Differences among cell types in NAD+ compartmentalization: A comparison of neurons, astrocytes, and cardiac myocytes†
This article is a US Government work and, as such, is in the public domain in the United States of America.
Abstract
Activation of the nuclear enzyme poly(ADP-ribose)-1 leads to the death of neurons and other types of cells by a mechanism involving NAD+ depletion and mitochondrial permeability transition. It has been proposed that the mitochondrial permeability transition (MPT) is required for NAD+ to be released from mitochondria and subsequently consumed by PARP-1. In the present study we used the MPT inhibitor cyclosporine-A (CsA) to preserve mitochondrial NAD+ pools during PARP-1 activation and thereby provide an estimate of mitochondrial NAD+ pool size in different cell types. Rat cardiac myocytes, mouse cardiac myocytes, mouse cortical neurons, and mouse cortical astrocytes were incubated with the genotoxin N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) in order to activate PARP-1. In all four cell types MNNG caused a reduction in total NAD+ content that was blocked by the PARP inhibitor 3,4-dihydro-5-[4-(1-piperidinyl)butoxy]-1(2H)-isoquinolinone. Inhibition of the mitochondrial permeability transition with cyclosporine-A (CsA) prevented PARP-1-induced NAD+ depletion to a varying degree in the four cell types tested. CsA preserved 83.5% ± 5.2% of total cellular NAD+ in rat cardiac myocytes, 85.7% ± 8.9% in mouse cardiac myocytes, 55.9% ± 12.9% in mouse neurons, and 22.4% ± 7.3% in mouse astrocytes. CsA preserved nearly 100% of NAD+ content in mitochondria isolated from these cells. These results confirm that it is the cytosolic NAD+ pool that is consumed by PARP-1 and that the mitochondrial NAD+ pool is consumed only after MPT permits mitochondrial NAD+ to exit into the cytosol. These results also suggest large differences in the mitochondrial and cytosolic compartmentalization of NAD+ in these cell types. © 2007 Wiley-Liss, Inc.
Nicotinamide adenine dinucleotide (NAD+) participates in many redox reactions in cells, including glycolysis and the citric acid cycle. The mitochondrial NAD+ pool is physically separated from the cytosolic NAD+ pool under normal conditions. The relative distribution of NAD+ between the cytosol and mitochondria is not easily measured and therefore is not known for most cell types. Evidence from cardiac myocytes suggests that most cellular NAD+ (>80%) is in the mitochondria (Di Lisa et al., 2001). However, cardiac myocytes may not be representative of all cell types in this respect because they have a uniquely high rate of oxidative metabolism.
NAD+ is also the substrate for the nuclear enzyme poly(ADP-ribose) polymerase-1 (PARP-1). DNA damage activates PARP-1, which consumes NAD+ as the substrate to form branched polymers of ADP-ribose [poly(ADP)-ribose, or PAR] on target proteins involved in DNA repair and transcription (D'Amours et al., 1999; Oei et al., 2005). However, excessive activation of PARP-1 depletes cellular NAD+ and results in the failure of NAD+-dependent processes and, ultimately, cell death (Berger, 1985; Zhang et al., 1994; Yu et al., 2002; Alano et al., 2004). It remains controversial how and to what extent activation of nuclear PARP-1 depletes NAD+ concentrations in the mitochondria. PARP-1 activation leads to opening of the mitochondrial permeability transition (MPT) pore (Alano et al., 2004; Xu et al., 2006). Di Lisa et al. (2001) reported that depletion of NAD+ in the cardiac myocytes of male rats was prevented by the MPT inhibitor cyclosporine A (CsA) and proposed that PARP-1-mediated NAD+ depletion requires opening of the MPT pore and the subsequent egress of mitochondrial NAD+ into the cytosol (Di Lisa et al., 2001). On the other hand, studies using cultured cortical astrocytes showed that more than 80% of cellular NAD+ was lost during PARP-1 activation even when MPT was blocked (Alano et al., 2004). One possible explanation for this discrepancy is the large difference in the proportions of cytosolic and mitochondrial NAD+ in different types of cells. In the present study we examined this point by comparing the effects of PARP-1 activation and CsA on cellular NAD+ concentration in astrocytes, cardiac myocytes, and neurons.
MATERIALS AND METHODS
Animals were handled in accordance with protocols approved by the San Francisco Veterans Affairs Medical Center Animal Studies Committee. The study was in conformance with the “Guide for the Care and Use of Laboratory Animals,” published by the U.S. National Institutes of Health (NIH Publication 85-23, revised 1996). Cell culture reagents for astrocytes and neurons were purchased from CellGro, and reagents for cardiac myocytes were purchased from the University of California, San Francisco, cell culture facility. Chemicals and reagents for experiments were purchased from Sigma (St. Louis, MO) except where noted otherwise.
Cortical Cell Cultures
Astrocyte cortical cultures were prepared from cerebral cortices of neonatal mice (Swiss-Webster) obtained from Simonsen (Gilroy, CA). In brief, the cortices were harvested, freed of meninges, dissociated with papain digestion (with DNAase) and subsequent trituration, and plated on 24-well Falcon culture plates or glass coverslips as previously described (Alano et al., 2004). Cells were treated for 48 hr with 20 μM cytosine arabinoside at confluence after 12–14 days in vitro in order to prevent microglial proliferation. This medium was replaced with Eagle's minimal essential medium supplemented with 3% fetal bovine serum (HyClone, Logan, UT), 2 mM glutamine, 100 nM sodium selenate, and 200 nM α-tocopherol. The astrocyte cultures were used when confluent after 19–24 days in vitro The growing conditions of these astrocyte cultures prevented neuronal proliferation, resulting in a cell culture that was virtually 100% astrocytes.
Neuronal cortical cultures were prepared from cerebral cortices of embryonic (E16) mouse pups (Swiss-Webster) as described previously (Alano et al., 2006). Mouse cortices were isolated, freed of meninges, dissociated with papain digestion (with DNAase) and subsequent trituration, and plated on 24-well Falcon culture plates coated with poly-D-lysine. Neurons were treated for 24 hr with 5 μM cytosine arabinoside after 1 day in vitro to prevent microglial proliferation. This medium was replaced with a conditioned medium, which was prepared from Eagle's minimal essential medium supplemented with 10% fetal bovine serum (HyClone, Logan, UT) and 2 mM glutamine and was conditioned in an astrocyte feeding layer for at least 48 hr. Primary cultured neurons were used when the neurons reached 7–10 days in vitro.
Cardiac Myocytes
Rat and mouse cardiac myocytes were prepared similarly. The myocytes were isolated and cultured from 250- to 300-g male Sprague-Dawley rats (Charles River) using a modification of the collagenase dissociation method described by Zhou et al. (2000). Rats were treated with heparin (50 U) and anesthetized by intraperitoneal injection of sodium pentobarbital (1.6 mg/10 g). The heart was quickly excised and the aorta cannulated for retrograde perfusion in a Langendorff apparatus at a constant flow rate (12 mL/min) at 37°C. The Langendorff system was cleaned prior to each use with 70% ethanol and rinsed thoroughly with sterile water to reduce contamination. All solutions used were sterilized using a 0.02 μM nitrocellulose filter. Primary cultures of cardiac myocytes were prepared from the cardiac ventricles. The heart was perfused for 9–10 min with isolation buffer consisting of 120 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 0.6 mM NaH2PO4, 0.6 mM KH2PO4, 5.6 mM glucose, 4.6 mM NaHCO3, 10 mM HEPES, and 5 mM taurine at a pH of 6.8, followed by digestion for 20 min with collagenase II (1.5 mg/mL, Worthington) in isolation buffer. Mouse cardiomyocytes were isolated from 20-g male C57BL6 mice (Charles River, Wilmington, MA) using the same approach as that used for the rat cells but with the addition of 10 mM 2,3-butanedione monoxime (BDM) to the perfusion buffer. After digestion, the soft and flaccid heart was removed, and myocytes were suspended in isolation buffer. Centrifugation (40g, 1 min) and resuspension were performed four times for the reintroduction of Ca2+ in 3 sequential steps from 50 μM to 1.0 mM, which was the final concentration Ca2+ in the medium. The cells were suspended in minimum essential medium (MEM) with Hanks Buffered Salt Solution (HBSS) supplemented with 2.5% fetal calf serum, 10 μg/mL penicillin, 1.5 μM vitamin B12, and 10 mM BDM. Myocytes were plated on 35-mm tissue culture dishes coated with laminin (10 μg/mL, coated for 2 hr) for a 1.5- to 2-hr attachment period in 2% CO2/air at 37°C, followed by washing out of the nonadhering (dead) cells. Experiments were performed on adherent cells 1 day following culture.
Experimental Procedures
For astrocytes and neurons, experiments were conducted in a balanced salt solution to mimic an artificial cerebrospinal fluid (aCSF) prepared as previously described (Alano et al., 2004). The aCSF contained 3.1 mM KCl, 134 mM NaCl, 1.2 mM CaCl2, 1.2 mM MgSO4, 0.25 mM KH2PO4, 15.7 mM NaHCO3, and 2 mM glucose. The pH was adjusted to 7.2, whereas the solution was equilibrated with 5% CO2 at 37°C. Osmolarity was verified at 290–300 mOsmol with a vapor-pressure osmometer (Wescor, Logan, UT). The cardiac myocytes were studied in a HEPES-buffered solution containing 120 mM NaCl, 5.4 mM KCl, 1.2 mM MgSO4, 1.2 mM NaH2PO4, 5.6 mM glucose, 5 mM NaHCO3, 10 mM HEPES, 2 mM CaCl2, and 1 mg/mL fatty acid-free bovine serum albumin at a pH of 7.4.
Exposure to N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) occurred at 37°C in a 5% CO2 atmosphere for astrocytes and neurons and in a 1% CO2 atmosphere for cardiac myocytes. The PARP inhibitor 3,4-dihydro-5-[4-(1-piperidinyl)butoxy]-1(2H)-isoquinolinone (DPQ; Calbiochem, San Diego, CA) and the mitochondrial permeability transition pore inhibitor cyclosporin A (CsA; Biomol, Plymouth Meeting, PA) were administered to the cells at least 1 hr prior to MNNG exposure and were present throughout the experiment.
Measurements of Cell Viability
Astrocyte death was quantified by measuring lactate dehydrogenase (LDH) activity in the cell lysates of surviving cells 20–24 hr after MNNG exposure as previously described (Alano et al., 2004). Cell death in each well was calculated as a percentage using the following equation: [(1 − LDH activity)/control LDH activity] × 100, with control LDH activity defined as the mean obtained in control cells from the same multiwell plate as the test wells. Control wells were treated with medium exchanges only.
Neuron death was assayed by a protocol for determining propidium iodide exclusion as a direct function of plasma membrane integrity as described previously (Ying et al., 2002; Alano et al., 2006) and was verified by retention of calcein. Cells were treated with cell-permeable calcein-AM (2 μM, 1 min), which is deesterified in the cell by cellular esterases into a cell-impermeable and fluorescent form (calcein). Viability was assayed 20–24 hr after MNNG exposure. Propidium iodide and calcein were added to the medium, and staining was visualized on a fluorescence microscope (Axiovert 40, Zeiss Microimaging) equipped with a digital camera (Axiocam, Zeiss Microimaging), using standard filter cube sets for rhodamine (ex 550 nm, em 590 nm) and FITC (ex 488 nm, em 520 nm) for propidium iodide and calcein, respectively.
Myocyte survival was determined by comparing the number of live cells in the experimental cultures to the number of live cells in the control cultures, as determined by a modified protocol for the propidium iodide exclusion assay described for neurons. Myocytes were counted in a medium containing propidium iodide (10 μM) after 10 min at room temperature using a fluorescence microscope equipped with a digital camera. Viability was assayed simultaneously using propidium iodide staining and morphological criteria. Live (unstained and rod-shaped) and dead (positive-stained and shrunken) myocytes in 10 random fields per condition per experiment were recorded at 40× magnification using a Zeiss inverted microscope. To validate the propidium iodide exclusion assay, the percentages of live and dead myocytes were also determined using trypan blue exclusion assay as previously described (Karliner et al., 2000, 2001), and these percentages were compared with the percentage of cells that excluded propidium iodide after 1 hr of culture.
Succinate Dehydrogenase Activity Assay
Succinate dehydrogenase (SDH) was assayed using a modified protocol (Pennington, 1961). SDH activity from whole-cell lysates (5 μg/mL protein) was measured in an assay medium containing 120 mM potassium phosphate buffer, 0.1 mM phenazine metasulfate, 0.1 mM cytochrome-c, and 0.25 mM freshly prepared KCN (pH 7.4). After incubation at 37°C for 1 min, the reaction was initiated by the addition of 25 mM sodium succinate, and a decrease in absorbance at 550 nm was recorded over time. At the end of the experiment, sodium hydrosulfite was added to the sample to completely reduce the cytochrome-c.
Isolation of Mitochondria
Isolation of mitochondria from cultured mouse neurons, astrocytes, and cardiac myocytes was performed according to a modified protocol (Almeida and Medina, 1998). Cells were grown on six-well plates as described and were harvested with a scraper in 0.5 mL of isolation medium [320 mM sucrose, 1 mM potassium EDTA, and 10 mM Tris-HCl (pH 7.4)]. Cells were homogenized in a glass Teflon homogenizer and were centrifuged at 500g for 5 min at 4°C. The supernatant fractions (S1) were saved (at 4°C) for the subsequent centrifugation step. The pellet was rehomogenized and centrifuged at 1,000g for 10 min at 4°C. The S2 supernatant was combined with S1 and centrifuged at 1,000g for 10 min at 4°C. This rehomogenization step increased the mitochondrial yield. The final supernatant was collected and centrifuged at 16,000g for 30 min. The final pellet (mitochondrial fraction) was resuspended in 200 μL of isolation medium and kept on ice for biochemical studies. Mitochondrial enrichment was quantified by Western blots using an antibody targeted to the mitochondrial protein voltage-dependent anion channel (VDAC).
Western Blots
Western blots were prepared as described previously (Alano et al., 2006) using 5–10 μg of total protein per lane. Cells were collected in RIPA lysing buffer, and cell extracts were mixed with a 1:4 volume of SDS-PAGE loading buffer [10% β-mercaptoethanol, 10% glycerol, 4% SDS, 0.01% bromophenol blue, and 62.5 mM Tris-HCl (pH 6.8)] and heated to 65°C for 15 min. Samples were loaded on a 10% resolving SDS-polyacrylamide gel; after electrophoresis, they were transferred to PVDF membranes. The membranes were blocked in blocking buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.1% Tween-20, and 5% blotting-grade nonfat dry milk] for 60 min. The membranes were then exposed to a 1:5,000 dilution of the VDAC Ab (polyclonal antibody from rabbit) overnight at 4°C, and after being washed three times for 10 min each with PBS + 0.1% Tween-20 buffer, they were exposed to a a 1:10,000 dilution of peroxidase-labeled antirabbit IgG antibody for 60 min at room temperature. The membranes were washed three times for 10 min each in PBS + 0.1% Tween-20 buffer, and bound antibody was detected via chemiluminescence using an ECL+ WB Detection kit (Amersham Pharmacia). Equal protein loading and transfer was verified by immunostaining for β-actin and by Ponceau S staining (not shown). VDAC immunoreactivity was measured in each lane from the 30-kDa VDAC band, and densitometry analysis was quantified with the ImageJ program (NIH). Values for each lane were normalized to protein loading as measured by β-actin immunoreactivity and expressed as the magnitude of increased VDAC staining normalized to astrocytes in each experiment. Immunoblots prepared in without primary or secondary antibodies as controls showed no signal.
NAD+ Measurement
NAD+ was assayed from whole cells and isolated mitochondria. Cells were washed four times with phosphate-buffered saline and collected by scraping after treatment with 0.5 N perchloric acid. For mitochondrial NAD+ measurements, isolated mitochondria were immediately treated with 0.5N perchloric acid. The acid treatment hydrolyzes NADH but does not affect NAD+. After 15 min on ice, the samples were centrifuged at 1,500g for 10 min at 4°C. The supernatant fractions were adjusted to pH 7.5 by adding 1.0N KOH and 75 μL of 0.33 M K2HPO4/KH2PO4 (pH 7.5). After 15 min on ice, the insoluble KClO4 was removed by centrifugation at 1,500g for 10 min at 4°C. NAD+ in the extracts was measured using an enzymatic cycling assay (Bernofsky and Swan, 1973; Jacobson and Jacobson, 1997) and modified as published previously (Ying et al., 2001; Alano et al., 2006). NAD+ standards were used to quantify NAD+ samples and were normalized to total protein as measured by the bicinchonic acid method.
Mitochondrial Labeling with Mitotracker and Fluorescence Microscopy
For Mitotracker staining of mitochondria, cells were incubated with 250 nM Mitotracker Green (Molecular Probes, Eugene, OR). Mitotracker Green was excited with a 488 laser, and fluorescence emission was captured on a photon multiplier tube. Cells were imaged after dye loading as previously described (Alano et al., 2004) using a Leica TCS-SP inverted confocal microscope (Leica, Bannockburn, IL) with a 40× objective lens. Images were collected using Leica TCS software with the same image acquisition parameters used for each cell type.
Statistics
The Student t test was used for single comparisons. For multiple comparisons, either one-way or repeated-measures analysis of variance was used. Post hoc analysis was performed with the Newman-Keuls test. A P value < 0.05 was considered significant.
RESULTS
Exposure to MNNG Led to PARP-1-Mediated Cell Death
Exposure to MNNG led to cell death in all four types of cells examined: mouse astrocytes, mouse neurons, mouse cardiac myocytes, and rat cardiac myocytes (Fig. 1). Cardiac myocytes from both rat and mouse were used to determine differences between these species as well as between the tissue types. In each case, cell death was prevented by the PARP inhibitor DPQ, confirming the role of PARP-1 in this process. The MPT has been shown to be a necessary downstream cell death event that is initiated by PARP-1 (Alano et al., 2004; Tanaka et al., 2005). In the present study, the MPT inhibitor CsA prevented cell death after incubation with MNNG in all four cell preparations, suggesting that the MPT is a similarly necessary event in PARP-1-mediated death of neurons and cardiac myocytes from both mouse and rat.
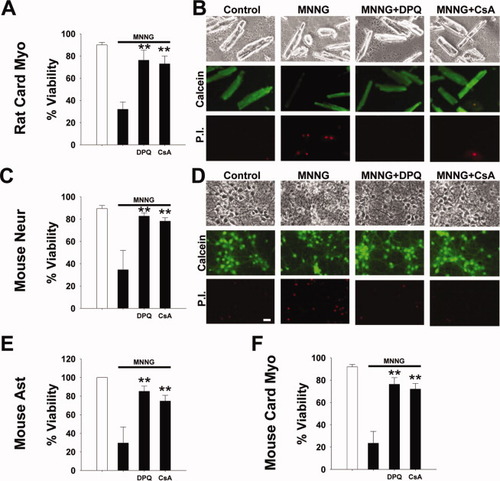
Treatment with MNNG caused PARP-1-mediated cell death. MNNG treatment of adult rat cardiac myocytes, mouse cardiac myocytes, mouse cortical astrocytes, and mouse cortical neurons led to PARP-1-mediated cell death. Representative images of cardiac myocytes (B) show that live cells were rod-shaped, whereas dead cells were contracted. Representative images of mouse cortical neurons (D) show live cells were phase-bright with distinct processes. Viability was verified by assaying plasma membrane integrity. Live cells retained calcein fluorescence (B, D, middle rows) and excluded the vital dye propidium iodide (PI; B, D, bottom rows), indicating an intact plasma membrane. Dead cells lost calcein fluorescence and stained positive for PI, indicating a compromised plasma membrane. Quantification of viability in cultured astrocytes was done with the LDH assay rather than by cell counting, as for the neurons and cardiac myocytes; therefore, images are not provided. Control-vehicle treatment—MNNG: 100 μM for 30 min for cardiac myocytes and astrocytes, 75 μM for 30 min for neurons; DPQ: 25 μM pretreated for at least 30 min prior to experiment; CsA: 250 nM pretreated for at least 60 min prior to experiment (n = 3 **P < 0.001).
PARP-1 Activation Causes NAD+ Depletion in Whole Cells
The effect of PARP-1 activation on intracellular NAD+ concentrations was evaluated with doses of MNNG for each cell type chosen on the basis of cytotoxicity studies (Fig. 1) to have roughly equipotent actions on all four cell types. MNNG incubation led to a decrease in total NAD+ that was blocked by the PARP inhibitor DPQ in all cell types (Fig. 2). CsA prevented the decrease in NAD+ in cardiac myocytes, consistent with the report of Di Lisa et al. (2001), suggesting that most of the NAD+ in these cells is normally in the mitochondria. In contrast, astrocytes treated with CsA preserved a much smaller portion of their NAD+ content, and neurons showed an intermediate effect.
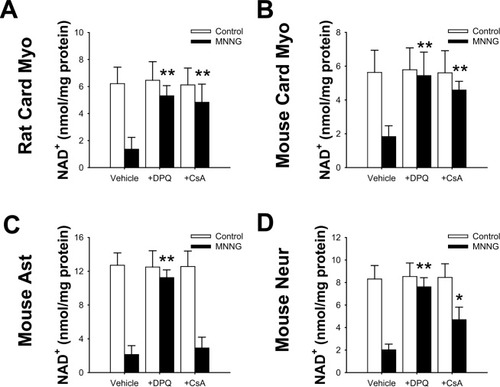
PARP-1 activation depleted cellular NAD+ levels. MNNG treatment led to decreased NAD+ levels. A: Decreased NAD+ level in rat cardiac myocytes. B: Decreased NAD+ level in mouse cardiac myocytes. C: Decreased NAD+ level in mouse cortical astrocytes. D: Decreased NAD+ level in mouse cortical neurons. The decrease was prevented by DPQ (25 μM). CsA prevented the MNNG-induced decrease in NAD+ in rat and mouse cardiac myocytes and in mouse cortical neurons, but not in mouse astrocytes (n = 3; *P < 0.01, **P < 0.001).
To exclude the possibility that the decrease in NAD+ was a result of ruptured cell membranes rather than consumption by PARP-1, we measured cell membrane integrity by the propidium iodide exclusion method in sister cultures at the time of the NAD+ assay. Cell membrane integrity did not significantly decrease at this point in any cell type (data not shown). These findings suggest that differences between cell types in the size of the mitochondrial NAD+ pools were relative to the total cellular NAD+ pool.
To confirm that CsA prevented NAD+ egress from mitochondria after PARP-1 activation, we measured NAD+ in mitochondria isolated from each cell type after incubation with MNNG and CsA. CsA prevented NAD+ depletion in mitochondria from mouse astrocytes, neurons, and cardiac myocytes (Fig. 3). Measures of VDAC confirmed comparable mitochondrial enrichment in the isolates. Mitochondria from astrocytes and neurons had similar concentrations of NAD+ (3.20 ± 1.04 and 4.66 ± 0.37, respectively), but mitochondria from cardiac myocytes had a much higher concentration (10.02 ± 1.82).
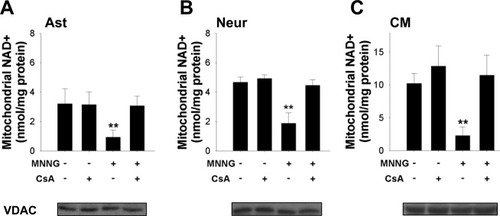
Cyclosporin A prevented PARP-1-mediated release of mitochondrial NAD+. Cells were treated with MNNG or vehicle in the presence or the absence of CsA. After 3 hr, mitochondria were isolated from the cells for NAD+ measurement. MNNG treatment led to decreased NAD+ levels. A: Decreased NAD+ level in mouse cortical astrocytes (Asts). B: Decreased NAD+ level in mouse cortical neurons (Neurs). C: Decreased NAD+ level in mouse cardiac myocytes (CMs). CsA prevented an MNNG-induced decrease in mitochondrial NAD+ in all three cell types (n = 3, **P < 0.001). Western blots for VDAC performed on aliquots of the mitochondrial isolates showed comparable mitochondrial enrichment in samples from the three cell types. Note: C has a different Y-axis scale than A and B.
These results indicate that the NAD+ pool in cardiac myocytes is predominantly in mitochondria, consistent with prior reports (Di Lisa et al., 2001). By contrast, the mitochondrial NAD+ pool in astrocytes is small relative to the cytosolic pool, and the NAD+ pool in neurons is more evenly split between mitochondria and the cytosol.
Quantification of Mitochondrial Content in Different Cell Types
An additional factor that could contribute to differing mitochondrial NAD+ pool sizes is differing numbers of mitochondria in these cell types. Mitochondria visualized with Mitotracker are shown in Figure 4. Cardiac myocytes from mice exhibited mitochondrial staining that was densely localized throughout the cells (Fig. 4A). Neurons exhibited a punctate pattern of staining that was highly localized in the cell bodies as well as the processes (Fig. 4C). Astrocytes also exhibited a punctate pattern that was less dense than that in the neurons (Fig. 4B). These photomicrographs qualitatively show that mitochondrial density was lowest in astrocytes and highest in cardiac myocytes.
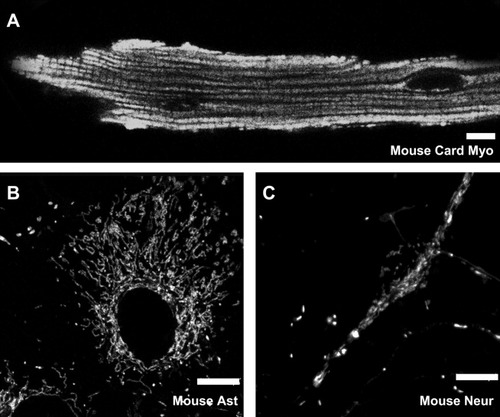
Representative confocal images of mitochondrial distribution. A: Mitotracker labeling of mitochondria in mouse cardiac myocytes. B: Mitotracker labeling of mitochondria in mouse cortical astrocytes. C: Mitotracker labeling of mitochondria in mouse cortical neurons. Scale bar = 10 μm.
Mitochondrial density was quantified by measuring the activity of a mitochondrial enzyme, succinate dehydrogenase (Fig. 5A). SDH activity in cardiac myocytes was significantly higher than that in neurons, and both were higher than in astrocytes. We also measured the levels of an outer mitochondrial membrane protein, voltage-dependent anion channel (VDAC) protein (Fig. 5B). The same pattern was observed: VDAC expression was highest in cardiac myocytes lowest in astrocytes, and intermediate in neurons.
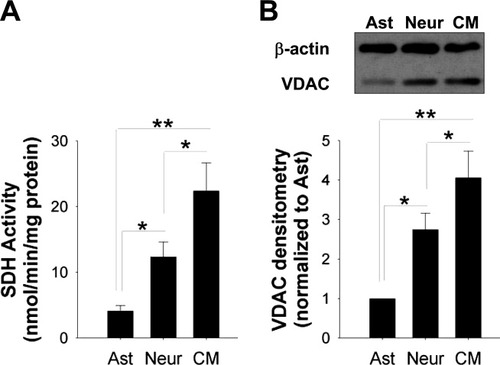
Mitochondrial density in astrocytes, neurons, and cardiac myocytes. A: Succinate dehydrogenase activity in mouse cortical astrocytes (Asts), mouse cortical neurons (Neurs), and mouse cardiac myocyte (CMs). Neuronal SDH activity was three times the level of astrocyte SDH activity (*P < 0.01), and cardiac myocyte SDH activity was five times the level of astrocyte SDH activity (n = 3; **P < 0.001). B: Western blots from mouse cortical Asts, mouse cortical Neurs, and mouse CMs (n = 3; *P < 0.01, **P < 0.001).
DISCUSSION
These data show that CsA can prevent PARP-1-mediated NAD+ consumption to varying degrees in different cell types. Because CsA completely prevented the loss of mitochondrial NAD+, these results confirm that it is the cytosolic NAD+ pool that is consumed by PARP-1 and that the mitochondrial NAD+ pool is consumed only after MPT permits mitochondrial NAD+ to exit into the cytosol. In addition, the large differences in total cellular NAD+ depletion under conditions in which the mitochondrial NAD+ pool is preserved by CsA suggest these cell types vary considerably in cytosolic NAD+ pool size.
In particular, astrocytes were found to have a total NAD+ concentration of 12.71 ± 1.76 nmol/mg protein. This concentration decreased to 2.13 ± 1.06 nmol/mg protein after PARP-1 activation, and there was only a negligible (nonsignificant) preservation of total NAD+ by CsA despite complete preservation of mitochondrial NAD+, indicating the vast majority of NAD+ is in the cytosol. By contrast, mouse cardiac myocytes had a total cellular NAD+ concentration of 5.63 ± 1.32 nmol/mg protein, with nearly all of it preserved in cells treated with CsA, indicating a large mitochondrial NAD+ pool. A large mitochondrial NAD pool in cardiac myocytes was corroborated by findings of both higher mitochondrial NAD+ concentration and increased mitochondrial density in the cardiac myocytes. Neurons were intermediate between astrocytes and cardiac myocytes in all these measures.
The functional significance of these differences remains to be established, but cardiac myocytes may be especially rich in mitochondria and mitochondrial NAD+ because they normally generate large amounts of ATP from nonglucose substrates in blood for cardiac contractile function. In direct contrast, astrocytes and neurons in the brain are completely dependent for energy production on glucose or possibly lactate (Chih et al., 2001), both of which require cytosolic NAD+ for metabolism. The greater cytosolic NAD+ pools and lower mitochondria density observed in astrocytes and neurons may reflect these differing substrate preferences. However, we cannot exclude the possibility that the mitochondrial density in these preparations is influenced by factors introduced by in vitro preparation. It should also be noted that neurons and probably astrocytes are heterogeneous, and a prior report comparing cortical astrocytes to cerebellar neurons found greater mitochondrial density in the astrocytes (Bambrick et al., 2006).
The large effect of CsA on NAD+ preservation in cardiac myocytes that we found agreed with the results reported by Di Lisa et al. (2001) that opening the MPT pore is necessary for the release and consumption of mitochondrial NAD+ during PARP-1 activation. An alternative possibility is that CsA either directly or indirectly inhibits PARP-1 activity; however, we and others have previously demonstrated no reduction in PARP-1 activity in cells treated with CsA (Alano et al., 2004; Xu et al., 2006). Similarly, the very small effect of CsA on PARP-1-mediated NAD+ depletion in cultured astrocytes agrees with our previous observations (Alano et al., 2004). The possibility that CsA is simply ineffective in astrocyte mitochondria can be excluded on the basis of the results of several previous studies showing that MPT induction in cultured astrocytes was inhibited by CsA (Alano et al., 2004; Dugan and Kim-Han, 2004; Jayakumar et al., 2004; Norenberg et al., 2004). Moreover, prior studies also showed that PARP-1 activation in astrocytes and other types of cells leads to a block in glycolysis at times when the utilization of mitochondrial substrates is not impaired (Ying et al., 2002, 2003, 2005; Zong et al., 2004). This provides strong independent evidence that only the cytosolic pool of NAD+ is consumed by PARP-1 until MPT occurs. Although the presence of mitochondrial PARP-1 activity has been reported (Du et al., 2003), both the present results and those of these prior studies argue against any significant PARP-1 activity in mitochondria.
A prior study reported that CsA had no effect on MNNG-triggered, PARP-1-mediated NAD+ depletion in HeLa cells (Cipriani et al., 2005). It is possible that MNNG failed to induce MPT in that cell type. Alternatively, like astrocytes, HeLa cells may have NAD+ partitioned primarily to the cytosol, such that the decrease in total NAD+ during PARP-1 activation is not significantly prevented by CsA treatment.
The measures of whole-cell NAD+ concentrations reported in this article are in rough agreement with those of previous reports. For cultured astrocytes, the published range is from 7 nmol/mg protein (Verderio et al., 2001) to 19 μmol/mg protein (Pieper et al., 2000). In a direct comparison, Pieper et al. (2000) reported that cultured astrocytes have roughly twice the amount of total NAD+ that cultured cortical neurons have, which is similar to our findings. Total NAD+ content in cultured cardiac myocytes has not been extensively quantified because all the findings were of decreased NAD+ levels normalized to control (as percent decrease), but one finding did report a value of 6nmol/mg protein (Pillai et al., 2006). Quantified mitochondrial NAD+ content from cultured cardiac myocytes are similarly limited because all the findings were of decreased NAD+ levels normalized to control, except for one finding that quantified mitochondrial NAD+ isolated from rat hearts of about 7 nmol/mg protein (Di Lisa et al., 2001), a result similar to our findings.
The changes in NAD+ levels observed after PARP-1 activation could, in principle, have resulted not only from PARP-1 consumption but also from the indirect effects of PARP-1 on the NAD+/NADH ratio in either the cytosol or mitochondria. However, evidence suggests this was not a significant factor in these studies. In both the cytosol and the mitochondria, the NADH concentration is very low relative to NAD+, such that even total oxidation of NADH to NAD+ would have only a negligible effect on total NAD+ concentration. Measured NAD+ levels could be influenced by NAD+ to reduce to NADH, but prior studies have shown a decrease, rather than an increase, in total NADH levels during PARP-1 activation (Nakamura et al., 2003; Ying and Swanson, unpublished observation). It should also be noted that mitochondrial NAD+ levels were measured after isolation from the cells, and during the isolation procedure the mitochondria were bathed in excess metabolic substrates. Thus, the NAD+ content of isolated mitochondria is unlikely to be affected by any change in the NAD+/NADH ratio that occurred prior to mitochondrial isolation.
Relatively high concentrations of MNNG (>250 μM) have been shown to have direct effects on mitochondria independent of PARP-1 activation (Dodoni et al., 2004). However, the MNNG concentrations used in the present study were well below this value and were shown by the same group not to directly cause MPT induction. More important, all the observed effects of MNNG were negated by the PARP-1 inhibitor DPQ, thus excluding a direct effect on mitochondria in these studies.
In summary, the present findings resolve the apparent discrepant results of studies of different cell types on the relationships between MPT induction and NAD+ depletion during PARP-1 activation. Whether PARP-1-dependent cell death exclusively depends on mitochondrial NAD+ depletion remains to be elucidated. The present results have also identified large differences between astrocytes, neurons, and cardiac myocytes in their relative cytosolic and mitochondrial NAD+ compartmentalization and mitochondrial densities.
Acknowledgements
This work was supported by the NIH (J.S.K., R.A.S.), the Department of Veterans Affairs (C.C.A., R.A.S.), and the Charitable Leadership Foundation (J.S.K.).




