Acute hypoxia differentially affects the γ-aminobutyric acid type A receptor α1, α2, β2, and γ2 subunit mRNA levels in the developing chick optic tectum: Stage-dependent sensitivity
Abstract
This investigation analyzes the effect of an acute hypoxic treatment on the level of four (α1, α2, β2, and γ2) subunit mRNAs of the GABAA receptor in layer “i” of the developing chick optic tectum. Our results show that 1 hr of normobaric acute hypoxia significantly changes the subunit mRNA levels. Different subunit mRNAs display different sensitivity to hypoxia: α1, β2, and γ2 mRNAs are highly sensitive, whereas α2 mRNA is almost not affected. The sensitivity of the mRNA levels to hypoxia is stage dependent. The mean percentages of variation produced by the hypoxia in the level of expression of the four subunits were 20% at ED12, 5% at ED16, and only 2% at ED18. These changes in the mean percentages of expression modify the probability of coexpression. In the case of double mRNA combinations, the hypoxia produced a mean variation in the probability of coexpression of 37% at ED12, 8% at ED16, and only 4% at ED18. With regard to the triple subunit mRNAs combinations, the variations were 206% at ED12, 11% at ED16, and only 7% at ED18. The quadruple combination values were 1,500% at ED12, 21% at ED16, and only 11% at ED18. This study demonstrates that the subunit mRNA levels are highly sensitive during the early stages, suggesting that GABAA receptor composition might undergo environment-dependent plastic changes providing a high degree of plasticity to the GABA neurotransmitter system development. © 2007 Wiley-Liss, Inc.
Hypoxia can cause severe central nervous system (CNS) dysfunction, including subsequent disorders such as loss of neuronal projections (Mallard et al., 1995), increased susceptibility to seizure (O'Reilly and Haddad, 1996), and apoptosis (Hill et al., 1995; Oo et al., 1995; Walton et al., 1997). It is known that, among other systems, the GABA neurotransmitter system is highly sensitive to hypoxic and ischemic conditions (Onodera et al., 1987; Ninomiya et al., 1988; Sher, 1990; Lutz and Leone-Kabler, 1995).
Previous studies from our laboratory have established the pattern of expression, heterogeneity, modulation properties, and functionality of the GABAAR complex in the developing chick OT (Fiszer de Plazas and Mitridate de Novara, 1990; Fiszer de Plazas et al., 1995; Gravielle et al., 1998; Viapiano et al., 1998). We have also characterized the time of appearance and the temporal evolution of high- and low-affinity GABA binding sites (Fiszer de Plazas et al., 1993).
More recently, by using a chick embryo model of normobaric acute hypoxic hypoxia, we have evaluated the changes induced by the hypoxic treatment both on the developmental pattern of the GABAAR and on the pharmacological properties of the receptor. These hypoxia-induced changes are stage dependent, being maximal during the early stages (ED12), decreasing during subsequent days, and disappearing by ED18 (Rodríguez Gil et al., 2000, 2002, 2004).
Several hypotheses can be proposed to account for the set of changes observed at ED12 and the absence of changes at ED18. One of them, with strong support in the literature, is that posttranslational changes such as kinase-mediated phosphorylation of the receptor complex subunits result in significant functional changes of the GABAAR complex. These differential effects depend on which consensus site is phophorylated and which specific kinases are involved in phosphorylation (Moss et al., 1992; Kellenberger et al., 1992; McDonald and Moss, 1994; Connolly et al., 1999). All these factors confer a high degree of plasticity on the GABAAR function.
The phosphorylation effect could also depend on which subunit is phosphorylated. Thus, changes in the receptor subunit composition could modify the phosphorylation effects. In this sense, it has been postulated that changes in the subunit mRNA levels produce corresponding changes in the GABAAR subunit composition relative to other isoforms (Yu et al., 1996).
If we try to explain the sensitivity to hypoxia at ED12 vs. the absence of sensitivity at ED18, in terms of differences in the receptor composition, at least three conditions should be fulfilled. First, the receptor complex composition at ED12 should be different from that at ED18. Second, during early stages (ED12), the “early” receptor complex should possess the ability to change its subunit composition under the hypoxic condition. Third, during the late stages, the receptor complex subunit composition should remain unchanged. In this case, the pattern of subunit mRNA levels should remain unchanged under the hypoxic condition. The first argument is supported by previous findings demonstrating that the level of mRNA expression of the α1, α2, β2, and γ2 subunits significantly changes in several tectal layers as a function of the developmental stage (Rodríguez Gil et al., 2005). The second and third points require experimental support.
Layer “i” of the chick embryo OT was selected for this analysis because it is the most prominent GABAergic layer in the adult OT (Dietl et al., 1988; Granda and Crossland, 1989). Layer i displays the maximal level of expression of the mRNAs encoding the four subunits (α1, α2, β2, and γ2) of the GABAA receptor during development and undergoes the most drastic changes between embryonic day (ED) 10 and hatching (Rodríguez Gil et al., 2005). The present work aims at 1) determining whether the mRNA level of the α1, α2, β2, and γ2 subunits are sensitive to acute hypoxia during development, 2) elucidating whether such sensitivity changes as a function of the embryonic age, and 3) finding out whether mRNA levels of all the subunits are equally sensitive to an acute hypoxic state.
MATERIALS AND METHODS
Preparation of Tissue
Fertile chicken (Gallus gallus domesticus) specific-pathogen-free (SPF) eggs from the white leghorn were obtained from a local hatchery and incubated at 37°C and 60% relative humidity. Global hypoxic treatment was induced as previously described (Rodríguez Gil et al., 2000). In brief, eggs at different embryonic days were placed vertically in a 10-liter plastic chamber inside the incubator under the same conditions and subjected to a stream of 8% O2/92% N2 for 60 min, at a flow rate of 1 liter/min. The chamber contained retention valves both to allow escape of excess gases and to avoid mixing with atmospheric air and a storage space with calcium hydroxide to absorb the CO2 formed during the hypoxic treatment. After 1 hr under normobaric hypoxic treatment, eggs were immediately processed for biochemical studies. Simultaneously, control embryos were maintained under normoxic conditions in the same incubator.
Embryos at different embryonic days (12–18) were killed by decapitation, frozen on dry ice, wrapped in Parafilm to prevent freeze drying, and stored at −70°C until use. The above protocol follows the Guide for the care and use of laboratory animals from the Institute of Laboratory Animals Resources, Commission of Life Sciences, National Research Council.
Cryostat sections (25 μm) coinciding with the longitudinal axis of the OT were used. Pairs of adjacent sections were thaw mounted on poly-L-lysine-coated slides. Dry sections were fixed for 5 min in 4% (w/v) paraformaldehyde in phosphate-buffered saline (PBS; 130 mM sodium chloride, 7 mM disodium hydrogen orthophosphate, and 3 mM sodium dihydrogen orthophosphate). Slides were washed twice for 2 min each in PBS, dehydrated for 5 min in 70% and 95% ethanol (v/v), and stored at 4°C in 95% ethanol. Prior to hybridization, sections were removed from ethanol and allowed to air dry.
In Situ Hybridization
Transcript-specific, antisense, 45-base oligonucleotides were acquired from Genset Corp. (La Jolla, CA), based on chicken sequences published previously: the α1 probe was complementary to nucleotides 1,356–1,400 (Bateson et al., 1991); the β2T probe was complementary to nucleotides 1,370–1,414 (Harvey et al., 1994); the γ2T probe was complementary to nucleotides 1,820–1,864 (Glencorse et al., 1990); the α2 probe was complementary to nucleotides 1,504–1,548 of rat. It has been demonstrated that this oligonucleotide recognizes chicken α2 subunit (Ho et al., 2001, 2002). In situ hybridization was performed as previously described (Wisden et al., 1991), with minor modifications (Rodríguez Gil et al., 2005).
Expression levels of the mRNAs encoding the four subunits (α1, α2, β2, and γ2) of the GABAAR were quantified in layer i by measuring the X-ray film optical density using an MCID-M1 V4.2 Rev.1.0 densitometric system (Imaging Research Inc., St. Catharines, Ontario, Canada). Tectal layers were identified according to criteria defined by Scicolone et al. (1995).
For microscopic analysis, the sections were dipped in photographic emulsion (Kodak NTB2 diluted 1:1 in water), allowed to dry, and exposed to darkness at 4°C for 4 weeks. Dipped sections were developed in D19 (Kodak) for 2 min, fixed, and subsequently counterstained with cresyl violet. Signal specificity was assessed by using competition experiments in which radiolabeled probes were hybridized to sections in the presence of excess (100-fold) of unlabeled probe. This resulted in blank autoradiographs.
To quantify the level of expression at the layer i neuronal population, cell counts was performed in the cephalic region of each chick OT. Ten (five hypoxic and five control) embryos (20 OTs) were used for each embryonic stage. Eight adjacent sections were obtained from each OT (one pair for each subunit). Ten 104 μm2 areas, all of them corresponding to homologous cephalic regions, were used for cell counting in each histological section. A cell was considered as positively labelled when it contained at least one clearly identifiable mark within the contour of the cytoplasm. To clearly visualize the cell contour, the cryostat sections were counterstained with cresyl violet. Data were express as density of labeled cells/104 μm2 ± SEM as well as percentage (n% ± SEM) of cells expressing each subunit mRNA. Because no statistically significant differences were found between right and left OTs, data were grouped. Statistical analyses for variation of OD, density of labeled cells, and percentage of labeled cells over time were carried out by using a two-way ANOVA, followed by a simple effect test and by a Tukey's posttest.
Minimal Probability of Coexpression
The minimal probability of coexpression (MPC) allows estimating the extent to which neuronal subpopulations of layer i that express different subunits overlap. The overlapping quantification allows a statistical estimation of coexpression. The rationale for this estimation resides in the fact that, if two different subunit mRNAs are expressed by more than 50% of a cell population, then some cells should coexpress them. As an example, if 80% of a cell population express A and B, then at least 60% of the cells coexpress them. To estimate the MPC, the percentage of cells expressing each of the four subunit mRNAs was calculated in series of four adjacent sections from each embryo. Five series of four adjacent sections obtained from five different embryos were used for this purpose. The mean of the percentage of cells expressing each subunit mRNA was used to estimate the MPC of each series. A simple calculus allows estimating the MPC for combinations of two subunits: (percentage of A + percentage of B) − 100% = MPC of both subunit mRNAs. The MPC for combinations of three subunits is given by the expression: [(percentage of A + percentage of B) − 100%] + percentage of C − 100%.
It can be noted that, given the procedure to estimate the MPC, the absence of overlapping between subpopulations that express different subunits (A and B) results in a negative number (–n). In this case, “n” also refers to a percentage of cells. The value “n” represents the probability that at least n% of cells do not coexpress A and B. Statistical analyses were carried out by using a two-way ANOVA, followed by a simple effect test and by a Tukey's posttest.
RESULTS
Densitometric Analysis
The X-ray film analysis allows a densitometric estimation of the differences in the intensity of expression of the four subunit mRNAs between controls and hypoxic embryos in layer i. Statistically significant differences in OD were found only at ED12. The hypoxic embryos showed 1) an increase in the OD corresponding to the α1 subunit mRNA and 2) a decrease in the OD corresponding to the β2 subunit mRNA. The other two subunits did not change under hypoxia. Neither at ED16 nor at ED18 were significant differences found when comparing the levels of the four subunit mRNAs between control and hypoxic embryos (data not shown).
Microscopic Analysis of In Situ Hybridization
Figure 1 shows photomicrographs of ED12 OTs from control and hypoxic embryos. The darkfield (Fig. 1A) and brightfield (Fig. 1B) images show qualitative differences in the expression of α1, β2, and γ2 mRNAs between control and hypoxic OTs. Figure 1C shows that light microscopy allowed a reliable identification of labelled cells and counting of grains per cell. These light microscopic preparations were used for the quantitative studies.

Light micrographs of in situ hybridization preparations illustrating the differences in α1, β2, and γ2 mRNA expression at ED12 between control and hypoxic embryos. A: Darkfield images along the OT radial axis illustrate the differences in the level of expression (compare control vs. hypoxia) in several OT layers; from the SAC to the TCC3. B: Brightfield images of layer i. C: Light microscopy allows a reliable identification of unlabeled and labeled cells with different numbers of grains. Scale bars = 45 μm in A; 10 μm in B; 5 μm in C.
Analysis of Density of Cells Expressing Different Subunit mRNAs
Under normal conditions, the number of cells expressing the α1 subunit mRNA in layer i significantly increased from ED12 to ED16 and ED18 (Fig. 2A). In addition, the hypoxic treatment produced a significant increase in the number of cells expressing the α1 mRNA on ED12. No significant changes took place either at ED16 or at ED18, suggesting that the expression of this subunit mRNA is not sensitive to acute hypoxia at the latest stages.
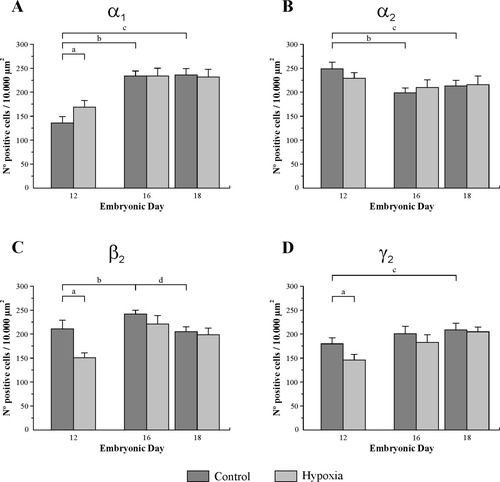
Each plot illustrates the changes in the level of expression of different subunit mRNAs in layer i of the OT along the normal development and the effect produced by an acute hypoxic treatment as a function of the stage. Each bar represents the mean ± SEM of the density (number of cells/104 μm2) of labeled cells. A: α1, B: α2. C: β2. D: γ2. Statistically significant differences during normal development between ED12 and ED16 (b), between ED12 and ED18 (c), and between ED16 and ED18 (d); a indicates the existence of statistically significant differences between control and hypoxic embryos at each developmental stage.
Under normal conditions, the number of α2 mRNA-expressing cells significantly decreased at ED12 vs. both ED16 and ED18 (Fig. 2B). No significant differences were found in the number of cells between controls and hypoxic embryos at the three stages analyzed.
During normal development, the density of β2 mRNA-expressing cells significantly increased between ED12 and ED16 (Fig. 2C) and then decreased between ED16 and ED18. The number of γ2 mRNA-expressing cells (Fig. 2D) gradually increased under normal conditions, and a significant difference could be estimated in comparing ED12 and ED18.
The number of cells expressing β2 and γ2 subunit mRNAs displayed similar patterns of response to hypoxia (compare Fig. 2C and D). The number of cells expressing these subunit mRNAs significantly decreased under hypoxia at ED12. At later stages, there are tendencies to decrease under hypoxia. The difference is greater at ED16 than at ED18, but this is not statistically significant.
Quantification of Subunit mRNA Expression Overlap and Estimation of the Minimal Probability of Coexpression
This section analyses the extent to which subpopulations of cells expressing different subunit mRNAs overlap. This procedure allows us 1) to investigate how the MPC changes as a function of the developmental stage and 2) to elucidate whether the hypoxic conditions affect the MPC. The percentage of cells expressing each subunit mRNA was used to estimate the MPC. Table I shows the values of mean percentages of cells expressing each subunit mRNA in control and hypoxic embryos.
| Subunit | ED12 | ED16 | ED18 | |||
|---|---|---|---|---|---|---|
| Control | Hypoxia | Control | Hypoxia | Control | Hypoxia | |
| α1 | 53 ± 5 | 66 ± 7a | 91 ± 5b | 91 ± 8b | 92 ± 7c | 90 ± 8c |
| α2 | 97 ± 6 | 89 ± 6 | 78 ± 5b | 82 ± 8 | 83 ± 6c | 84 ± 9 |
| β2 | 82 ± 9 | 59 ± 5a | 94 ± 4b | 86 ± 9b | 80 ± 5d | 77 ± 7c |
| γ2 | 70 ± 6 | 57 ± 6a | 76 ± 8 | 71 ± 8b | 81 ± 7c | 80 ± 5c |
- * Ten fields per sections were counted for each subunit, and these values were averaged to obtain the percentage of each embryo (see Materials and Methods). The table shows values of mean percentage ± SEM obtained from five embryos.
- a Statistically significant differences between control and hypoxia at each embryonic day.
- b Statistically significant differences between ED12 and ED16 under control or hypoxic conditions.
- c Statistically significant differences between ED12 and ED18 under control or hypoxic conditions.
- d Statistically significant differences between ED16 and ED18 under control or hypoxic conditions.
Figure 3 graphically illustrates the changes in the MPC of the α1 subunit mRNA in combinations with the other subunit mRNAs in normal development and the changes in the MPC induced by acute hypoxia at ED12 and ED18. At ED12, the MPC of the α1 mRNA in double, triple, and quadruple combinations with the other mRNA undergoes remarkable changes under hypoxic conditions [Fig. 3A; compare left (normal) and right (hypoxia) panels]. By contrast, at ED18, the MPC of the α1 mRNA undergoes only minor modifications (Fig. 3B; compare normal and hypoxic embryos). Tables II–IV show the MPC values corresponding to the whole set of possible combinations (double, triple, and quadruple) of the four types of mRNA. The statistical significance of the differences between stages and between controls and hypoxic embryos is indicated in these tables.
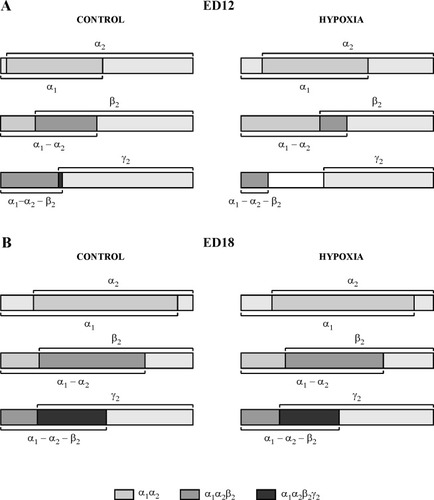
Schematic representation of the changes in the MPC of 1 subunit mRNA in defined combination with other subunits under control and hypoxic conditions at ED12 and ED18. Each bar represents the entire (100%) neuronal population counted in the layer i. The segments indicated on each bar labeled as α1, α2, β2, or γ2 correspond to the percentages of the entire population that express each subunit mRNA. The zones of overlap, indicated with different colors, correspond to the MPC of the α1 α2 subunits mRNA combination (light grey), α1 α2 β2 combination (grey) and α1 α2 β2 γ2 combination (dark grey). The absence of overlapping of the four subunit mRNAs observed at ED12 under hypoxic conditions, indicated as an empty space (white segment), corresponds to a negative value (−29), indicating that at least 29% of the cells do not coexpress the four subunits.
| Subunits | ED12 | ED16 | ED18 | |||
|---|---|---|---|---|---|---|
| Control | Hypoxia | Control | Hypoxia | Control | Hypoxia | |
| α1-α2 | 50 ± 4 | 55 ± 3a | 68 ± 5b | 73 ± 5ab | 74 ± 6cd | 74 ± 7c |
| α1-β2 | 35 ± 4 | 25 ± 4a | 85 ± 6b | 77 ± 6ab | 71 ± 7cd | 67 ± 6cd |
| α1-γ2 | 23 ± 3 | 23 ± 7 | 67 ± 8b | 62 ± 7b | 73 ± 7c | 70 ± 7cd |
| α2-β2 | 79 ± 7 | 48 ± 7a | 72 ± 9 | 68 ± 8b | 62 ± 7c | 61 ± 7c |
| α2-γ2 | 67 ± 9 | 46 ± 7a | 54 ± 10b | 53 ± 7 | 64 ± 8 | 64 ± 5cd |
| β2-γ2 | 52 ± 6 | 16 ± 4a | 70 ± 9b | 57 ± 7ab | 61 ± 6cd | 57 ± 8c |
- * Mean ± SEM of the MPC obtained from five embryos.
- a Statistically significant differences between control and hypoxia at each embryonic day.
- b Statistically significant differences between ED12 and ED16 under control or hypoxic conditions.
- c Statistically significant differences between ED12 and ED18 under control or hypoxic conditions.
- d Statistically significant differences between ED16 and ED18 under control or hypoxic conditions.
| Subunits | ED12 | ED16 | ED18 | |||
|---|---|---|---|---|---|---|
| Control | Hypoxia | Control | Hypoxia | Control | Hypoxia | |
| α1-α2-β2 | 32 ± 7 | 14 ± 5a | 62 ± 8b | 59 ± 4b | 54 ± 6c | 51 ± 6c |
| α1-α2-γ2 | 20 ± 6 | 12 ± 5a | 44 ± 6b | 44 ± 7b | 56 ± 5cd | 54 ± 5c |
| α1-β2-γ2 | 5 ± 3 | −18 ± 7a | 61 ± 6b | 48 ± 5ab | 52 ± 5cd | 47 ± 4c |
| α2-β2-γ2 | 49 ± 5 | −1 ± 6a | 48 ± 5 | 39 ± 6b | 44 ± 3c | 41 ± 4c |
- * Mean ± SEM of the MPC obtained from five embryos.
- a Statistically significant differences between control and hypoxia at each embryonic day.
- b Statistically significant differences between ED12 and ED16 under control or hypoxic conditions.
- c Statistically significant differences between ED12 and ED18 under control or hypoxic conditions.
- d Statistically significant differences between ED16 and ED18 under control or hypoxic conditions.
| Subunits | ED12 | ED16 | ED18 | |||
|---|---|---|---|---|---|---|
| Control | Hypoxia | Control | Hypoxia | Control | Hypoxia | |
| α1-α2-β2-γ2 | 2 ± 3 | −29 ± 10a | 38 ± 4b | 30 ± 4b | 35 ± 6c | 31 ± 5c |
- * Mean ± SEM of the MPC obtained from five embryos.
- a Statistically significant differences between control and hypoxia at each embryonic day.
- b Statistically significant differences between ED12 and ED16 under control or hypoxic conditions.
- c Statistically significant differences between ED12 and ED18 under control or hypoxic conditions.
Changes in the MPC During Normal Development
The MPC of the double combinations α1α2, α1β2, and α1γ2 increased during normal development, especially between ED12 and ED16 (Fig. 3, Table II). The differences between ED16 and ED18, although smaller, are also significant except for α1γ2. The changes in the MPC of the other double combinations, i.e., α2β2, α2γ2, and β2γ2, display complex patterns that can not be described by a simple rule. The statistically significant differences (P < 0.05) are indicated in Table II.
The MPC of the triple combinations α1α2β2, α1α2γ2, and α1β2γ2 also significantly increased from ED12 to ED16 and ED18 (Table III). The MPC of α2β2γ2, by contrast, gradually decreased during development and displayed significant differences only between ED12 and ED18. The MPC of the quadruple combination α1α2β2γ2 displayed a significant increase from ED12 to ED16 and ED18, with the maximal increase between ED12 and ED16.
From this picture, it is clear that 1) during normal development the MPC of the different subunit mRNAs combinations differentially changes as a function of the stage, 2) any combination involving the α1 subunit significantly increased as a function of the stage, and 3) the combinations excluding the α1 subunit only occasionally show characteristic patterns; as specific cases, the MPC of α2β2 and α2β2γ2 display decreasing trends as a function of the stage. This is due to the fact that only the mean percentage of the α1 subunit mRNA-expressing cells consistently increased between ED12 and ED18.
Changes in the MPC Induced by Acute Hypoxia
The MPC drastically changed under hypoxia at ED12; the hypoxic influence decreased by ED16 and was minimal by ED18 (Fig. 3, Table II). The acute hypoxia differentially affects the MPC of different subunit mRNA combinations as a function of the stage. At ED12, except for α1γ2, the MPC of all the other double combinations significantly changed. The α1α2 was the only double combination whose MPC increased with hypoxic treatment. This is explained by the increase in the mean percentage of the α1 mRNA-expressing cells under hypoxia, with the mean percentage of the α2 mRNA-expressing cells remaining almost unchanged. The absence of difference in the MPC of α1γ2 between control and hypoxia results from the fact that the increase in the mean percentage of cells expressing the α1 mRNA was counteracted by a concomitant decrease in the mean percentage of cells expressing the γ2 mRNA. The MPC of β2γ2 was particularly affected, because the hypoxia significantly reduced the mean percentage of cells expressing both subunit mRNAs. The MPC of the other combinations significantly decreased, although with different intensity. The statistical significant differences are indicated in Table II.
At ED12, the differences in MPC for triple combinations between normal and hypoxic embryos were even greater (Table III). Given that the mean percentages of β2 and γ2 mRNA-expressing cells simultaneously decreased under hypoxia, the MPC of the triple combinations involving these subunit mRNAs showed the maximal differences. The negative values of MPC calculated for such combinations indicate the probability that at least that percentage of cells do not coexpress those triple combinations. No coexpression of the four subunit mRNAs was calculated at this stage under the hypoxic condition (Table IV). The negative value of the MPC indicates the probability that at least 29% of cells do not coexpress the four types of mRNA.
At ED16, the pattern of changes in the MPC induced by hypoxia was similar to that described at ED12. The differences, however, were smaller, and only three of the six double combinations displayed significant differences. The MPC of α1α2 increased, whereas that of α1β2 and β2γ2 significantly decreased. By ED16, the decrease induced by hypoxia in the MPC of the triple combinations was smaller than that estimated at ED12; only the MPC of α1β2γ2 significantly decreased under hypoxia.
At ED18, the values of the MPC for combinations of two, three, and four subunit mRNAs consistently decreased under hypoxia. The differences, however, were very small and nonsignificant on this statistical test.
Mean Percentage of Variation Between Control and Hypoxia as a Function of Stage
This complex picture is easily explained by calculating the differential changes produced by acute hypoxia in the mean percentages of cells expressing the four different subunit mRNAs. These differences depend on both the mRNA type and the developmental stage.
To estimate the differences in the effect produced by hypoxia in different developmental stages, we calculated the absolute differences in the mean percentage of cells expressing each of the four subunits between control and hypoxia. These differences were expressed in terms of percentages with respect to the control value and then averaged to obtain a mean percentage of variation as a function of the stage. The same procedure was used to estimate the mean percentage of variation in the MPC as a function of the stage.
Data presented in Table I show that the hypoxia produced a mean percentage of variation of 20% at ED12, 5% at ED16, and only 2% at ED18. The differences between ED12 vs ED16 and ED18 were statistically significant (P < 0.003).
With regard to the mean percentage of variation of the MPC of the double combinations, the hypoxia produced a variation of 37% at ED12, 8% at ED16, and only 4% at ED18. The differences between ED12 and ED16 and ED18 were statistically significant (P < 0.01). With regard to the MPC of the triple combinations, the hypoxia produced a variation of 206% on ED12, 11% at ED16, and only a 7% at ED18. For the comparison of the mean percentage of variation in the MPC of the quadruple combination between ED12 vs. ED16 and ED18, the differences as a function of stage were even higher: about 1,500% at ED12, 21% at ED16, and only 11% at ED18. From this picture it is clear that the sensitivity to acute hypoxia decreases as a function of the developmental stage.
As was pointed out in this and in the preceding section, the differences in values of MPC between control and hypoxia calculated at ED18 were small. However, in comparing the values of MPC of the double combinations between control and hypoxia at ED18 as two statistical populations, a paired Student's t-test indicated a highly significant difference (P < 0.007). A similar analysis performed with the values of triple combinations also gave a significant difference (P < 0.01). Taking the values of MPC of all the combinations estimated in normal vs. hypoxic conditions as two statistical populations, the analysis indicated the existence of a highly significant difference (P < 0.00003).
DISCUSSION
Previous binding studies in the developing OT demonstrate that acute hypoxia induces a significant decrease in the binding capacity (Bmax) of the low-affinity binding site and an increase in the GABAA receptor sensitivity to binding modulators such as pentobarbital and 3α-hydroxy-5α-pregnan-20 one, without any effect on the binding affinity (Kd) (Rodríguez Gil et al., 2002). These hypoxia-induced changes are maximal at ED12, decrease during subsequent days, and disappear by ED18. We have proposed that the receptor subunit composition at ED12 differs from that at ED18. Given that the receptor stoichiometry could depend, in part, on the availability of receptor subunits, a differential change in the subunit mRNA levels during the early stages could result in significant differences in the receptor complex composition. In this paper we propose that the mechanisms involved in regulating the stability of the subunit mRNAs could be sensitive to the hypoxic condition during the early stages and less sensitive during the last stages.
The rate of transcription is the most relevant step in the regulation of the GABAAR subunit mRNAs expression and the differential transcription of the receptor subunits seems to explain the differences in GABAAR subtypes found in different CNS areas and/or different developmental stages (Steiger and Russek, 2004). The level of expression of the different receptor subunit mRNAs significantly changes as a function of the developmental stage: in the OT the α1 subunit levels increase during development (Yin and Lee, 1994), and the expression of four subunit mRNAs (α1, α2, β2 and γ2) significantly changes during development in the different layers of the OT between ED10 and ED20 (Rodríguez Gil et al., 2005).
The present paper demonstrates that a controlled level of acute hypoxia during development produces significant changes in the level of several receptor subunit mRNAs. This study also demonstrates that the sensitivity to acute hypoxia is stage-dependent, the earlier stages being more sensitive than the later ones. We also demonstrate that the different subunit mRNAs are differentially affected. The level of the α1, β2, and γ2 mRNA undergoes drastic changes, whereas α2 mRNA is not affected at all.
During the early stage (ED12), acute hypoxia induces significant changes in the density and percentage of neurons expressing the different subunit mRNAs. The changes in the percentage explain the differences in the degree of mRNA expression overlap measured in neurons of layer i subjected to hypoxia.
It is known that a large variety of GABAergic neuron populations from different CNS areas coexpress different repertoires of GABAAR subunits. There are reports suggesting that the most common pentameric combination includes 2α, 2β, and 1γ subunits (Chang et al., 1996) or 2α, 1β, and 2γ subunits (Backus et al., 1993). A remarkable feature of the GABAAR is the high variability and plasticity of the receptor assembly. In the rat brain, different subunit mRNAs combinations are expressed in different regions, and there are varying degrees of overlap in their distribution. In the OT of 1-day-old chick embryo, α, β, and γ mRNA are coexpressed in dissimilar proportions (Bateson et al., 1991; Glencorse et al., 1990, 1992; Harvey and Darlison, 1997). The existence of several GABAAR subtypes with different subunit compositions probably depends, in part, on the differential transcription of each mRNA and on the differential availability or proportion of the different subunit proteins.
The quantification of the mRNAs expression overlap presented in this paper allows estimation of the MPC. In this sense, two different features must be considered: 1) the changes in the MPC that takes place during the normal development and 2) the changes in the MPC produced by acute hypoxia in different developmental stages. The changes in the MPC calculated for different combinations of subunit mRNAs during normal development probably depend on changes in the rate of transcription of the different subunit mRNAs as a function of the developmental stage. It has been proposed that the differential transcription in different CNS areas or in different developmental stages has the potential to modify the subunit composition of the GABAAR generating different GABAAR subtypes (Steiger and Russek, 2004).
There are several other posttranscriptional mechanisms that could explain the variety of combinations of subunit expression that can originate the several GABAAR subtypes. The changes in MPC reported in this paper are established with only 1 hr of hypoxia. These changes are so fast that one of the most plausible explanations is that hypoxia could change the level of the different subunits by modifying the mRNA stability. There are examples in the literature demonstrating that fast changes in the level of different mRNA species depend on changes in the mRNA stability (Levy et al., 1996; McGary et al., 1997; Li et al., 1999; Zulueta et al., 2002; Zhang et al., 2006).
The MPC of the double subunit mRNA combinations involving the α1 subunit (α1α2, α1β2, α1γ2) increases significantly during normal development. The MPC of the triple combinations involving the α1 subunit mRNA also significantly increases between ED12 and ED16. This is due to the fact that the mean percentage of cells expressing the α1 mRNA strikingly increases between ED12 and ED16. The mean percentages of cells expressing β2 and γ2 mRNA, by contrast, significantly decrease under hypoxia. The interplay between these changes in the mean percentages of cells expressing the different mRNA (an increase in α1, a decrease in β2 and γ2) explains the changes in the values of the MPC observed in Table II.
It must be remarked that, although we cannot detect statistically significant differences in the density of cells expressing each subunit mRNA between normal and hypoxic embryos at ED18, there is a highly statistically significant difference in the MPC between control and hypoxic embryos, and this is true for the MPC estimated for double, triple, and also quadruple combinations of mRNA. This result indicates that, at ED18, rather than being insensitive, the regulation of mRNA level is only less sensitive than that at the earlier stages. It must also be noted that, although highly significant, the differences in the MPC at ED18 are probably too small to affect the receptor complex composition significantly.
All these results give indirect support to our hypothesis that hypoxia, by changing the level of the different subunit mRNAs, could affect the receptor complex subunit composition. In theory, a change in the subunit mRNA availability could alter the proportion of the subunit protein available for receptor complex assembly, and changes in the receptor subunit composition could alter the potential effects of phosphorylation by different kinases. In this sense, it has already been postulated that the decrease in the β and the α3 subunit mRNA level, observed in chronic neurosteroid treatment, could produce a corresponding decrease in the presence of β and α3 subunit in the composition of GABAAR isoforms, relative to other isoforms (Yu et al., 1996). The present data together with our previous results demonstrating significant changes in the pharmacological properties of the GABAAR under hypoxia strongly suggest that during the early developmental stages the GABAAR is able to undergo environment-dependent plastic changes.




