High potassium induces taurine release by osmosensitive and osmoresistant mechanisms in the rat hippocampus in vivo
Abstract
The high potassium-evoked taurine efflux in the nervous tissue has been entirely considered to be the result of the cell swelling produced by KCl influx via passive Donnan forces. However, the extracellular taurine increase evoked in the hippocampus by applying 6–100 mM KCl through microdialysis probes, which saturates at a concentration of 25 mM KCl, is not congruent with the mentioned osmosensitive release of taurine stimulated by high potassium. Therefore, we studied whether the taurine release elicited by different high KCl concentrations (25, 50, 75, or 100 mM) was blocked under hypertonic conditions (+100 mM sucrose). Taurine release stimulated by 25 mM KCl was totally osmosensitive, but that released by higher KCl concentrations became progressively osmoresistant, achieving more than the 60% of the extracellular taurine enhancement during 100 mM KCl perfusion. The osmoresistant taurine release evoked by 100 mM KCl perfusion was partially reduced by a solution without Ca2+ and with high Mg2+, or by D,L-2-amino-5-phosphopentanoic acid, an N-methyl-D-aspartic acid (NMDA) receptor antagonist. Moreover, the release of taurine induced by a hypoosmotic solution was reduced by the presence ofeither high K+ (75 mM) or NMDA (100 μM). These results indicate that although moderately high [K+] evoke the osmosensitive release of taurine, higher [K+] inhibit it and trigger the release of taurine by an osmoresistant mechanism. This last component is partially mediated by NMDA receptors activated by the glutamate released during potassium-induced depolarization. © 2008 Wiley-Liss, Inc.
Taurine (2-aminoethanesulphonic acid) is an ubiquitous free amino acid in the brain accumulated in many cell types, including neurons and glial cells (Clements et al., 1989; Pow et al., 2002), where it can reach concentrations in the millimolar range (Palkovits et al., 1986). The origin of these high amounts of taurine is caused by the confluence of two processes: its biosynthesis from cysteine through the cysteine sulfinate pathway (Wu, 1982), and its intracellular uptake by specific taurine transporters (Smith et al., 1992). The existence of high amounts of free intracellular taurine makes this amino acid especially suited to behave as an organic osmolyte. In fact, it has been shown in a great variety of experiments that the stimulation of taurine release is associate with cell swelling induced by, among other things, hypotonic stress (Pasantes-Morales and Schousboe, 1988; Solís et al., 1988; Kimelberg et al., 1990), high potassium (Pasantes-Morales and Schousboe, 1989; Oja and Saransaari, 1992; Vitarella et al., 1994), weak organic acids (Lehmann and Sandberg, 1990; Solís et al., 1990), and glutamate receptor activation (Lehmann et al., 1985; Menéndez et al., 1990; Saransaari and Oja, 1991; Shibanoki et al., 1993). Such extensive data have led to the consensus that taurine probably plays a role in cell volume regulation in the vast majority of cells. Nevertheless, an osmoresistant release of taurine evoked by activation of glutamate receptors of N-methyl-D-aspartic acid (NMDA) type has been reported (Menéndez et al., 1990; Shibanoki et al., 1993; Tranberg et al., 2004). Although its physiological meaning is presently unknown, it has been proposed to serve as a protective mechanism against excitotoxicity (Oja and Saransaari, 2000).
Depolarization induced by the application of solutions containing high K+ is one of the stimuli most frequently used to evoke the exocitotic release of neurotransmitters. High potassium has been proven to also stimulate the release of taurine in cell cultures (Pasantes-Morales and Schousboe, 1989; Schousboe and Pasantes-Morales, 1989; Martin et al., 1990; Vitarella et al., 1994), brain slices (Oja and Kontro, 1987; Franco et al., 2001), synaptosomes (Kontro, 1979; Kontro et al., 1980; Hanretta and Lombardini, 1986; Tuz et al., 2004) and in vivo microdialysis studies (Solís et al., 1986; Fujikawa et al., 1996; Bockelmann et al., 1998). However, the K+-evoked taurine efflux has been entirely considered to be the result of the cell swelling produced by high KCl via passive Donnan forces because it was abolished when chloride was replaced by an impermeant anion or by increasing medium osmolality (Pasantes-Morales and Schousboe, 1989; Schousboe et al., 1990). However, it was only partially reduced by maneuvers interfering with calcium influx (Oja and Kontro, 1987; Schousboe and Pasantes-Morales, 1989). Curiously, in a previous study that used microdialysis probes implanted in the hippocampus in vivo (Solís et al., 1986), we found that the perfusion of isotonic solutions containing high concentrations of KCl (6–100 mM) evoked an extracellular taurine increase attaining saturation at an intermediate concentration of KCl (25–50 mM). This result is difficult to reconcile with the mentioned osmosensitive release of taurine stimulated by high potassium application because cell volume is positively correlated with the extracellular concentration of KCl (Walz, 1987). Therefore, we studied whether the taurine release elicited by different high concentrations of KCl were blocked under hypertonic perfusion conditions. We found that taurine release stimulated by 25 mM KCl was totally osmosensitive, but became progressively osmoresistant with further increments of KCl concentration. This component can explain more than a 60% of the extracellular taurine enhancement during 100 mM KCl perfusion. The osmoresistant release of taurine evoked by high potassium was partially mediated by NMDA receptors activated by the glutamate released during potassium-induced exocytosis.
MATERIALS AND METHODS
Reagents and Materials
The standard perfusion liquid was a solution of Krebs-Ringer bicarbonate (KRB) composed of the following (in mM): 122 NaCl, 3 KCl, 25 NaHCO3, 0.4 KH2PO4, 1.2 MgSO4, and 1.2 CaCl2. In some experiments the KRB was made either hypertonic by adding 100 or 150 mM sucrose or hypoosmotic by reducing the concentration of NaCl in 50-mM. In the solutions containing high concentrations of KCl, the concentration of NaCl was reduced in a equimolar amount. Drugs applied by addition to a standard or modified KRB solution included D,L-2-amino-5-phosphopentanoic acid (AP5), 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX) and NMDA were obtained from Sigma (Tres Cantos, Madrid, Spain). The drugs were prepared as stock solutions in distilled water (30 mM AP5, 20 mM CNQX, and 10 mM NMDA), stored frozen in the dark, and diluted to a final concentration in the perfusion solution immediately before use. All solutions were prepared with deionized water obtained from a Millipore Milli-Q water purification system (Bedford, MA). The osmolality of the perfusion solutions was determined by a microosmometer (Advanced Instruments Mod.3MO, Norwood, MA). The mean value of standard KRB solution was 285 ± 1 mOsm (n = 85) and the solution containing 100 mM KCl was 281 ± 2 mOsm (n = 13).
Animals and Surgical Procedure
Male Sprague Dawley rats (200–300 g) were anesthetized with an intraperitoneal injection of urethane (1.2 g/kg) and fixed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). The skin wounds and pressure points were infiltrated with mepivacaine (2%). Body temperature was maintained at 37°C with a heating pad and monitored by a rectal probe. The care and use of animals was carried out following the European Communities Council Directive (86/609/ECC).
Microdialysis Procedure
Microdialysis probes of side-by-side type were made in-house with a cuprophan membrane (Disscap, Hospal, CO) of 200 μm inner diameter and 1-mm active dialysis length. Each probe was reused no more than three times. Active area of the probe was implanted in the CA1 field of the dorsal hippocampus following the coordinates: 4.5 mm caudal to bregma, 2.6 mm lateral to midline and 2.5 mm bellow the dura (Paxinos and Watson, 1997). The probes were perfused with a standard or modified KRB solution at a flow rate of 2 μL/min with a syringe minipump (Bionalytical Systems, West Lafayette, ID). A perfusion period of 90 min without sampling was done to enable the recovery of basal levels of amino acids after microdialysis probe implantation. Afterward, perfusate samples were manually collected every 10 min and stored at −20°C until amino acid analysis.
Amino Acids Determination
The samples were derivatized with ortho-phthal-dialdehyde (OPA). The fluorescent derivatized amino acids were separated by reversed-phase chromatography on a Micra C18 column (33 × 4.6 mm, particle size 1.5 mm) by gradient elution. The mobile phase comprised of solvent A (0.05 M sodium acetate; pH 5.88) and solvent B (methanol) with a binary gradient (time = 0 min %B 2, time = 0.1 min %B 15, time = 1%B 47, time = 6%B 100, time = 9%B 2), at time = 13 min the column is ready for a new sample injection. Ultra pure water and solvents were filtered through 0.2 μm filters from Millipore (Bedford, MA). The solvent flow rate was adjusted to 0.5 mL/min and the injection volume was 10 μL. Fluorescence detection (Perkin-Elmer LS4, Norwalk, CT) was performed at 365 and 455 nm for excitation and emission wavelengths, respectively. Amino acids were identified by their retention times, and their concentrations were calculated by comparing them to calibrated amino acid external standard solutions (1.5 μM). Baseline extracellular levels of the amino acids studied in the present study were (in pmol/sample): 3.0 ± 0.3 for glutamate, 29.2 ± 2.1 for glutamine, and 5.5 ± 0.5 for taurine (n = 46).
Statistical Analysis
The results were statistically evaluated with two-way analysis of variance (ANOVA) followed by Bonferroni test as a post hoc evaluation to compare correlative samples under different experimental conditions over time. The hypothesis that taurine release induced by a hypoosmotic solution containing NMDA was different from the sum of the means of the taurine released during NMDA and hypoosmotic application, was tested by a linear combination of the factor level means with coefficients (− 1, 1, 1) for NMDA + hypoosmotic, NMDA and hypoosmotic, respectively. One-way ANOVA was used for this analysis. Differences were considered statistically significant when P < 0.05.
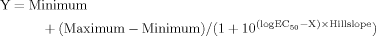
RESULTS
Extracellular Taurine Increase Stimulated by Different KCl Concentrations Under Isotonic or Hypertonic Conditions
After a baseline perfusion period of 1 hr with a standard KRB, an isotonic solution in which KCl concentration was enhanced to 25, 50, 75, or 100 mM was perfused for 1 hr. Afterward, the perfusion with the solution containing the standard KCl concentration was resumed for two additional hours. In these experiments the levels of taurine, glutamate and glutamine were analyzed in the 10-min perfusate samples. Changes in the extracellular levels of glutamate were regarded as representative of the stimulation of neurotransmitters release process by K+-induced depolarization. Furthermore, the extracellular concentration of glutamine was also determined in order to detect unspecific efflux of amino acids due to cell lysis because the interstitial levels of this amino acid are usually reduced under high K+ exposure (Szerb, 1991). Perfusion with different high-K+ solutions induced the increase of extracellular concentration of taurine that peaked during the second 10-min sample, remaining enhanced until the end of high-K+ application (Fig. 1A). The dose dependence of the high K+ effect, considering the total amount of taurine obtained during the 1 hr of K+ perfusion, yielded an apparent EC50 of 33 mM (Fig. 2A). In contrast, glutamine content in the perfusate was inversely related with the K+ concentration in the perfusion solution (Fig. 1C). Significant and transitory increments in extracellular glutamate levels were only observed during 75 and 100 mM KCl perfusion (maximum peak of 258% ± 116% and 267% ± 50%, respectively; Fig. 1B), a concentration range during which taurine increase attained saturation (maximum peak of 782% ± 123% and 730% ± 71%, at 75 and 100 mM KCl, respectively; Fig. 2). When the perfusion with the standard KRB solution was resumed, the high K+-induced changes in the extracellular levels of amino acids returned to baseline values between 30 and 90 min depending on the concentration of KCl that was applied and the amino acid analyzed, with the recovery of glutamine faster than that of taurine.
Effect of high K+ concentrations on extracellular levels of taurine, glutamate and glutamine under isotonic and hypertonic conditions. The concentration of KCl in the perfusion solution was increased to 25, 50, 75, or 100 mM for 1 hr (indicated by the horizontal bar) and the extracellular levels of taurine (A), glutamate (B), and glutamine (C) were analyzed in the 10-min dialysate samples. These experiments were carried under isotonic conditions (open circles, n = 6–12 per group) or in the continuous presence of a hyperosmotic solution made by adding 100 mM sucrose (solid circles, n = 6–9 per group). Data were normalized to the mean value of the six 10-min samples obtained before high K+ application and are expressed as means ± SEM. ☆P < 0.05; ☆☆P < 0.01; ☆☆☆P < 0.001 comparing correlative samples under isotonic and hypertonic conditions.
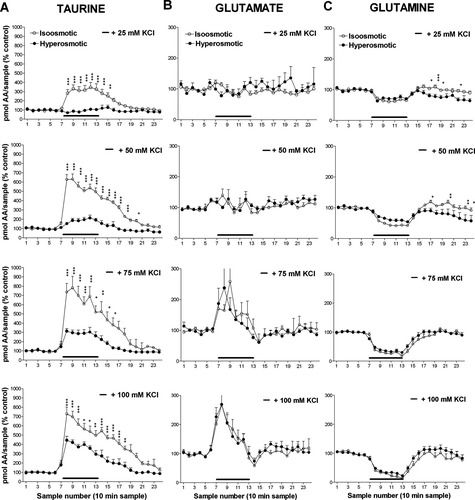
Effect of K+ concentration on the osmosensitive and osmoresistant components of the K+-induced taurine release. A: Data represent total taurine release obtained during the six samples with high K+ perfusion under isotonic (open circles) or hypertonic (solid circles) conditions from the experiments shown in Figure 1. B: The amount of taurine released during 1-hr high K+ perfusion under isotonic conditions was considered to be 100%. That taurine released under hypertonic conditions was assumed to be the osmoresistant component (clear bars). The difference between total taurine release and the osmoresistant component gave the osmosensitive fraction (dark bars) of the high K+-induced taurine release.
The effects of different high [K+] on the extracellular levels of glutamate and glutamine were not modified when the experiments were carried out under hypertonic conditions, made by adding 100 mM sucrose to the perfusion solution (Fig. 1B,C). In contrast, this hypertonic medium reduced the taurine increase produced by high K+ solutions, with an efficiency that was inversely correlated with the concentration of KCl perfused (Fig. 2). So whereas the taurine release evoked by 25 mM KCl was totally due to osmotic-sensitive mechanisms, that stimulated by 100 mM KCl displayed a 60% component resistant to osmotic changes. This osmoresistant component of the K+-induced taurine release did not appear to be due to an inefficient blockade of swelling by 100 mM sucrose because when, in another set of experiments, 100 mM KCl was applied in a hypertonic medium of 150 mM sucrose, taurine increase (2,284% ± 566% measured during 1 hr high K+ perfusion; data not shown) was statistically indistinguishable (P > 0.05) from that found in the presence of 100 mM sucrose (2,259% ± 140%).
Glutamate Receptors Involvement in the High-K+-induced Release of Taurine
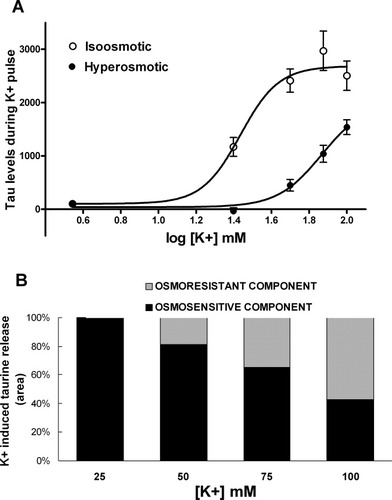
Exocytotic release of neurotransmitters stimulated by high K+ application depends on the extracellular concentration of Ca2+. To assess whether such a mechanism participate in the increase of extracellular taurine during 100 mM KCl perfusion, we carried out a group of experiments in which Ca2+ was omitted from the perfusion medium and the concentration of Mg2+ was enhanced to 20 mM, a condition known to block the voltage-activated calcium channels required for exocytosis (Katz and Miledi, 1965). The perfusion of this solution containing low Ca2+ and high Mg2+ for 3 hr did not cause significant modifications of extracellular baseline levels of neither glutamate nor taurine (11 ± 10% and 6 ± 15%, respectively; n = 4; P > 0.05; data not shown), but strongly reduced the extracellular increase of glutamate stimulated by 100 mM KCl (Fig. 3B). Interestingly, under this experimental condition, the K+-induced taurine increase was inhibited only during the first 20 min of 100 mM KCl perfusion (Fig. 3A), indicating that taurine release during this period of time was triggered by a calcium-mediated exocytotic process.
Effect of extracellular Ca2+ lowering on the release of taurine and glutamate evoked by 100 mM KCl. A: Data represent the mean ± SEM of the taurine levels obtained from the experiments performed with a standard perfusion solution (open circles; n = 9; corresponding to the experiments shown in Fig. 1A), or under a solution without Ca2+ and with 20 mM Mg2+ (closed circles; n = 4). In these experiments the solution containing 100 mM K+ was perfused from sample 6 to sample 12. B: Extracellular levels of glutamate obtained in the experiments presented in (A). ☆P < 0.05; ☆☆P < 0.01; ☆☆☆P < 0.001 comparing correlative samples in the absence and the presence of Ca2+.
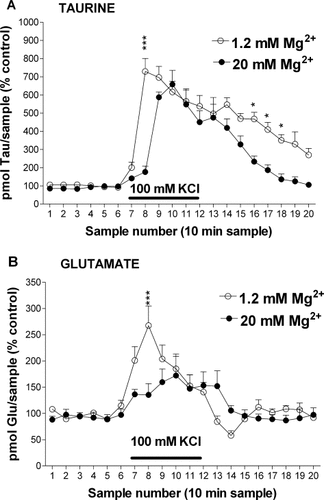
Alternatively, taurine release could have been indirectly caused after the activation of glutamate receptors by the glutamate released during high K+ stimulation because previous works show that glutamate receptors activation enhances extracellular taurine levels (Lehmann et al., 1985; Menéndez et al., 1989). We evaluated this possibility in a group of perfusion experiments conducted in the continuous presence of CNQX (200 μM) and AP5 (1 mM), antagonists of AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid)/kainate and NMDA receptors, respectively. Under these conditions, the extracellular taurine increase evoked by 100 mM KCl perfusion was significantly smaller than that obtained in the absence of the antagonists, especially at the peak occurring during the second sample (Fig. 4A).
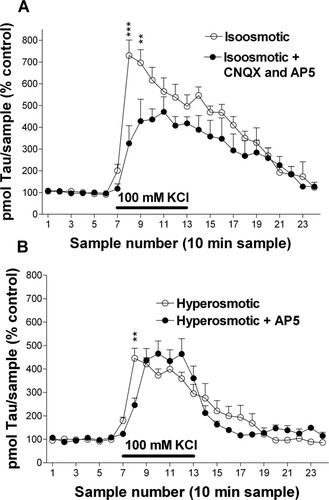
Glutamate receptor antagonists partially reduce osmosensitive and osmoresistant components of the 100 mM KCl-induced release of taurine. The horizontal bar represents the period of high K+ application. A: Extracellular taurine levels obtained under isoosmotic conditions in the continuous presence of glutamate antagonists, CNQX (200 μM) and AP5 (1 mM), (n = 4; solid circles), or without antagonists (n = 12; open circles; data presented in Fig. 1A). B: Extracellular taurine levels analyzed in perfusates obtained with hypertonic solutions (+100 mM sucrose) containing AP5 (1 mM), a NMDA receptor antagonist, (n = 5; solid circles), or without AP5 (n = 9; open circles; data presented in Fig. 1A). ☆P < 0.05; ☆☆P < 0.01; ☆☆☆P < 0.001 comparing correlative samples in the absence and the presence of glutamate receptor antagonists.
Considering this result and knowing that NMDA receptor activation produces taurine release by an osmotically insensitive process (Menéndez et al., 1990; Shibanoki et al., 1993), we next wanted to elucidate whether the osmoresistant component of the high K+-induced taurine release was attributable to NMDA receptor activation secondary to the release of endogenous glutamate. This task was carried out in a group of experiments in which a hyperosmotic medium (100 mM sucrose) containing the NMDA antagonist AP5, was continuously perfused. We found that the presence of AP5 affect taurine release only during the first 20 min but not during the rest of the time (1 hr) of the 100 mM KCl application (Fig. 4B). These results demonstrate that an early component of the osmoresistant release of taurine induced by 100 mM KCl involves NMDA receptor activation.
High K+ Application Reduces Osmosensitive Taurine Release
In the next group of experiments we wanted to confirm more directly the possible inhibitory effect of high K+ conditions on the osmosensitive component of taurine release. To this end, we perfused a KRB with reduced osmolality (−100 mOsm) during 40 min, which elicited a 10-fold increase of extracellular taurine levels (Fig. 5A) but only marginally modified the levels of other amino acids as presented for the case of glutamate (152% ± 15%; Fig. 5B) and glutamine (107% ± 32%; Fig. 5C). During the subsequent 40 min, the concentration of K+ in the hypotonic solution was increased to 75 mM while reducing NaCl concentration in an equimolar amount, which caused a significant reduction of the hypoosmotic-induced taurine increase. In contrast, extracellular concentration of amino acids with putative neurotransmitter role such as glutamate and gamma-aminobutyric acid (GABA), was much increased during high K+ application (to 734% ± 100% for glutamate, and from a value lower than 0.5 pmol/sample to 3.9 ± 0.3 pmol/sample for GABA, data not shown). These increases were even larger than those obtained in the experiments where K+ was applied under isotonic conditions. Nevertheless, the reduction of glutamine levels caused by 75 mM K+ was similar under isotonic and hypotonic conditions (to 29% ± 7% and 33% ± 4% of baseline values, respectively). These experiments indicate that the application of high concentrations of K+ has a negative effect on the osmosensitive release of taurine.
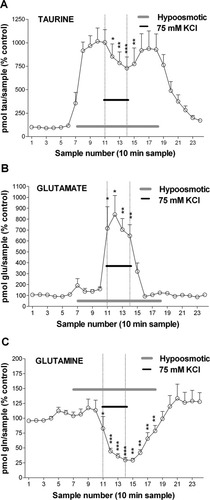
Effect of hypoosmotic conditions on the changes in extracellular levels of taurine, glutamate and glutamine induced by high K+. The graphs represent the time course of changes caused by a hypoosmotic solution (−50 mM NaCl; horizontal clear bar) alone or with a high concentration of KCl (75 mM; horizontal black bar) on the levels of taurine (A), glutamate (B), and glutamine (C). ☆P < 0.05; ☆☆P < 0.01; ☆☆☆P < 0.001, compared with the mean of the two values previous to high K+ perfusion (n = 5–7).
Hypoosmotic-induced Taurine Release was Reduced by NMDA Receptor Activation
Churchwell et al. (1996) found that the activation of NMDA receptors inhibited the regulatory volume decrease (RVD) triggered by veratridine-induced swelling in cortical neurons. This observation led us to test whether the activation of this type of receptors, although inducing an osmoresistant release of taurine as shown above, were affecting negatively the osmosensitive release of taurine. To address this possibility, we activated NMDA receptors by perfusing trough the microdialysis probe a solution containing 100 μM NMDA. Twenty minutes after the beginning of NMDA application, the osmolality of perfusion solution was reduced (−100 mOsmol) to evoke a pure osmosensitive release of taurine. The time course of taurine release induced during 1 hr perfusion of NMDA alone was studied in an independent group of experiments (n = 5), where we observed a peak of taurine release during the first 10-min sample (325% ± 63%) that progressively decay toward baseline values during subsequent samples, as reported before (Menéndez et al., 1989; Shibanoki et al., 1993). As shown in Figure 6, the hypoosmotic-induced release of taurine in the presence of NMDA (n = 7) was reduced when compared with that obtained in interleaved experiments in the absence of the glutamate agonist (n = 6). This was more evident when the algebraic addition of extracellular taurine levels obtained during the last four samples of NMDA perfusion experiments with the four first samples of hypoosmotic experiments (4,677 ± 434) was statistically compared (P = 0.016) with the values obtained in those experiments in which hypoosmotic medium was applied in the presence of NMDA (2,766 ± 319). This result strongly indicates that the osmosensitive release of taurine is reduced by the activation of NMDA receptors.
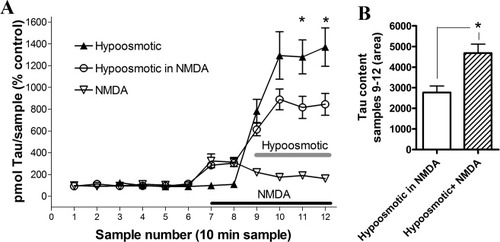
NMDA receptor activation reduces hypoosmotic-induced taurine release. A: Time course of changes on the extracellular taurine levels induced by a hypoosmotic solution (−100 mOsmol, horizontal gray bar) in the presence (open circles, n = 7) and absence (solid triangles, n = 6) of NMDA (100 μM; horizontal black bar). Open triangles depict taurine changes caused by NMDA application alone (n = 5). ☆P < 0.05 comparing correlative samples under hypoosmotic conditions in the presence and absence of NMDA. B: Open bar represents the area of taurine content obtained during the whole period of hypoosmotic perfusion in the presence of NMDA (open circles in A), and the dashed bar represents the algebraic addition of taurine levels obtained during the last four samples of NMDA perfusion experiments (open triangles in A) with the four first samples of hypoosmotic experiments (solid triangles in A). ☆P = 0.016 (see Materials and Methods for statistical analysis of this comparison).
DISCUSSION
Taurine is known to act in a large number of cellular types as an organic osmolyte participating in cell volume regulation. Taurine release stimulated by exposure to high concentrations of potassium has usually been regarded as the consequence of cellular swelling produced under this condition. In the present work, we have found two components of the high-potassium-induced taurine efflux that can be distinguished by their sensitivity to hypertonic solutions. Although moderately high potassium solutions (25 mM) evoke taurine release by a process totally blocked by hypertonic media, much higher K+ concentrations (75–100 mM) induce extracellular taurine increases that are largely resistant to such hypertonic conditions and that are partially due to the activation of NMDA receptors. In addition, we found that the release of taurine induced by a hypotonic solution was reduced when the extracellular concentration of K+ was enhanced to 75 mM or in the presence of NMDA. We have to emphasize that although we considered the increase of extracellular taurine levels as resulting mainly from the intensification of taurine release, changes in taurine uptake might also contribute.
It is widely known that high K+ media cause the extracellular increase of neurotransmitters by acting mainly at three levels: 1) depolarizing synaptic terminals and triggering vesicular exocytosis, 2) entering the cell and allowing the influx of chloride ions and water, which results in isoosmotic swelling, and 3) collapsing the ionic gradients required for neurotransmitter uptake through membrane transporters. In the case of taurine, its release induced by high K+ appears to be mainly related to its osmolyte role (Pasantes-Morales et al., 1989; Martin et al., 1990; Schousboe et al., 1990; Vitarella et al., 1994; Cardin et al., 1999). An exocytosis-mediated release of taurine from synaptic terminals has also been reported (Kontro, 1979; Kontro et al., 1980), but even in this case it has been suggested that is associated with the osmotic regulation of the vesicular pool (Tuz et al., 2004).
In our experiments with the application of 100 mM K+ in the hippocampus by in vivo microdialysis, we detected an early component (during the first 20 min of perfusion) that was totally inhibited by Mg2+ and partially reduced by hypertonic media. These results could be interpreted as the existence of a pool of taurine released by exocytosis. However, because this early component was also reduced by glutamate receptors antagonists, we consider more plausible the possibility that it was indirectly produced through the activation of glutamate receptors by the glutamate released by exocytosis.
The extraction fraction of potassium from a microdialysis probe was estimated by Fujikawa et al. (1996) in rat amygdala. They reported that when perfusing 100 mM KCl, 12% of potassium was extracted by the tissue. This small increase of extracellular potassium concentration in brain tissue exposed to high concentrations of KCl through a microdialysis probe seems to be due to the accumulation of potassium by glial cells in the vicinity of the probe (Largo et al., 1996). Considering these data, our findings of osmosensitive taurine release induced with KCl concentrations of 25–50 mM might be viewed as the result of glial swelling caused by K+ buffering. Nevertheless, this possibility is difficult to confirm because it is really impossible to know what the sources of extracellular taurine are with microdialysis in vivo.
We found that the extracellular taurine increase induced by high K+ containing solutions, have an osmotic sensitive-component that was negatively correlated with the concentration of K+ in the perfusion liquid. This result contrasts with the strong association of the K+-stimulated taurine release with cell swelling demonstrated in both cultured astrocytes (Pasantes-Morales and Schousboe, 1989; Martin et al., 1990; Vitarella et al., 1994) and neurons (Schousboe et al., 1990). However, our data are congruent with observations obtained in slices from cerebral cortex where a component of the K+-induced taurine release was not explained by cell volume adjustments (Oja and Saransaari, 1992). Thus, it seems that an osmoresistant release of taurine is especially evident when whole tissue preparations are exposed to high K+ concentrations. The close proximity among cells in the tissue might enable other neuroactive substances, released during high potassium stimulation, to activate the osmoresistant release of taurine. In our experiments the extracellular overflow of glutamate, which was unaltered by hypertonic media, was clearly detected in the presence of solutions containing 75 or 100 mM K+, just when the osmoresistant component of taurine release appears. The extracellular glutamate increase observed under these conditions might be the result of two processes: the enhanced release of glutamate and the inhibition of glutamate reuptake caused by the low extracellular sodium concentration and membrane depolarization imposed with high K+-containing solutions. Because the activation of glutamate receptors of NMDA type produce the release of taurine by a process independent of cell swelling (Menéndez et al., 1990; Shibanoki et al., 1993; Tranberg et al., 2004), it might be possible that the activation of NMDA receptors by the released glutamate was the cause of the osmoresistant release of taurine. In fact, we have detected the participation of NMDA receptors during an early phase (first 20 min) of the osmoresistant release of taurine. The pathway for the osmoresistant release of taurine induced by NMDA receptor activation has not been identified, although it has been proposed that Ca2+-dependent Cl− channels could be a possible pathway for the taurine efflux independent of cell swelling (Tranberg et al., 2004).
Taurine transporters working in reverse mode by the depolarizing and low extracellular Na+ conditions imposed during 100 mM K+ perfusion, could also be the source for a possible osmoresistant release of taurine. Unfortunately, the lack of specific compounds blocking taurine transporters -the available GES, is a substrate of the transporter causing taurine release by heteroexchange (Huxtable et al., 1979; Herranz et al., 1990), has precluded the assessment of the relative impact of taurine transporters in the extracellular taurine increase evoked by high-potassium containing solutions. Nevertheless, the collapse of ionic gradient under high K+ conditions might have contributed to enhance extracellular taurine levels by impairing the reuptake of taurine released by both osmosensitive and osmoresistant mechanisms.
High K+ concentrations above 50 mM induce taurine release by an osmoresistant process but also affect negatively the osmosensitive release of taurine, as can be deduced from our observation that the hypoosmotic-induced taurine release was reduced in the presence of a high concentration of K+ (75 mM). The reduction of the osmosensitive component does not appear to be due to the depletion of taurine in the cellular pool from where it is released because an hypoosmotic solution evoked a constant taurine increase with higher magnitude than that observed with 100 mM K+ perfusion (Figs. 5 and 6). On the other hand, cultured cells exposed to a hypoosmotic solution containing high concentrations of potassium accumulates KCl imposed by Donnan equilibrium, which leads the cells to swell normally but without showing RVD (Pasantes-Morales et al., 1993, 1994; Olson et al., 1995) and to induce a noninactivating taurine efflux (Cardin et al., 1999).
Another possibility is that the appearance of the osmoresistant release of taurine and the reduction of the osmosensitive component are linked. This possibility is supported by our data showing that NMDA receptor activation reduces the osmosensitive taurine release evoked by hypoosmotic-induced swelling (Fig. 6) because, as pointed out before, the osmoresistant component of high K+–induced taurine release was partially due to NMDA receptor activation, and it emerged when the osmosensitive part began to diminish. Interestingly, it has been found in cultures of cortical neurons that RVD activated during the isotonic swelling induced by veratridine was inhibited by NMDA receptor activation through an unidentified mechanism (Churchwell et al., 1996).
Although there is general consensus that the osmosensitive release of taurine plays a role in cell volume regulation (Schousboe and Pasantes-Morales, 1992) and even in neurotransmission between glial cells and neurons in the supraoptic nucleus (Hussy et al., 2001), the physiological meaning of the osmoresistant release of taurine is unknown. We propose that the osmoresistant release of taurine, which is mainly stimulated by NMDA receptor activation, may be involved in some forms of synaptic facilitation phenomena where both NMDA receptors and taurine participate (del Olmo et al., 2004; Súrez and Solís, 2006).
During cerebral ischemia and traumatic brain injury, extracellular concentration of K+ can rise to the 50–80 mM range (for review, see Leis et al., 2005), which is accompanied by the extracellular enhancement of glutamate concentration (Benveniste et al., 1984; Bullock et al., 1998). According to our findings, and considering that taurine has been proposed to protect against excitotoxicity (Oja and Saransaari, 2000), the possible reduction of the osmosensitive release of taurine caused under these pathological situations might contribute to excitotoxic cell injury.
Acknowledgements
We thank Dr. R. Martín del Rio for helpful comments, Javier Zamora for help with data analysis, and María J. Asensio and José Barbado for technical assistance.




