Dynamics of lactate concentration and blood oxygen level-dependent effect in the human visual cortex during repeated identical stimuli
Abstract
In vivo 1H NMR spectroscopy at 7 T was utilized to measure the changes in lactate concentration upon repeated identical visual stimuli, each lasting for 2 min. The average amplitude of these increases was found to be reduced over time (P < 0.01), from 0.13 ± 0.02 μmol/g during the first half of the stimulation paradigm, to 0.06 ± 0.02 μmol/g during the second half of the stimulation paradigm. In contrast, the blood oxygen level-dependent (BOLD) effect on the fMRI water signal and on the height of the total creatine signal at 3.03 ppm was persistent during the whole observation period. This finding may suggest a differential adaptation of cortical output that is not reflected at the level of the global excitation-inhibition activity of the cortical canonical circuits. Alternative possibilities that could account for an adaptation of [Lac] changes are also discussed. © 2007 Wiley-Liss, Inc.
During the past 15 years, the time course of lactate concentration ([Lac]) in the human brain has been measured by means of 1H NMR spectroscopy under a variety of experimental conditions and functional paradigms (for review see Mangia et al., 2006a). The interest in investigating the [Lac] dynamics during increased neuronal activity is due to the fact that lactate is an intermediate of glucose metabolism and is an important marker of metabolic activity under either aerobic or anaerobic conditions (Siesjö, 1978); therefore, its role during brain activation has been at the center of many considerations (Chih et al., 2001; Dienel and Hertz, 2001; Shulman et al., 2001; Dienel and Cruz, 2004; Pellerin and Magistretti, 2004; Schurr, 2006).
In our own first extensive application of temporally resolved functional NMR spectroscopy (fMRS) at 7 T (Mangia et al., 2006b), we concentrated on the dynamics of [Lac] during visual stimulations lasting for approximately 5 min and 10 min. We found that [Lac] increased by ∼0.2 μmol/g within the first minute of activation, reaching a new steady-state value, and came back to baseline after the end of the stimulus. Such a finding, together with other experimental evidence reported either in that work and previously in the literature, suggested that the increased energy needs were fulfilled by oxidative metabolism that reached a new steady-state level during the entire prolonged stimulation. In the Mangia et al. (2006b) study, the amplitude of [Lac] changes was slightly decreased during the second repetition of 5-min stimulation periods (see Fig. 3a in Mangia et al., 2006b), but the difference compared with the first stimulation period was not statistically significant and therefore was not taken into account in further discussion. That observation nonetheless suggested the possibility of variable lactate response with repeated identical stimuli and led to the current study, in which we investigated in detail the changes of [Lac] during repeated stimuli.
Lower stimulus-induced lactate increases with repeated stimuli may reflect reduced metabolic needs arising from neuronal adaptation. It is well established that the adaptation response is ubiquitously expressed in the properties of single neurons throughout the nervous system. In the visual system, specifically, the firing rate of feature-sensitive neurons (e.g., neurons selective to pattern orientation or motion direction) of a wide range of species typically decreases during repeated presentations of a stimulus with the same orientation or motion (Barlow and Hill, 1963; Vautin and Berkley, 1977; Movshon and Lennie, 1979; Maddess et al., 1988; Ibbotson et al., 1998; Carandini et al., 1998; Dragoi et al., 2000; Kohn and Movshon, 2003). This very property of neuronal populations has also been cleverly exploited for designing fMRI experiments aimed at differentiating responses of overlapping populations (for review see Grill-Spector and Malach, 2001; Krekelberg et al., 2006). The logic of these experiments relies on the fact that adaptation affects the activity of only the feature-specific neurons. Thus, presentation of a new stimulus, differing in sensory attributes, is likely to activate a new, not-fatigued neural population, resulting in a “rebound” in the fMRI signal. Such adaptation fMRI designs showed that changes in the blood oxygen level-dependent (BOLD) fMRI signal depend on the time scale of the adaptation designs (for review see Krekelberg et al., 2006). The exact conditions that would generate an fMRI signal rebound, as well as the mechanisms involved in different types of adaptation behaviors, remain unknown. The adaptation mechanisms in a given cortical area may involve simple fatigue or disinhibition resulting from feature opponency (e.g., motion or color opponency; see Simoncelli and Heeger, 1998), or they may be local or inherited from changes in other areas' activity (e.g., Kohn and Movshon, 2003). In other words, adaptation may be reflected in the input of an area, in its output (activity of the projection neurons), or both.
The aim of the present work was to gain some insight into the neural mechanisms of adaptation. By using high-field fMRS, we examined two types of response variants during repeated identical 2-min-long visual stimuli. The first examined variable, as mentioned above, was the concentration of lactate [Lac]; the second was the height of one of the strongest singlets of the spectrum, that is, the total creatine (Cr) signal at 3.03 ppm. The variations of the height of the total Cr signal during stimulation periods reflect the line width changes induced by the BOLD effect on metabolites (Zhu and Chen, 2001; Mangia et al., 2006b). In addition, we measured the BOLD effect on the water signal from fMRI time series.
MATERIALS AND METHODS
Measurements were performed on a 7 T/90 cm magnet (Magnex Scientific) interfaced to Varian INOVA console. Details of the system equipment, data acquisition, and data processing have been given elsewhere (Mangia et al., 2006a, b). Briefly, after automatic adjustment of all first- and second-order shim terms by FASTMAP (Gruetter and Tkàc̆, 2000), in vivo 1H NMR spectra were acquired by using a stimulated echo acquisition mode (STEAM) sequence with echo time (TE) = 6 msec and repetition time (TR) = 5 sec, as described previously (Tkàc̆ et al., 2001; Tkàc̆ and Gruetter, 2005). Localization of the volume of interest (VOI = 20 × 22 × 20 mm3) in the activated region of the visual cortex was guided by initial fMRI maps (parameters are in the legend to Fig. 1).
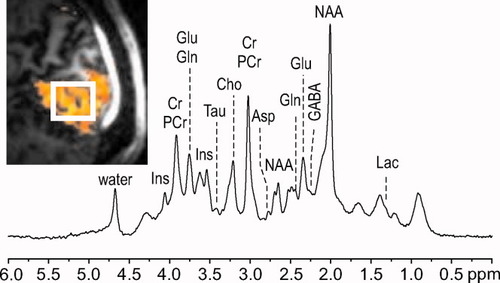
Representative 1H NMR spectrum (summed from 12 subjects) used for generating the time courses reported in Figure 2A,B. The inset depicts the localization of the voxel (20 × 22 × 20 mm3) in the activated visual cortex, based on functional maps. fMRI parameters: GE-EPI, TE = 22 msec, TR = 2.4 sec, spatial resolution 2.5 × 2.5 × 2.5 mm3; eight trials with 5-sec stimulation on and 22.5-sec stimulation off; activated pixels correspond to cc = 0.3, cluster size = 6 contiguous pixels. Functional maps were overlaid on T1-weighted anatomical images. Spectroscopic parameters: STEAM, TE = 6 msec, TR = 5 sec. Processing: frequency and phase corrections of individual scans, summation of four scans from each subject, residual eddy currents correction, summation across the 12 subjects (for a total of 48 scans), further frequency correction, gaussian multiplication (σ = 0.0865 sec), Fourier transform, and zero-order phase correction. No other postprocessing, such as water signal removal or baseline correction, was performed. Labels indicate the resonances of main brain metabolites in the spectrum.
Twelve healthy volunteers (aged 19–26 years) gave informed consent according to procedures approved by the Institutional Review Board and the FDA. A radial red/black checkerboard covering the entire visual field and flickering at a frequency of 8 Hz was used to activate the visual cortex, as described previously (Mangia et al., 2006b). The stimulus was turned on and off every 2 min during a 32-min stimulation paradigm for a total of eight functional trials (i.e., eight stimulation-rest episodes). An initial ∼2.7 min of rest condition was used before the 32-min paradigm as baseline reference, so the entire observation period was almost 35-min long. Data were acquired consecutively every 5 sec from each subject, and all FIDs were saved separately, without any averaging. Subsequently, they were individually frequency and phase corrected and averaged in different ways. To obtain time courses for the 35-min observation period (see Fig. 2) with 20-sec time resolution, four consecutive FIDs were summed for each subject (e.g., scans 1–4, 5–8, etc.), and the resulting FIDs corresponding to the same time interval in the stimulation paradigm were summed across subjects, thus generating a total of 104 spectra of 48 scans each (e.g., 48-scan/20-sec spectra). In a separate analysis, spectra were further summed over the first four stimulation-rest episodes; this generated a single time course corresponding to one stimulation-rest cycle. Every point of this time course resulted from the sum of four 48-scan/20-sec spectra (e.g., 192-scan/20-sec spectra) acquired at the same relative position in each trial, except the very first point, which instead indicated the baseline reference (averaged over the initial eight consecutive 48-scan/20-sec spectra). LCModel analysis (Provencher, 1993) was then utilized for [Lac] quantification on either the 48-scan/20-sec and 192-scan/20-sec spectra, using a simulated basis set and the spectrum of fast-relaxing macromolecules experimentally measured from the human brain.
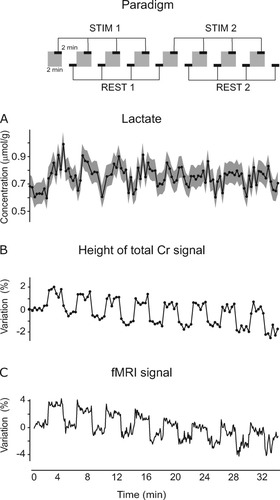
A: Time courses of [Lac] during the ∼35-min observation period covering eight stimulation-rest episodes. Spectra were first summed across the 12 subjects in groups of four scans (a representative spectrum from this time series is shown in Fig. 1), then quantified with LCModel. The gray shading through the curve indicates the uncertainty of [Lac] quantification identified by the CRLB (on average, CRLB were 9%). Time resolution: 20 sec. B: Time course of the height of the total creatine (Cr) signal (at 3.03 ppm) during the functional paradigm, quantified from the same time series of spectra used in A. Time resolution: 20 sec. C: Averaged time course of the BOLD-based fMRI signal acquired from the same VOI used for spectroscopy from one representative subject. Time resolution: 5 sec. The schematic of the functional paradigm illustrates that only the spectra acquired during the second half of each activation/rest episode were used for further spectra summation (as shown in Fig. 3) and for the statistical tests. Gray regions indicate the stimulation periods.
Several statistical tests were performed to compare [Lac] between activation and rest conditions and to compare the amplitude of [Lac] changes between the first half and the second half of the stimulation paradigm. For this purpose, [Lac] levels were evaluated only from the last three 48-scan/20-sec spectra of each activation/rest condition (to avoid transient effects), as shown in Figure 2. Averaged [Lac] levels were thus calculated from a total of 12 values corresponding to rest and activation over trials 1–4 and, separately, over trials 5–8. Unpaired t-tests between rest and activation were performed to evaluate the average amplitude of [Lac] changes, relative to the first and second half of the stimulation paradigm; these two average amplitudes of [Lac] changes were then compared between each other. In addition, averaged [Lac] levels during activation conditions were compared between trials 1–4 and 5–8, and the same was done for [Lac] during rest conditions. To check the stability of the baseline over the entire observation period, the initial [Lac] reference value was compared with [Lac] during rest conditions over trials 1–8. Data were presented as average ± sem.
Similarly to our previous study (Mangia et al., 2006b), two difference spectra between activation and rest conditions were generated using data from all subjects, respectively, during the first half and the second half of the stimulation paradigm. Specifically, the same spectra used for the statistical tests were summed accordingly and then subtracted. In details, this corresponded to four episodes, three spectra per episode and 48 scans per spectra, which resulted in a total of 576 scans per each activation-rest condition (called REST 1, REST 2, STIM 1, and STIM 2 in Figs. 2, 3). Exponential multiplication corresponding to 0.3-Hz line broadening was applied to the summed FIDs acquired during stimulation in order to match the line width of both spectra prior to subtraction. The resulting “BOLD-free” difference spectra were then analyzed by LCModel.
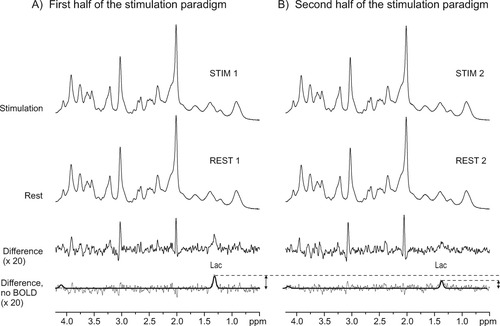
Comparison of spectra acquired from all subjects during the first half (A) and the second half (B) of the stimulation paradigm. Spectra were summed as shown in Figure 2 and as explained in Materials and Methods. From the top: summed spectra acquired during stimulation periods (STIM 1 and STIM 2); summed spectra acquired during rest periods (REST 1, REST 2); difference spectra between STIM and REST; difference spectra without the BOLD effect (obtained by 0.3-Hz line-broadening of the STIM spectra before subtraction), with superimposed LCModel fits of [Lac]. Vertical scales of the difference spectra are increased by 20 times compared with the sum of spectra acquired during rest and activation.
The BOLD effect on metabolites (Zhou and Chen, 2001) was evaluated throughout the entire session by quantifying the height of the total Cr signal (at 3.03 ppm) from the time series of 48-scans/20-sec spectra. In a different session, the BOLD-based fMRI signal was acquired from one of the subject during the same stimulation paradigm utilized for fMRS, the signal being averaged in the same VOI used for the spectroscopic measurements (fMRI acquisition parameters as in the legend to Fig. 1, except TR = 5 sec).
RESULTS
In vivo 1H NMR spectrum acquired from the visual cortex is shown in Figure 1 as an example of the quality that was routinely achieved throughout the whole study. Shimming resulted in water line widths around 13–14 Hz, with concomitant total Cr line widths of 11–12 Hz. The acquired spectra were artifact-free in the entire chemical shift range, and contamination by signals from extracerebral lipids was not observed in spite of the ultra-short echo time (TE = 6 msec). Spectra were highly reproducible within each session as well as between sessions of different subjects. The inset in Figure 1 shows the localization of the VOI in the activated region of the occipital lobe of a representative volunteer.
[Lac] was quantified with an average Cramér-Rao lower bound (CRLB) equal to 9% when utilizing the 48-scan/20-sec spectra and to 7% when utilizing the 192-scan/20-sec spectra. These CRLB corresponded to average estimated errors equal to 0.08 μmol/g and 0.05 μmol/g, respectively. As expected from previous work, stimulus presentation induced an increase in [Lac]. However, the observed [Lac] changes decreased in amplitude over time (Fig. 2A) and gradually adapted to a value not higher than 0.1 μmol/g. Specifically, during the first half of the paradigm, the average amplitude of [Lac] changes was 0.13 ± 0.02 μmol/g (P < 0.0001), whereas, during the second half of the paradigm, it was 0.06 ± 0.02 μmol/g (P = 0.0005). These two average amplitudes of [Lac] changes relative to the first and second halves of the stimulation paradigm were statistically different from each other (P < 0.01). [Lac] in activation conditions over trials 1–4 was higher than that over trials 5–8 (P = 0.0002). [Lac] under rest conditions over trials 1–4 was not different from that over trials 5–8 (P = 0.8). In addition, the initial reference value of [Lac] was slightly lower (by 7%) than [Lac] in rest conditions over trials 1–8 (P < 0.02).
The difference spectra calculated between stimulation and rest periods from all subjects (Fig. 3) further confirmed the decrease in the amplitude of [Lac] changes. The peaks in these difference spectra were indeed attributed to [Lac] changes as well as to line width changes ascribed to the BOLD effect. The effects of line width changes were minimized by applying appropriate line broadening to the stimulation spectra prior to subtraction (upper traces in Fig. 3). LCModel quantification of the remaining signals of these line-width-matched difference spectra provided [Lac] changes between stimulation and rest periods. These [Lac] changes were estimated to be 0.16 ± 0.01 μmol/g and 0.09 ± 0.01 μmol/g (errors are CRLB), respectively, in the first and second halves of the stimulation paradigm. These concentration changes were in very good agreement with those estimated by the analysis of the [Lac] time course shown in Figure 2A.
When spectra were summed from trial to trial (see Materials and Methods) during the first half of the stimulation paradigm (Fig. 4), [Lac] was found to reach the new steady-state value within the first minute of activation, recovering to baseline within 1–2 min after the end of the stimulus. Based on the estimated errors of the fit (CRLB), the baseline recovered after the end of the stimulus was not different from the baseline reference acquired before the beginning of the functional paradigm. The features of this time course (rise time and baseline recovery) were in excellent agreement with previous findings (Mangia et al., 2006b).
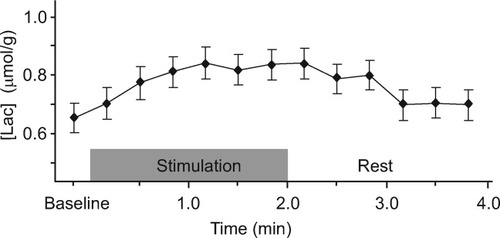
Time course of [Lac] over four averaged trials relative to the first half of the stimulation paradigm (first four stimulation-rest episodes). Every point of this time course results from the sum of four 48-scan spectra acquired at the same relative position in each trial (for a total of 192 scans). Time resolution: 20 sec. The very first point indicates the baseline reference (averaged over the initial eight consecutive 48-scan spectra). Error bars indicate CRLB.
In contrast to [Lac] time course, the signal height of the singlet of total Cr at 3.03 ppm increased by ∼2% during the stimulation periods for the entire functional paradigm (Fig. 2B). This phenomenon was associated with the signal-width decrease induced by the BOLD effect (Zhu and Chen, 2001) and confirmed that the VOI was correctly localized in the activated region of the visual cortex. The time course of the signal height of total Cr was in perfect agreement with the time course of the BOLD-based fMRI signal (Fig. 3C). As opposed to the baseline of [Lac], the BOLD-related signals decreased in the rest periods over time. Finally, all volunteers were able to perceive the repeated visual stimuli with identical performances for the entire experimental session, as guaranteed by the attentional task (described in Mangia et al., 2006b) that was performed by each subject during the study.
DISCUSSION
Functional MRS was applied here with a block-design paradigm characterized by repeated 2-min stimulation periods. This stimulation paradigm was closer to the paradigms used for fMRI studies than for typical fMRS experiments (for review see Mangia et al., 2006a). To a large extent, this is due to the reduction of signal averaging based on the high sensitivity of fMRS at 7 T (Fig. 1). The present study suggests an effect of repetitive stimuli on the increases in [Lac] (Fig. 2A), which decreased in amplitude over the course of a long observation period (P < 0.01), from an average of 0.13 ± 0.02 μmol/g to an average of 0.06 ± 0.02 μmol/g. The stimulation-induced increases in [Lac] occurred on top of a very stable baseline (P = 0.8), whereas the average [Lac] during stimulation conditions was reduced over time (P = 0.0002). These findings demonstrate that 1) 2-min-long rest periods were sufficient to guarantee a recovery of [Lac] to the baseline level after each stimulus (as also confirmed by the time course shown in Fig. 4, in agreement with Mangia et al., 2006b) and 2) the decreased amplitude of [Lac] changes was not due to a baseline drift. The adaptation of [Lac] changes was further confirmed by the analysis of the difference spectra between spectra acquired during rest and stimulation conditions from all subjects, averaged over the first and second halves of the functional paradigm (Fig. 3).
Only one other study has reported [Lac] changes upon repeated stimuli (Hu and Wilson, 1997). That study explored the extracellular [Lac] in the dentate gyrus of the hippocampus of the rat brain after several 5-sec electrical stimulations of the perforant pathway, interleaved by 2-min rest periods. As opposed to what observed in the present study, the authors found that the stimulation-induced increases in [Lac] occurred on top of a major global drifting of the baseline (up to 80%) during the entire paradigm: the stimulation-induced increases in extracellular [Lac] were smaller by three- to fourfold than the increase in baseline. This was due to the fact that the extracellular [Lac] takes almost 10 min to recover the baseline after one stimulus (Hu and Wilson, 1997; Fig. 1), so 2-min-long rest periods were not sufficient to guarantee a recover of the baseline after each stimulus.
In contrast to the adaptation of [Lac] changes, the modulation of the BOLD effect remained unchanged (Fig. 2B,C). This discrepancy indicates that the BOLD effect and the [Lac] response may be sensitive to different aspects of neuronal activation and/or metabolic long-term alteration. Specifically, we suggest that the two measurements may be preferentially sensitive to the global level of excitation-inhibition activity in a cortical microcolumn, and the deviation from this balance that commonly results in the firing of projection neurons (e.g., the pyramidal cells in cortex), respectively.
The reduction of neuronal electrical activity decreases energy metabolism in the brain. Indeed, several studies conducted in awake animals and under different levels of anesthesia (which is known to affect neurotransmission rates) have suggested that energy consumption increases with glutamatergic neurotransmission (Sibson et al., 1998; Choi et al., 2002; Oz et al., 2004). Theoretical work (Attwell and Laughlin, 2001; Lennie, 2003) and experimental evidence (for review see Raichle and Mintun, 2006; see also Sokoloff, 1999) indicate that the majority of energy usage not related to housekeeping work is devoted to reversing ion movements generated by postsynaptic current and action potentials, which ultimately results in neural firing. In our previous work (Mangia et al., 2006b), we concluded that the observed increase in [Lac] was occurring together with an increase of energy demands reflected in the increased oxidative metabolism. Together, these observations suggest that increases in [Lac] may indicate increased neuronal firing. Under these premises, the reduced amplitude of [Lac] changes upon repeated stimuli (Figs. 2, 3) would reflect a progressive decrease of energy consumption and consequently a progressive decrease of neuronal signaling in the region investigated. This is consistent with other findings suggesting that neuronal adaptation reduces firing (see, for example, Khon and Movshon, 2003).
On the other hand, measurements of intracortical electrical activity and BOLD fMRI in monkeys (Logothetis et al., 2001) lead to the conclusion that the BOLD contrast reflects the input and intracortical processing of a given area (the so-called perisynaptic activity), identified by local field potentials (LFPs), rather than its spiking output identified by multiunit activity (MUA). A similar conclusion applies also for cerebral blood flow (Lauritzen, 2001). Some authors argue that BOLD actually reflects spiking activity as well (on this topic, see review by Raichle and Mintun, 2006). However, it is worth noting that, if spike rate is increased (not always the case) during certain behavioral or stimulation conditions, these increases are always correlated with LFPs and of course with BOLD. Of additional interest are the cases of dissociation in cortex, where converging inputs in an area result in cross-inhibition and abolishment of spiking (no output). The conclusion drawn by Logothetis et al. (2001) relied on the observation that recording sites characterized by strong response-adaptation showed that, in the absence of any change in the MUA and single spikes, the LFPs were the only regressor that could be used to estimate BOLD. Therefore, here we propose to interpret the observed persistent BOLD effect during repeated identical stimuli in terms of persistent global synaptic activity, rather than persistent spiking output of that region.
A decrease of the spiking output concomitant with a persistently elevated synaptic activity, as suggested by the time courses of [Lac] and BOLD, is feasible based on how neurons integrate synaptic input, in other words, based on the balance between recurrent excitation and inhibition of the cortical canonical circuits. In this context, our findings suggest the existence of a dynamic interplay between activation and inhibition during repeated identical visual stimuli. In fact, if the activation-inhibition balance changes over time but the total inhibition-activation activity remains constant, the neuronal firing (and therefore the energy demands and the [Lac] response) would be dynamically affected, but the BOLD contrast would remain unchanged. This phenomenon could resemble a type of adaptation that results in a drop of energy requirements but not in modulations of the BOLD contrast.
It is of course also possible that the [Lac] time course does not reflect changes in oxygen consumption (to be measured) upon repeated visual stimuli and/or that a direct recording of the spiking output (to be measured) upon repeated visual stimuli reveals constant firing (absence of adaptation). In these cases, then, the data of the present study would imply a dynamic interplay of glycolysis compared with TCA cycle rates (a sort of metabolic adaptation) in the presence of unaffected neural firing, the origin of which would nonetheless still require further investigation. Alternatively, the lactate accumulated at the beginning under the first stimulation episodes could be removed or cleared from the observed zone by the circulation. To explain a reduction in the amplitude of [Lac] changes upon repeated stimuli, this possibility implies that stimulus-induced changes in CBF have to be higher after repeated stimuli. We do not have a measurement of CBF during the functional paradigm adopted in this study, but, from the stability of the stimulus-induced BOLD responses, we can conclude that increased amplitudes of CBF changes should be accompanied by similar changes of oxygen consumption. At the moment, there is no reason to predict that changes in oxygen consumption are higher after repeated stimuli (on the contrary, we suggest that these changes decrease after few stimuli because of adaptation). In addition, it is not established from a quantitative point of view how much a local change in blood flow would affect lactate clearance.
CONCLUSIONS
We conclude that the neuronal response of the primary visual cortex to repeated identical visual stimuli has different outcomes in terms of the amplitude of [Lac] changes (which are reduced over time) and BOLD effect (which does not exhibit any modulation in amplitude). Based on the premises that [Lac] increases originate from energy needs linked to neuronal signaling and that BOLD reflects mainly perisynaptic activity, our findings imply a differential adaptation of the cortical output that is not reflected at the level of the global excitation-inhibition activity. Further investigations are nonetheless needed to corroborate this interpretation and to elucidate the specific mechanisms that generate such an effect in the cortical canonical circuits.




