trypan blue-positive cells increased (15%, P < 0.05).
PARP-Mediated Apoptotic Pathway
To determine whether the treatment with different concentrations of CdCl2 can affect the expression of protein involved in DNA repair and in programmed cell death, we investigated the expression of poly-(ADP-ribose) polymerase (PARP), a marker of the caspase 3-mediated pathway of apoptosis. Human PARP is a 116-kDa nuclear protein involved in repair of DNA nicks induced by various stressors, and it is one of the substrates for caspase 3, which cleaves PARP at Asp 214 and Gly 215, leading to formation of 85-kDa and 25-kDa fragments during apoptosis. Cleavage of PARP correlates with DNA fragmentation and other morphological changes, making it a critical marker of apoptosis. In FNC-B4 cells treated with CdCl2, the PARP-mediated apoptotic pathway is activated in a dose-dependent manner: the cleaved form of PARP progressively increases (and the full length of PARP decreases) when cells are treated with 10 and 100 μM CdCl2 in comparison with control, untreated cells (Fig. 3).
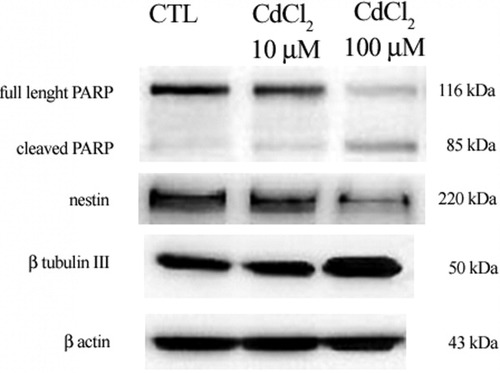
Western blotting analysis of nestin, β-tubulin III, and PARP in FNC-B4 neuroblsts before and after stimulation with CdCl2. Western blotting analysis for nestin and β-tubulin III expression reveals bands migrating approximately at the expected 220- and 50-kDa molecular sizes, respectively. With the increasing CdCl2 doses, the expression of nestin progressively decreases, whereas the expression of β-tubulin III increases. Western blotting analysis for full-length (116 kDa) and cleaved (85 kDa) PARP shows a significant increase in the cleaved form expression when FNC-B4 cells are treated with 100 μM CdCl2.
Gene and Protein Expression
FNC-B4 cells, obtained from the human fetal olfactory epithelium, have at the same time the properties of immature neurons and the ability to differentiate and express both neuronal proteins as well as olfactory-specific markers (Vannelli et al., 1995). When FNC-B4 cells are exposed to 10 and 100 μM CdCl2, the expression of several somatic markers such as Nanog (a homeodomain transcription factor, marker of pluripotency), CD15 (a carbohydrate antigen associated with the extracellular matrix and marker of highly proliferative cells), and nestin (a protein associated with neural stem cell) appears significantly decreased, with a concentration-dependent pathway, in comparison with untreated, control cells (Fig. 4A). Nevertheless, after 10 and 100 μM CdCl2 treatments, glial fibrillary acid protein (GFAP; Fig. 4B) and β-tubulin III (Fig. 4C) expression increase, suggesting the possibility of a multi-differentiation into different cell subtypes, neurons or astrocytes. To confirm the trend of FNC-B4 cells that differentiate in response to CdCl2 exposure, the expression of β-tubulin III and nestin proteins was evaluated. Western blotting analysis confirmed that, whereas β-tubulin III expression increases in a dose-dependent manner, the expression of nestin significantly decreases (Fig. 3).
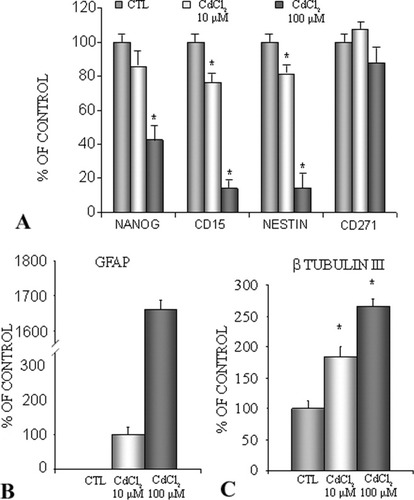
Quantitative gene expression analysis (Nanog, CD15, nestin, GFAP, and β-tubulin III) in FNC-B4 before and after stimulation with CdCl2. A: mRNA expression of neural markers. Nanog, CD15, and nestin decrease in 10 and 100 μM CdCl2-treated neuroblasts. mRNA expression of CD271 (nerve growth factor receptor) is high both in control (CTL) and in treated cells, confirming the viability of control and CdCl2-exposed neuroblasts. B: mRNA expression of GFAP progressively increases when FNC-B4 cells are stimulated with 10 and 100 μM CdCl2. C: mRNA expression of β-tubulin III increases when neuroblasts are stimulated with 10 and 100 μM CdCl2. Results are calculated accordingly to the comparative cycle threshold method using the GAPDH as reference gene for normalization and are expressed as percentage of data obtained in the untreated, control cells (CTL). Data are reported as mean ± SEM from at least three separate experiments; ☆P < 0.05 vs. control.
The simultaneous overexpression of GFAP and β-tubulin III was somewhat intriguing, so we analyzed, by confocal laser scanning microscopy, the localization of GFAP and β-tubulin III in control and treated cells. Data obtained demonstrated that GFAP and β-tubulin III co-localize in CdCl2 treated cells, with a dose-dependent pathway (Fig. 5). In particular, control cells (Fig. 5A–C,J) are positive to β-tubulin III (43.38% ± 2.83%, n = 3) and virtually to GFAP (0.42% ± 0.12%, n = 3); 10 μM CdCl2-treated cells (Fig. 5D–F,J) are positive both to β-tubulin III (66.42% ± 3.24%, n = 3) and to GFAP (7% ± 2%, n = 3). Treatment with 100 μM CdCl2 (Fig. 5G–I,J) increased the number of cells positive to β-tubulin III (93.7% ± 3.8%, n = 3) and GFAP (47% ± 3%, n = 3). After 10 and 100 μM CdCl2 treatment, the co-localization of β-tubulin III and GFAP in FNC-B4 neuroblasts was 7% ± 2% (n = 3) and 47% ± 3% (n = 3), respectively. In the merged images (Fig. 5C,F,I,J) the yellow color indicates a co-localization of β-tubulin III with GFAP in the treated cells.
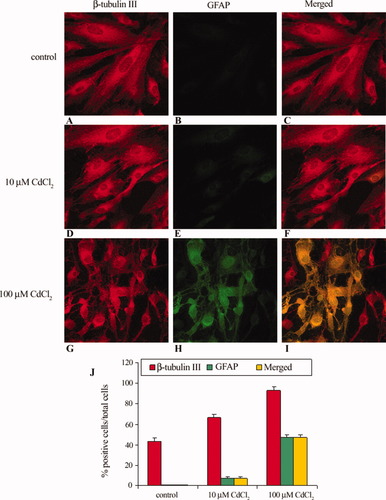
β-Tubulin III and GFAP expression in FNC-B4 neuroblsts before and after stimulation with CdCl2. A–C: Control, untreated cells. Only cells positive to β-tubulin III but no cells positive to GFAP have been detected. Merged image shows no colocalization of β-tubulin III and GFAP in the same cells. D–F: 10 μM CdCl2-treated cells. After the treatment, numerous cells positive to β-tubulin III and some cells positive to GFAP have been detected. In the merged image, the yellow color indicates the colocalization of β-tubulin III and GFAP in the same cells. G–I: 100 μM CdCl2-treated cells. Most of the cells are positive both to β-tubulin III and to GFAP; colocalization of β-tubulin III and GFAP is evident in the merged image (yellow). Immunohistochemistry and confocal laser scanning microscopy. Total magnification: ×630. J: Cells were scored as either positive or negative for β-tubulin III and/or GFAP, and results were expressed as the percentage of positive cells ± SE (calculated by counting the number of stained cells over total in 15 separate fields per slide). In controls, only cells positive to β-tubulin III were detected; the number of cells positive both to β-tubulin III and to GFAP after stimulation with 10 and 100 μM CdCl2 significantly increased (☆P < 0.01 vs. control cells) with a dose-dependent pathway. Ordinate: cells positive to β-tubulin III and to GFAP (% over total cells); columns are mean ± SE. Results were obtained from three separate experiments (n = 3).
Among cell markers suggesting a proliferative cell phenotype, the expression of the CD117 gene (encoding a receptor for stem cell factor) was analyzed, because it identifies and characterizes neurons with proliferative and stem phenotypes. CD117 is overexpressed in FNC-B4 cells treated with 10 μM CdCl2, but the decrease in expression of this marker is dramatic when cells are stimulated with 100 μM CdCl2 (Fig. 6A).
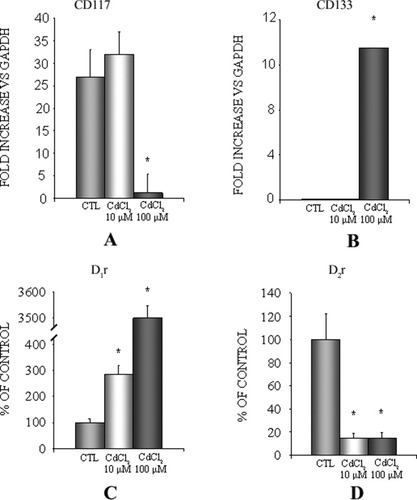
Quantitative gene expression analysis of CD117 and of dopamine D1 and D2 receptors in FNC-B4 before and after stimulation with CdCl2. A: mRNA expression of CD117 is significantly decreased after 100 μM CdCl2 treatment in comparison with 10 μM CdCl2 exposure and with control (CTL) cells. B: The exposure of FNC-B4 cells to CdCl2 causes a dose-dependent increase in mRNA expression of dopamine D1 receptor. C: mRNA expression of dopamine D2 receptor is decreased in FNC-B4 cells after CdCl2 treatments.
Because dopamine, in parallel to its action in odor processing, plays a growth factor-like role in the permanent neurogenesis observed in the olfactory epithelium, by a direct dopamine D1r- or D2r-mediated action, we evaluated the expression levels of dopamine D1r and dopamine D2r before and after CdCl2 treatments. Our data show that CdCl2 induced in FNC-B4 neuroblasts an increase in dopamine D1r expression in a dose-dependent pathway (Fig. 6B); on the contrary, dopamine D2r expression dramatically decreased after stimulation both with 10 and with 100 μM CdCl2 (Fig. 6C).
DISCUSSION
This study provides the first evidence that CdCl2 is simultaneously able to inhibit strongly the growth of developing neurons (FNC-B4) and induce differentiation of neural progenitor cells into distinct neuronal and glial cell lineages. Environmental pollutants and transition metals such as cadmium have short-term and long-term effects on neuron development. Some studies demonstrate that cadmium may disturb the natural oxidation/reduction balance in cells through various mechanisms, which interferes with cellular signaling and gene expression systems (Buzard and Kasprazak, 2000).
In fact, in some cell lines, such as human neuroblastoma NB-1 cells, exposure to CdCl2 induces a significant neurite outgrowth, suggesting a role in neuron differentiation for cadmium (Pramanik et al., 2001; Chow et al., 2008). Furthermore, administration for 3 hr of 100 or 150 μM CdCl2 inhibits proliferation and induces several functional differentiation markers in some cell lines (Lee et al., 2006).
Our data show that the treatment of human immature neuron FNC-B4 cells with different concentrations of CdCl2 significantly affects cell growth. Indeed, low concentrations of CdCl2 stimulate cell growth, whereas higher concentrations cause a decrease in cell proliferation. The latter effect, according to previous study (Mao et al., 2007), is associated with morphological and biochemical alterations as well as induction of apoptotic cell death consistent with the induction of cell differentiation toward a glial/neural precursor.
Low concentrations of CdCl2 induce the appearance of a proliferative phenotype in FNC-B4 cells associated with an increase in CD117 gene expression, a marker associated with cellular proliferative potential (Miettinen and Lasota, 2005). The exposure of FNC-B4 neuroblasts to high concentrations of CdCl2 induced significant morphological and cytoskeletal alterations and remarkable reduction of proliferation and of CD117 gene expression. Several cells also show signs of apoptosis as confirmed by morphological observation and by the increased level of cleaved PARP. These findings suggest that CdCl2, depending on its concentration, is able to change and thus regulate distinct biological responses. The exposure of FNC-B4 to increasing concentrations of CdCl2 induces a significant decrease in the expression of some neural markers, such as Nanog, nestin, and CD15. This latter gene distinguishes highly proliferative cells and is strongly expressed in neuronal regions with prolonged neurogenesis, i.e., the olfactory epithelium (Capela and Temple, 2006). On the other hand, neural/glial differentiation markers such as GFAP and β-tubulin III became robust. Recent studies suggest that dopamine may play a crucial role as a modulator in olfactory discrimination and in olfactory processing (Tillerson et al., 2006) as well as in several disorders with olfactory deficits, such as Parkinson disease, characterized by altered dopamine homeostasis in olfaction-related brain regions (Berendse et al., 2001). However, dopamine is present in the brain early in development, and functional dopamine receptors are expressed in the central nervous system prior to the onset of synaptogenesis, suggesting a role for dopamine in brain development that may be independent of its role at the synapse in the mature CNS. Neurotransmitters such as dopamine can influence brain development by modulating neurogenesis or neuronal and glial cell differentiation (Popolo et al., 2004). Recent studies show that dopamine receptor activation influences the cell cycle of neuroepithelial cells in the lateral ganglionic eminence and in the cerebral wall (Araki et al., 2006). Our findings demonstrate that CdCl2 simultaneously up-regulates dopamine D1r expression and down-regulates dopamine D2r expression. In particular, increasing CdCl2 concentrations induced a progressive increase in dopamine D1r expression, whereas low and high CdCl2 concentrations caused a significant decrease in dopamine D2r expression. Because dopamine D1r and D2r activation produces opposite effects on precursor cell activity, we hypothesize that dopamine's overall effects correlate with relative numbers and activity of each receptor subtype expressed on the FNC-B4 neuroblast cell line.
During development of the nervous system, neural stem cells give rise to both the neuronal and the glial populations. A dominant model of neural development is that neuronal and glial lineages diverge early, with neuroepithelial precursor giving rise to neuron-restricted and glial-restricted progenitors (Götz and Huttner, 2005). Although several molecular markers have been defined for neural progenitors, their identification, characterization, and function in neuronal and glial lineages have generated much discussion and are still not fully understood. Recent studies have demonstrated that some radial glia, the first cells differentiated within the embryonic neuroepithelium, have stem cell characteristics and produce neurons and astrocytes (Alvarez-Buylla et al., 2001). In the adult brain, neural stem cells are found in two regions of the periventricular germinal matrix, the subventricular or subependymal zone of the lateral ventricles and the subgranular zone of the hippocampal dentate gyrus (Watts et al., 2005). In addition to the “neurogenic” regions of the brain, multipotential neural stem cells are isolated from postnatal cerebellum (Lee et al., 2005). These cells express markers associated with neural progenitor and stem cells as well as GFAP, a defining marker of astroglia (Lee et al., 2005). GFAP-positive cells with characteristics of neural stem cells are obtained from fetal human brain parenchyma. These cells co-express both glial and neuronal markers, and they are able to differentiate into distinct neuronal and glial cell lineages (Götz and Huttner, 2005; Rieske et al., 2007).
From our data, it emerges that FNC-B4 neuroblasts treated with a high concentration of CdCl2 are forced to differentiate toward cells expressing both neuronal (β-tubulin III) and glial (GFAP) markers; this neuronal/glial precursor still expresses vimentin, though differently arranged in comparison with control cells and with neuroblasts treated with low concentrations of CdCl2, suggesting the ability to evolve both toward neuron and toward glial cells. As a matter of fact, vimentin is a critical cytoskeletal protein involved in the oxidative stress response (Choi et al., 2003) and initially is expressed by nearly all neuronal precursor but is substituted by neurofilaments in postmitotic neurons; the presence of vimentin in postmitotic cells is considered a typical marker of glial cells (Yabe at al., 2003). In addition, the increase in GFAP expression in FNC-B4 neuroblasts after CdCl2 exposure is in agreement with the hypothesis that one of the pathways of neurogenesis can begin from GFAP-positive cells (Maurer at al., 2008). Furthermore, the migration of neural progenitor cells from the subventricular zone to the olfactory bulb is allowed by the rostral migratory stream; in this circumscribed pathway, in a typical chain-like manner, migrating neural progenitors are guided by tubular formations of astrocytes, expressing GFAP, from their origin to the olfactory bulb, where they differentiate (Schachner, 1982; Alvarez-Buylla and García-Verdugo, 2002; Doetsch, 2003).
A remarkable feature of olfactory neurons is that they have a half-life in the range of weeks and are replaced by new neurons that differentiate from a progenitor present in the olfactory epithelium (Graziadei, 1973). Because FNC-B4 cell exposure to CdCl2 induces a decrease in some neural markers and an increase in neural differentiation marker expression, we hypothesize that CdCl2 might represent a specific signal for olfactory-lineage differentiation. We also speculate that CdCl2 in FNC-B4 neuroblasts might represent a selective cue by which, in a heterogeneous degree of maturity of the primary cell culture, the more committed progenitors and/or differentiated mature neurons rapidly move toward apoptosis, whereas the undifferentiated neural precursors are positively selected and forced into a state of active differentiation into glial cells and/or neurons, via an intermediate precursor expressing both glial and neuronal markers (Doetsch, 2003). The identification of the neuronal/glial cell precursor, the signaling that regulates self-renewal and differentiation of FNC-B4 cells, is basic and crucial for understanding the mechanisms of pathological processes leading to neurological diseases.




