Neuroprotective effects of acetyl-L-carnitine on neuropathic pain and apoptosis: A role for the nicotinic receptor
Abstract
Several pathologies related to nervous tissue alterations are characterized by a chronic pain syndrome defined by persistent or paroxysmal pain independent or dependent on a stimulus. Pathophysiological mechanisms related to neuropathic disease are associated with mitochondrial dysfunctions that lead to an activation of the apoptotic cascade. In a model of peripheral neuropathy obtained by the loose ligation of the rat sciatic nerve, acetyl-L-Carnitine (ALCAR; 100 mg/kg intraperitoneally [i.p.] twice daily for 14 days) was able to reduce hyperalgesia and apoptosis. In the present study, different mechanisms for the analgesic and the antineuropathic effect of ALCAR are described. The muscarinic blocker atropine (5 mg/kg i.p.) injected simultaneously with ALCAR did not antagonize the ALCAR antihyperalgesic effect on the paw-pressure test but significantly reduced the analgesic effect of ALCAR. Conversely, the antineuropathic effect of ALCAR was prevented by cotreatment with the nicotinic antagonist mecamylamine (2 mg/kg i.p. twice daily for 14 days). A pharmacological silencing of the nicotinic receptors significantly reduced the X-linked inhibitor of apoptosis protein–related protective effect of ALCAR on the apoptosis induced by ligation of the sciatic nerve. Taken together, these data highlight the relevance of nicotinic modulation in neuropathy treatment. © 2008 Wiley-Liss, Inc.
Neuropathic pain is an unpleasant, abnormal signaling associated with injury or malfunction in the peripheral or central nervous system. Characteristically, it can persist in the absence of visible injury or clinically measurable inflammation. This painful experience can originate from peripheral, spinal, or supraspinal sites as a result of conditions such as focal trauma neuropathies, toxic neuropathies, or postherpetic neuralgia; chronic lower back pain or other spinal injury; or pain associated with stroke, brain tumors, and acquired immunodeficiency syndrome (Jensen et al., 2001).
The acetyl ester of L-carnitine isomer (ALCAR) is able to raise the pain threshold, showing an analgesic effect in acute pain conditions (Ghelardini et al., 2002; Galeotti et al., 2004) and an antihyperalgesic effect both in animal and human neuropathic conditions (Chiechio et al., 2002; Osio et al., 2006). Moreover, ALCAR shows a protective action profile on the nervous tissue. It has been demonstrated that in vitro, ALCAR is able to attenuate the rate of neuronal mortality (Manfridi et al., 1992) and to decrease the neurotoxicity evoked by mitochondrial uncoupling factors (Virmani et al., 1995). In vivo, an ALCAR protective effect was shown in rats that underwent sciatic nerve transection (Fernandez et al., 1989) or division (Hart et al., 2002), and after they underwent chemotherapy (Pisano et al., 2003). McKay-Hart et al. (2002) described an ALCAR proregenerative effect on peripheral nerve after trauma. In addition, antiapoptotic effects of ALCAR in a peripheral mononeuropathy model were described in our laboratory (Di Cesare Mannelli et al., 2007).
The pharmacological effects of ALCAR can be attributed to the physiological properties of L-carnitine transport of small-, medium-, and long-chain fatty acids across the mitochondrial membranes. Furthermore, the presence of the acetyl group makes ALCAR a strategic partner for normal mitochondrial function as an important donor of acetyl-group donor during high-energy metabolism and in anabolic reactions (Bremer, 1990; Pettegrew et al., 2000). Finally, ALCAR has also an antioxidant activity (Mansour, 2006) and enhances acetylcholine (ACh) production. Microdialysis studies demonstrated increased ACh release in both rat striatum and hippocampus after ALCAR administration (Imperato et al., 1989). Such increase in ACh levels could be due to the capability of ALCAR to function as precursor of ACh. In fact, the synthesis of [14C]ACh from [14C]ALCAR was observed in rat brain (White and Scates, 1990). The cholinotropic and cholinoprotective activities of ALCAR might depend on its ability to increase acetyl-CoA levels (Bigini et al., 2002). The cholinergic pathways, activated by the muscarinic receptor M1-subtype, are likely to be responsible for ALCAR antinociceptive activity observed in the mouse hot plate and abdominal constriction tests, as well as in the rat paw-pressure test (Ghelardini et al., 2002; Galeotti et al., 2004). Nevertheless, the cholinergic component of the effects elicited by ALCAR in neuropathic pain and neuroprotection remain unknown. Moreover, study of the modulation of nicotinic acetylcholine receptors (nAChR) for neuropathic pain treatment is drawing interest (Bunnelle et al., 2007).
To explore the pharmacodynamic profile of ALCAR, we evaluated the role of the muscarinic and nicotinic components of the antihyperalgesic effects of ALCAR after repeated administration in a rat model of chronic constriction injury (CCI). A relationship between the mechanisms of action of ALCAR antineuropathic and antiapoptotic effects is proposed.
MATERIALS AND METHODS
Animals
Male Sprague Dawley rats from Harlan-Italia were used. Four rats were housed per cage (size 26 × 41 cm) and placed in the experimental room for acclimatization 24 hr before the test. The animals were fed with standard laboratory diet and with tap water ad libitum, and they were kept at 23 ± 1°C with a 12 hr light/dark cycle, light at 7 A.M. All experiments were carried out in accordance with the European Community Council Directive of November 24, 1986, for the care and use of laboratory animals. All efforts were made to minimize animal suffering and reduce the number of animals used.
Peripheral Rat Mononeuropathy
Neuropathy was induced according to the procedure described by Bennett and Xie (1988). Briefly, rats were intraperitoneally (i.p.) anesthetized with 400 mg/kg chloral hydrate. Under aseptic conditions, the right common sciatic nerve was exposed at the level of the middle thigh by blunt dissection. Proximal to the trifurcation, the nerve was carefully freed from the surrounding connective tissue and four chromic catgut ligatures (4-0; Ethicon) were tied loosely around it with about 1-mm spacing. After hemostasis was confirmed, the incision was closed in layers. The animals were allowed to recover from surgery and then housed one per cage with free access to water and standard laboratory chow.
Drug Administration
ALCAR was purchased from Sigma-Tau, atropine and mecamylamine from Sigma. The administration was performed twice a day i.p. For cotreatment with ALCAR and atropine or mecamylamine, two consecutive injections were provided.
Paw-Pressure Test
The nociceptive threshold in the rat was determined with an analgesimeter (Ugo Basile) according to the method described by Leighton et al. (1988). Briefly, a constantly increasing pressure was applied to a small area of the dorsal surface of the paw with a blunt conical probe by a mechanical device to a small area of the paw. Mechanical pressure was increased until vocalization or a withdrawal reflex occurred while rats were lightly restrained. Vocalization or withdrawal reflex thresholds were expressed in grams. Rats scoring below 40 g or over 75 g during the test before drug administration (25%) were rejected. An arbitrary cutoff value of 250 g was adopted. The data were collected by an observer who was blinded to the protocol.
Tissue Explants
After treatment, animals were killed, and the right sciatic nerves 1 cm upstream the ligation and trifurcation were explanted. The same procedure was performed for the left unoperated nerve. Tissue extracted was approximately 20 mg. All the experiments were performed using the entire portion of the nerve, including the ligature site, except for the TUNEL (terminal deoxynucleotidyl transferase dUTP nick-end labeling) assay. For this assay, portions of the nerve, approximately 1 cm upstream (proximal) and 1 cm downstream (distal) the ligation were processed, and the nerve fragments carrying the ligature were left out. Each experiment was performed on a single rat nerve.
Western Blot Test
Tissue was homogenized in lysis buffer containing 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, and Complete Protease Inhibitor (Roche). The homogenate was incubated on ice for 30 min, and then the suspension was sonicated on ice with three 10-sec bursts at high intensity with a 10-sec cooling period between each burst. The samples were centrifuged at 13,000g for 15 min at 4°C. Protein concentrations in the supernatant were measured by bicinchoninic acid (Sigma-Aldrich) assay. Thirty micrograms of each protein extracts were separated on a 4%–12% sodium dodecyl sulfate–polyacrylamide gel by electrophoresis and transferred onto nitrocellulose membranes (Bio-Rad). Membranes were blocked with 5% nonfat dry milk in phosphate-buffered saline containing 0.1% Tween-20 (PBST) and then probed overnight with primary antibodies (caspase 3, cytochrome c, poly(ADP-ribose)polymerase (PARP), and X-linked inhibitor of apoptosis protein (XIAP) antibodies; Cell Signaling; 1:1,000 in PBST/5% milk). After washing with PBST, the membranes were incubated for 1 hr in PBST containing the appropriate horseradish peroxidase–conjugated secondary antibody (1:5,000) and washed again. Enhanced chemiluminescence (Pierce) was used to visualize the peroxidase-coated bands. Densitometric analysis was performed by Scion Image analysis software. β-Actin normalization was performed for each sample.
Caspase 3 Enzymatic Activity
Nerve segments (20 mg each) were homogenized in 200 mM Tris-HCl buffer (pH 7.5) containing 2 M NaCl, 20 mM EDTA, and 0.2% Triton X-100 and centrifuged at 5,000g for 5 min at 4°C. Protein concentrations in the supernatants were determined as reported for Western blot testing. Fifty microliters of the supernatants were incubated with 0.025 mM fluorogenic peptide caspase substrate 0.025 mM rhodamine 110 bis-(N-CBZ-L-aspartyl-L-glutamyl-L-valyl-L-asparticacid amide) (Z-DEVD-R110; Molecular Probes) in a buffer containing 10 mM PIPES pH 7.4, 10 mM EDTA, and 0.5% CHAPS at 25°C for 30 min. The amount of cleaved substrate in each sample was measured in a 96-well plate fluorescent spectrometer (Perkin-Elmer; excitation at 496 nm and emission at 520 nm). Each experiment was performed in duplicate.
Cytochrome c: Cytosolic Fraction Extraction
Extraction was performed as reported by Cervia and coworkers (2006) with minor modifications. Briefly, nerve tissue was homogenized in 10 mM Tris-HCl buffer (pH 7.5), 0.3 M sucrose, 1 mM EDTA, and Complete Protease Inhibitor (Roche). Samples were centrifuged at 10,000g for 60 min at 4°C, and the supernatants containing the cytosolic fraction were collected. Pellets were resuspended in a cold buffer containing 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and Complete Protease Inhibitor (Roche) and sonicated on ice with three 20-sec bursts at high intensity with a 10-sec cooling period between each burst. Supernatants containing the mitochondrial fraction were obtained by centrifugation at 10,000g (30 min, 4°C). Protein concentrations were determined as reported. Western blot test with cytochrome c oxidase subunit IV (COX IV) antibody was performed to verify the absence of mitochondrial contaminants in the cytosol preparation.
DNA Fragmentation: TUNEL
Sections (7 μm) obtained from paraffin-embedded sciatic nerves were rehydrated and then digested with 20 μg/mL proteinase K for 20 min at room temperature (RT). After rinsing in Tris-buffered saline, endogenous peroxidases were inactivated with 30% H2O2, 1:10 in methanol for 5 min at RT. After equilibration with a 0.5 M Tris-HCl buffer (pH 8.0) containing 0.5 M NaCl and 0.1 M MgCl2, sections were incubated with reaction mix containing labeled and unlabeled deoxynucleotides and Klenow enzyme (Calbiochem) at 37°C for 1.5 hours. Labeling reactions were stopped by incubating for 5 min with 0.5 M EDTA (pH 8.0) at RT. Subsequently a peroxidase–streptavidin conjugate was applied for 30 min at RT, and detection was obtained by incubation with 3,3′-diaminobenzidine-H2O2/urea solution (15 min, RT). Sections were then treated with 0.3% methyl green for 6 min.
Statistical Analysis
Results were expressed as the means ± SEM, and an analysis of variance was performed. A Fisher's protected least significant difference procedure was used as post hoc comparison. P values of less than 0.01 were considered significant. Data were analyzed by Origin 7.5 software.
RESULTS
CCI is a model of mononeuropathy and elicits a pain syndrome characterized by hyperalgesia that begins about 3 days after nerve injury, reaches a plateau between 7 and 15 days, and then decreases (Takaishi et al., 1996). Fifteen days after the operation, rats that underwent the right sciatic nerve ligation showed a nociceptive threshold of 35.2 ± 4.5 g (Fig. 1) in response to the paw-pressure test, compared with a threshold of 63.6 ± 4.5 g in the contralateral part (Fig. 1). Repeated treatments with ALCAR (100 mg/kg i.p. twice per day) for 15 days starting on the day of the operation significantly increased the threshold of the nociceptive response to a mechanical stimuli both in the left (121.3 ± 5.8 g; analgesic effect) and in the right paw (55.8 ± 4.7 g; antihyperalgesic effect; Fig. 1). Administration of the muscarinic receptor blocker atropine (5 mg/kg i.p. twice per day for 15 days) together with ALCAR significantly reduced the analgesic effect of the acetyl derivative on the left paw (67.2 ± 5.1 g) but did not alter the antihyperalgesic effect on the right paw (52.8 ± 3.9 g; Fig. 1). Atropine did not alter the pain threshold in saline-treated CCI rats (Fig. 1). Similar results were obtained after 7 days' treatment with ALCAR with or without atropine (Fig. 1).
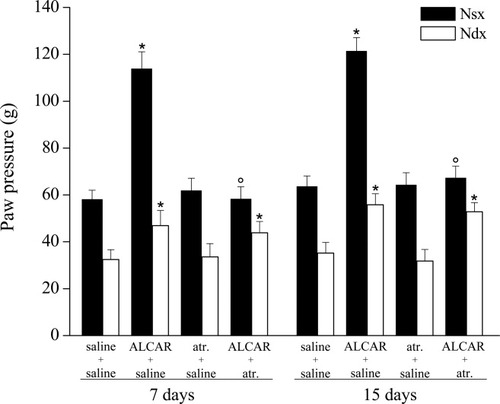
Effect of atropine (atr.) on paw-pressure test. Response to noxious stimuli on the right paw (Ndx) that underwent CCI respect to the contralateral unoperated paw (Nsx). Effects of treatments with saline alone, ALCAR (100 mg/kg twice daily for 7 and 15 days), atropine (5 mg/kg twice daily for 7 and 15 days), or coadministration of ALCAR and atropine. The paw-pressure test was performed 30 min after the last injection. Each value represents the mean of two experiments with 12 rats per group. *P < 0.01 vs. control (saline + saline) rats. °P < 0.01 vs. ALCAR + saline–treated rats.
Coadministration of ALCAR with the nicotinic antagonist mecamylamine (2 mg/kg i.p. twice per day) for 15 days abolished ALCAR's antihyperalgesic effects on the right paw (32.2 ± 4.8 g) without altering the analgesic profile on the contralateral one (127.8 ± 6.3 g) (Fig. 2). The same figure shows the absence of effects of mecamylamine on saline treatment for 15 days (Fig. 2). Similar results were obtained after 7 days' treatment (Fig. 2).
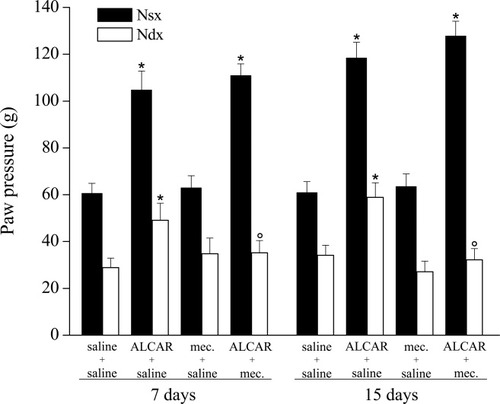
Effect of mecamylamine (mec.) on paw-pressure test. Response to noxious stimuli on the right paw (Ndx) that underwent CCI respect to the contralateral unoperated paw (Nsx). Effects of treatments for 7 and 15 days with saline alone, ALCAR (100 mg/kg twice daily), mecamylamine (2 mg/kg twice daily), or coadministration of ALCAR and mecamylamine. The paw-pressure test was performed 30 min after the last injection. Each value represents the mean of two experiments with 12 rats per group. *P < 0.01 vs. control (saline + saline) rats. °P < 0.01 vs. ALCAR + saline–treated rats.
Previously, in the CCI rat model, we have demonstrated an apoptotic state of the ligated nerve characterized by cytosolic cytochrome c release, activation of caspase 3, subsequent cleavage of PARP, and DNA fragmentation (Di Cesare Mannelli et al., 2007). Repeated ALCAR administration exerted a protective action on the peripheral nerve regulated cell death through a XIAP-related mechanism (Di Cesare Mannelli et al., 2007). Given that the effect of ALCAR on neuropathic pain is mediated by the nicotinic receptor activation, we paid attention to the involvement of this component in the protective properties of ALCAR. Apoptosis in the ligated nerve was evaluated after 15 days' treatment with ALCAR (100 mg/kg i.p. twice per day) and the nicotinic receptor antagonist mecamylamine (2 mg/kg i.p. twice per day). Figure 3 shows Western blot analysis of pro–caspase 3 (36 kDa) and its cleaved active fragments (19 and 16 kDa). Treatment of CCI rats with ALCAR prevented caspase 3 cleavage in the ligated right sciatic nerve (Fig. 3a). The expression of the 19- and 16-kDa fragments (Fig. 3b) was significantly decreased in the right nerve after ALCAR treatment compared with saline-treated rats (Fig. 3b). Administration of mecamylamine together with ALCAR significantly reduced the protective effect of the carnitine derivative (Fig. 3a). Rats treated with mecamylamine alone (Fig. 3a,b) did not show differences from the saline-treated group. Next, we evaluated whether the increased cleavage of pro–caspase 3 in the ALCAR + mecamylamine group was accompanied by increased caspase 3 activity. The cleavage of the specific fluorogenic substrate Z-DEVD-R110 allowed us to observe a twofold increase in caspase 3 activity in the right nerve of ALCAR + mecamylamine-treated rats compared with ALCAR + saline group (Fig. 4). Caspase activity was not altered when mecamylamine was coadministered with saline (Fig. 4).
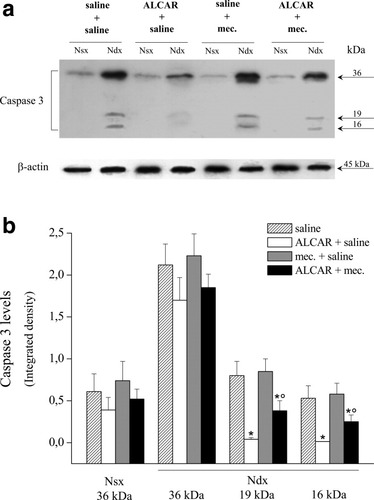
Caspase 3 protein levels. a: Representative Western blot of the effects of ALCAR, mecamylamine, or ALCAR + mecamylamine on the protein levels of pro–caspase 3 (36 kDa) and its two cleaved forms (19 and 16 kDa) in the right (Ndx) and left (Nsx) sciatic nerves. Right nerves were processed for loose ligation, and the administration of ALCAR and mecamylamine was started on the day of the operation and continued for 15 days. b: Densitometric analysis of Western blots. Results are expressed as mean ± SEM of five different animals. *P < 0.01, significantly different from saline + saline-treated rats. °P < 0.01, significantly different from ALCAR + saline–treated rats.
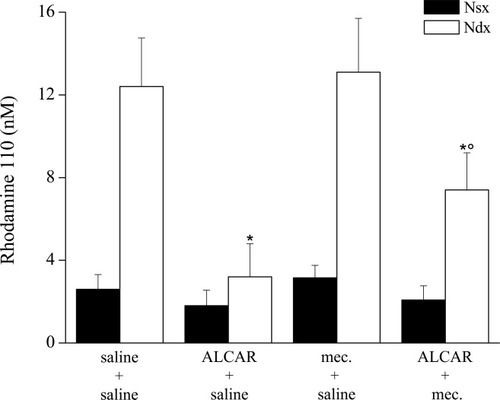
Caspase 3 activity. Catalytic activity was evaluated by measuring the fluorescent compound rhodamine Z-DEVD-R110 derivative in nerve homogenates. The CCI effects (Ndx) were evaluated in respect to nonoperated nerve (Nsx) for rats treated with saline, ALCAR, mecamylamine, or ALCAR plus mecamylamine for 15 days. All data are expressed as the mean ± SEM of five different animals. *P < 0.01 vs. saline + saline-treated animals. °P < 0.01 vs. ALCAR + saline–treated animals.
Cytochrome c, a mitochondrial intermembrane protein released after changes in the permeability of the mitochondrial membrane, activates caspase 3. We have previously demonstrated that repeated administrations of ALCAR prevents CCI-induced mitochondrial damage and cytochrome c release (Di Cesare Mannelli et al., 2007). In agreement with our previous results, treatment with ALCAR for 15 days significantly decreased cytochrome c protein levels in the ligated right nerve (Fig. 5). Such an effect was significantly reduced by coadministration of mecamylamine. In fact, a 14-kDa band, corresponding to cytochrome c, was detected in the purified cytosolic fractions from the right ligated nerve of the ALCAR + mecamylamine group, indicating that cytochrome c release was significantly increased in this animals compared with ALCAR + saline–treated animals (Fig. 5). Mecamylamine treatment did not modify cytosolic cytochrome c levels in saline-treated rats (Fig. 5). Western blot analysis with anti–COX IV, a protein belonging to the inner mitochondrial membrane, was performed to confirm separation of mitochondria from the cytosolic fraction (data not shown).
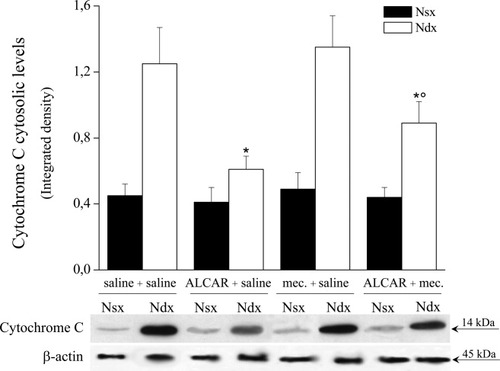
Cytochrome c cytosolic levels. Cytosolic fraction was extracted and processed by Western blot analysis with specific antibody to cytochrome c. Enhanced chemiluminescence detection images and densitometric analysis show the effects of a 15-day treatment with saline, ALCAR, and ALCAR plus mecamylamine on the right ligated nerve of CCI rats (Ndx); for each group, the left intact nerve (Nsx) was considered to be the control. Quantitative analysis is reported as the mean ± SEM of five animals. *P < 0.01 vs. saline + saline-treated rats. °P < 0.01 vs. animals treated with ALCAR + saline.
The downstream effects of caspase 3 activation were then evaluated by measuring the expression levels of the-89 kDa fragment of PARP, a 116-kDa protein known to be cleaved by active caspase 3. As shown in Figure 6, treatment with ALCAR significantly prevented PARP cleavage in the right nerve of CCI rats, an effect that was reduced by mecamylamine, which was ineffective when coadministered with saline (Fig. 6).
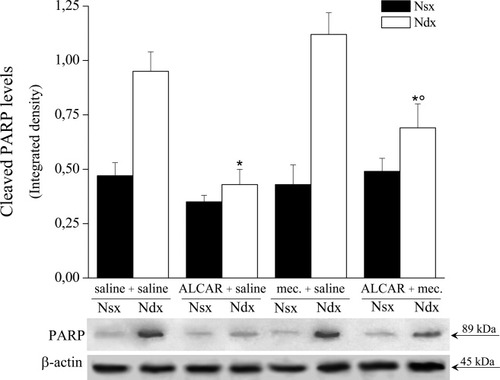
Cleaved PARP levels. Western blot evaluation of the 89-kDa PARP fragment in the left intact and right ligated nerve homogenates of CCI rats. Densitometric analysis was performed for each treatment group and expressed as the mean ± SEM of five animals. *P < 0.01 vs. saline + saline-treated rats. °P < 0.01 vs. ALCAR + saline–treated rats.
Hence, we examined the last event of the programmed cell death cascade: DNA fragmentation. Endonuclease-related gene disruption generates free 3′-OH group at the ends of DNA fragments, which were revealed by colorimetric TUNEL technique on 7-μm paraffin-embedded tissue. As shown in Figure 7, ALCAR significantly decreased the number of apoptotic nuclei in the right ligated nerve compared with saline-treated animals. Cotreatment with mecamylamine caused a significant reduction of ALCAR's protective effect both in the distal and in the proximal portions of the right nerve (Fig. 7).
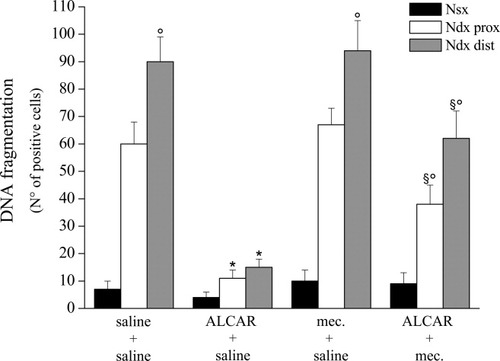
TUNEL assay. Evaluation of DNA fragmentation was performed in nerve sections (7 μm) from CCI rats. Positive nuclei number was reported for the left nerve and for the proximal and distal regions of the right nerve with respect to ligation. The effects of 15 days' treatment with saline, ALCAR alone, mecamylamine alone, or ALCAR + mecamylamine were analyzed. Quantitative analysis was performed evaluating five animals for each group and analyzing six sections for each nerve and six fields for section. Data are expressed as mean ± SEM of TUNEL-positive cells. *P < 0.01 vs. saline-treated rats. °P < 0.01 vs. the proximal part of the ligated nerve. §P < 0.01 vs. saline and ALCAR + saline–treated rats.
Finally, the protective effect of ALCAR was related to XIAP (Di Cesare Mannelli et al., 2007). Although treatment with ALCAR increased the XIAP level only in the right nerve after ligation (Fig. 8), the pharmacological blockade of the nicotinic receptor significantly reduced ALCAR-related XIAP increase (Fig. 8). Mecamylamine did not alter XIAP level in saline-treated rats (Fig. 8).
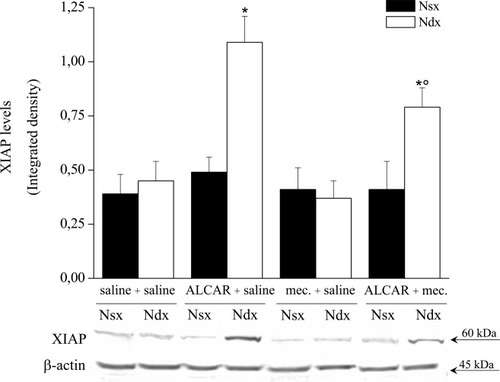
XIAP expression levels. Right ligated (Ndx) and left intact (Nsx) nerves were homogenate and processed by Western blot analysis with antibody specific to XIAP. Enhanced chemiluminescence detection images and densitometric analysis show the effect obtained by 15 days' treatments with saline, ALCAR, mecamylamine, and ALCAR plus mecamylamine. Quantitative analysis is shown as the mean ± SEM of five animals. *P < 0.01 vs. saline-treated rats. °P < 0.01 vs. ALCAR + saline–treated rats.
DISCUSSION
The acetyl derivative of L-carnitine induces a cholinergic enhancement (Rapoport, 1999) promoting ACh synthesis (Imperato et al., 1989; White and Scates, 1990). The cholinotropic and cholinoprotective activities of ALCAR might depend on its ability to increase acetyl-CoA levels (Bigini et al., 2002). ALCAR seems to determine regionally specific activation of different cholinergic receptors with a predominant stimulation of nicotinic receptors in subcortical nuclei and of muscarinic receptors in cortical areas. The spontaneous ACh- and 5-HT-evoked electrophysiological responses in neurons of reticular formation were facilitated after ALCAR, and the effect was blocked by the nicotinic antagonist dihydro-beta-erythroidine but not by atropine (Tempesta et al., 1985); also, ALCAR increases the release of dopamine and excitatory amino acid in different brain regions, an effect that was blocked by atropine (Ori et al., 2002).
The relevance of the cholinergic element is well established for the effect of ALCAR on acute pain. Ghelardini et al. (2002) have demonstrated that knocking down the muscarinic M1 receptor abolishes the analgesic effect of ALCAR. Moreover, Galeotti et al. (2004) have shown that downstream to the receptor activation, this antinociceptive activity is dependent on phospholipase C and inositol 1,4,5-trisphosphate.
According to previous results, these data show that the muscarinic receptor blocker atropine reverts the analgesic effect of ALCAR, in terms of nociceptive threshold increase of the left unoperated paw. Surprisingly, the antihyperalgesic effect on the right paw is not altered by atropine. Conversely, the cotreatment with the nicotinic antagonist mecamylamine completely reverts the antihyperalgesic effect of ALCAR, whereas the increase of the nociceptive threshold in the left paw remains intact.
The initial observations of the nicotinic potential on pain management date to 1932 (Davis et al., 1932). More recently, nicotine and nAChR agonists have been demonstrated to exhibit antinociceptive, antihyperalgesic, and antiallodynic effects (Bannon et al., 1998; Bunnelle et al., 2007; Ji et al., 2007) across a range of preclinical models of pain, involving peripheral and central sites of action (Ji et al., 2007).
Clinical validation of the nAChR approach has been achieved, but development of nicotinic agents for the management of moderate-to-severe pain has been limited to cardiovascular and gastrointestinal adverse effects (Andrews et al., 2005; Bunnelle et al., 2007). Current hypothesis attributes these side effects to poor selectivity of the nicotinic agents for the different nAChR subtypes. To date, no drugs have been successfully developed and approved for the treatment of pain from this pharmacological class.
On the other hand, nicotine and nicotinic agonists (Rosa et al., 2006; Copeland et al., 2007) are able to protect from apoptosis. In particular, Dasgupta et al. (2006) have demonstrated that nicotine inhibits the apoptosis induced by the drugs gemcitabine, cisplatin, and Taxol, which are used to treat non–small cell lung cancer. This protection correlates with the induction of XIAP and survivin by nicotine. Also ALCAR has an antiapoptotic activity, that correlates with the induction of XIAP (Di Cesare Mannelli et al., 2007).
A relationship between chronic pain and apoptosis can be hypothesized. Joseph and Levine (2004) demonstrated that apoptotic phenomena are involved in the neuropathic pain-related behavior hyperalgesia. The inhibition of caspases 1, 2, 3, 8, and 9 reduced hyperalgesia in rat models of neuropathy induced by cancer or antiviral chemotherapy. In agreement with this evidence, both nicotine and ALCAR show a protective effect on both hyperalgesia and apoptosis. Moreover, ALCAR shows a mecamylamine-reverted antihyperalgesic effect and a XIAP-related antiapoptotic effect similar to nicotine (Dasgupta et al., 2006). Aimed to inquire the nicotinic involvement in ALCAR antiapoptotic properties, we have analyzed the effect of mecamylamine. This classical, nonselective, nicotinic blocker reduces the protective effect of ALCAR on all steps of the apoptotic cascade, starting from the cytochrome c cytosolic release up to the DNA fragmentation, including XIAP induction. Nevertheless, the reversion of ALCAR protective effects by mecamylamine is not complete, suggesting that nicotinic receptor activation is likely not the only factor responsible for preventing ALCAR effects on apoptosis. On the other hand, the mechanism of the antiapoptotic effect of nicotine is debated. In models of programmed cell death induced by glutamate, hypoxia or β-amyloid protein (Aβ) some studies indicate that nicotine-induced protection is primarily mediated by both the α4β2 (Kihara et al., 1998; Rosa et al., 2006) and α7 nicotinic receptor (Kihara et al., 2001; Rosa et al., 2006). Moreover, cytisine (a selective α4β2 nicotinic receptor agonist) and 3-(2,4)-dimethoxybenzylidene anabasine (DMXB, a selective α7 nicotinic receptor agonist) were able to mimic nicotine protective effects on Aβ-induced neurotoxicity in cultured neuronal cells (Kihara et al., 2001). Nonetheless, Liu and Zhao (2004) have showed that mecamylamine did not completely revert nicotine protective effect against Aβ-induced caspase 3 activation; therefore, the protection of nicotine is partly via nicotinic receptors.
Furthermore, ALCAR is an endogenous molecule with a pivotal role in improving the energetic state of the cell, and it may prevent the failure of mitochondrial oxidative metabolism (Budd and Nicholls, 1998). Moreover, its antioxidant role may be of benefit in preventing cell damage by the reactive oxygen species, whose generation is thought to be of great importance in the effector mechanism of cell death (Tesco et al., 1992).
In conclusion, ALCAR presents both analgesic and antihyperalgesic properties mediated by the cholinergic pathway. Nevertheless, although analgesia can be viewed as a muscarinic effect, the antineuropathic effect is completely reverted by mecamylamine-mediated blockade of the nicotinic receptor. The nicotinic mechanism is also responsible for a significant, but not complete, part of the apoptotic-preventing effect of ALCAR. ALCAR is a clinical compound with a good safety profile, so the nicotinic component of ALCAR pharmacodynamic properties is free of compromising adverse effects.




