Neuronal PAS domain protein 1 regulates tyrosine hydroxylase level in dopaminergic neurons
Abstract
Catecholamines (dopamine, norepinephrine, and epinephrine) are all synthesized from a common pathway in which tyrosine hydroxylase (TH) is the rate-limiting enzyme. Dopamine is the main neurotransmitter present in dopaminergic neurons of the ventral midbrain, where dysfunction of these neurons can lead to Parkinson's disease and schizophrenia. Neuronal PAS domain protein 1 (NPAS1) was identified as one of the genes up-regulated during dopaminergic MN9D cell differentiation. We found that there was a corresponding decrease in TH level during MN9D differentiation. Overexpression and siRNA experiments revealed that NPAS1, in concert with ARNT, negatively regulates the expression of TH and that this regulation is mediated by a direct binding of NPAS1 on the TH promoter. Expression studies also confirmed a decrease in TH level in the ventral midbrain during mouse development, concomitant with an increase in NPAS1 level. These results suggest that NPAS1 plays a novel and important role in regulating TH level of dopaminergic neurons in the ventral midbrain during development. © 2007 Wiley-Liss, Inc.
Neuronal PAS domain protein 1 (NPAS1) belongs to a family of basic helix-loop-helix (bHLH) PAS (Per, ARNT, Sim) transcription factors that play important roles in several physiological functions that include xenobiotic metabolism [the dioxin or Ah receptor (AhR); Whitlock, 1999], maintenance of circadian rhythms (Clock; King et al., 1997), hypoxic responses such as angiogenesis and erythropoiesis (HIF-α and HLF; Hochachka et al., 1996; Guillemin and Krasnow, 1997; Wenger and Gassmann, 1997), neurogenesis (Single-minded; Thomas et al., 1988; Nambu et al., 1996), and embryonic tubulogenesis (Trachealess; Isaac and Andrew, 1996). More recently, NPAS3 has been shown to play a role in fibroblast growth factor (FGF)-mediated hippocampal neurogenesis (Pieper et al., 2005). Each member of this family consists of an N-terminal basic region that binds DNA, the HLH and PAS domains for dimerization and specificity of interacting partner, and a highly divergent C-terminal region (Reisz-Porszasz et al., 1994; Jiang et al., 1996). Many of these proteins form heterodimer with another bHLH-PAS domain protein, the AhR nuclear translocator (ARNT; Hoffman et al., 1991), which is ubiquitously expressed. The basic region of each subunit then contacts a half-site of the asymmetric E-box (Mason et al., 1994; Fukunaga and Hankinson, 1996) to regulate transcription (Whitelaw et al., 1993; Salceda et al., 1996; Swanson, 2002).
Tyrosine hydroxylase (TH; EC 1.14.16.2) is an enzyme that catalyzes the first and rate-limiting step in the biosynthesis of catecholamines, which include dopamine, epinephrine, and norepinephrine (Levitt et al., 1965). Dysfunctioning of dopaminergic neurons is hypothesized to be critically involved in the etiology and/or pathophysiology of Parkinson's disease (PD) and schizophrenia. In PD, the degeneration of dopaminergic neurons in the substantia nigra pars compacta results in a decreased level of dopamine, which leads to PD. Because of its crucial role in dopamine production, many studies have focused on getting stem cells to express TH or trying to increase the TH expression in progenitor neuronal cells for PD treatment. Transcription factors known to regulate TH expression include CREB (Ghee et al., 1998; Piech-Dumas and Tank, 1999; Lim et al., 2000), c-fos (Gizang-Ginsberg and Ziff, 1990), Nurr1 (Sakurada et al., 1999; Iwawaki et al., 2000; Kim et al., 2003), Pitx3 (Lebel et al., 2001; Maxwell et al., 2005), Egr1 and AP1 (Guo et al., 1998; Papanikolaou and Sabban, 2000; Nakashima et al., 2003; Maharjan et al., 2005), AP2 (Kim et al., 2001), Sry (Milsted et al., 2004), Sp1 (Papanikolaou and Sabban, 1999), and HIF1 (Schnell et al., 2003).
During mammalian development, most of the dopaminergic neurons are found in the ventral midbrain (VM). Different groups have shown that, during prenatal development in the VM, TH level increases and then decreases. This is followed by an increase during postnatal life, suggesting that there is a dynamic change in TH level during prenatal development, the cause and significance of which are not known (Solberg et al., 1993; Matsushita et al., 2002).
Previously, we have identified neuronal NPAS1 as a gene up-regulated in differentiating dopaminergic MN9D. We also found that there is a decrease in TH level during MN9D differentiation. Because of the high degree of similarity between NPAS1 and HIF, we hypothesized that NPAS1 is able to regulate TH expression, which is known to be a target gene of HIF (Schnell et al., 2003). From overexpression and siRNA experiments, our results show that NPAS1 actually negatively regulates TH expression. This regulation is mediated by a direct repressive effect on the TH promoter. To correlate this function of NPAS1 with a physiological role during development, we investigated the expression profile of NPAS1 in mouse embryos. Interestingly, we found that NPAS1 level in the VM increases, with a concomitant decrease of TH level. Thus NPAS1 may play an important role in modulating TH level in VM dopaminergic neurons during development.
MATERIALS AND METHODS
Cell Culture
The MN9D cell (a kind gift from Dr. Alfred Heller) is a mouse dopaminergic neuronal cell line derived from the fusion of rostral mesencephalic neurons from embryonic C57BL/6J mice with the N18TG2 neuroblastoma cells (Choi et al., 1991). MN9D was deemed suitable in this study because it is dopaminergic and neuronal in nature (Chee et al., 2005). The cells were grown as previously reported (Teh et al., 2006; Zhou et al., 2006). After treatment with 1 mM n-butyrate (Sigma, St. Louis, MO) to induce differentiation, MN9D cells were split after every 3 days over a period of 12 days.
Immunocytochemistry
Cells were fixed in ice-cold 3% paraformaldehyde in PBS for 30 min, followed by permeabilization with 2% Triton X-100 in PBS. The cells were then blocked in 5% goat serum in PBS for 1 hr, followed by incubation with GAP43 (Chemicon, Temecula, CA), NPAS1 (Biogenes, Germany), TH (Immunostar, Hudson, WI) or FLAG (Sigma) antibodies in dilution buffer (2% goat serum in PBS) for 1 hr at room temperature. After this, three washes were carried out before incubating the cells with rhodamine-coupled goat anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) or FITC-coupled goat anti-mouse IgG (Sigma) in dilution buffer for 1 hr at room temperature. The cells were then washed thrice and stained with Hoechst stain for 5 min before the images were acquired.
Flow Cytometry
Cells were plated at a density of 105–106 on 100- × 20-mm polystyrene-treated Corning plates and differentiated with n-butyric acid as mentioned. The distribution of the cells in G1, S, and G2/M cell cycle phases was determined by DNA flow cytometry. After 48 hr posttreatment, cells were harvested, washed in PBS, fixed in 0.5% paraformaldehyde for 5 min, rewashed in PBS, resuspended in 70% ice-cold ethanol, and stored at −20°C. Cells were stained with propidium iodide (50 μg/ml) in the presence of RNase A (50 mg/ml). Flow cytometric analysis was carried out on 20,000 cells using an EPICS Elite ESP cytometer (Beckman Coulter).
Construction of Plasmids
All plasmids used have been described previously (Teh et al., 2006).
Transfection of Cells
For transient transfection in overexpression and dual-luciferase experiments, cells were grown to the appropriate confluence and transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as recommended by the manufacturer. After 48 hr, the cells were collected and processed for the experiments.
The siRNAs were transfected into cells according to instructions given by the manufacturer (Qiagen, Valencia, CA). For each well, 0.6 μg of siRNA was used. The ratio of amount of siRNA used to RNAiFect transfection reagent is 1:9. First, the siRNA stock was diluted with buffer EC-R and mixed by vortexing. Next, RNAiFect transfection reagent was added and mixed by pipetting up and down five times. The mix was then incubated for 10–15 min at room temperature to allow for complex formation before adding to the MN9D cells.
Dissection of Animals
Pregnant Swiss albino mice were sacrificed by cervical dislocation and the embryos were dissected out in ice-cold PBS. The midbrains of the embryos were then dissected under the microscope and subsequently separated into the dorsal and ventral halves. The dissected tissues were collected either in RLT buffer for RNA isolation or cell lysis buffer for protein isolation.
Construction of cDNAs for Real-Time PCR
RNA was isolated using the RNAeasy Mini kit (Qiagen). Random priming was carried out by adding 0.5 μl of random primers and incubating at 65°C for 10 min and then placing on ice. For reverse transcription, the random-primed RNA was split into two tubes: RT+ and RT− (6 μl each), and 6 μl of deionized water, 4 μl of 5× first strand buffer, 2 μl of 0.1 M DTT, and 1 μl dNTPs were added to each tube. The tubes were then incubated at 25°C for 10 min, followed by 42°C for 2 min, after which 1 μl of reverse transcriptase was added to the RT+ tube. The tubes were then incubated at 42°C for 50 min and 70°C for 10 min and stored at −20°C.
Real-Time and Reverse Transcription PCR
The SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) was used. Standards were undiluted cDNAs synthesized from total RNA of untreated MN9D cells. The PCR started with 50°C for 2 min and 95°C for 10 min and then continued with 40 cycles of 15 sec at 95°C and 1 min at 60°C on the ABI Prism detection system (Applied Biosystems). A melting curve was obtained for each PCR product after each run to confirm that the signal corresponded to a unique amplicon of the predicted size. The specificity of the PCR product was verified by sequencing. Expression levels were obtained by subtracting the value for each sample in the absence of reverse transcriptase from the corresponding value in the presence of reverse transcriptase and then normalizing to the housekeeping gene encoding HPRT, obtained for every sample in parallel assays in two to four independent experiments.
For reverse transcription PCR, the Access RT-PCR system (Promega, Madison, WI) was used. The primers used for NPAS1 gene were 5′-ACCTAGGTGGACACATCCTACAGT-3′ and 5′-CAGGTAGATGGACACTGTCTCTGA-3′, for TH gene were 5′-AGTACTTTGTGCGCTTCGAGGTG-3′ and 5′-CTTGGGAACCAGGGAACCTTG-3′, and for HPRT gene were 5′-GAATCTGCAAATACGAGGAGTCCT-3′ and 5′-CTTTACTAGGCAGATGGCCACA-3′ respectively.
Dual Luciferase Assay
The pTH_Luc plasmid was a kind gift from Prof. Gerald Thiel (Department of Medical Biochemistry and Molecular Biology, University of Saarland Medical Center, Homburg, Germany). The dual Luciferase assay was carried out using the Promega kit (USA).
Isolation of Proteins and Western Blot Analysis
Control and treated cells were washed twice with PBS and lysed with the lysis buffer (100 mM HEPES, 150 mM NaCl, 5 mM MgCl2, 1 mM EDTA, and 1% Triton X-100). Total cell lysates were collected and incubated overnight at −20°C. The extracted protein was separated by SDS-PAGE and transferred onto a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). Membranes were probed with the respective primary antibodies (Santa Cruz Biotechnology) and the corresponding HRP-conjugated secondary antibodies (Santa Cruz Biotechnology). Proteins were visualized with an Enhanced Chemiluminescence detection kit (Pierce, Rockford, IL).
Chromatin Immunoprecipitation
Chromatin immunoprecipitation was carried using the EZ ChIP chromatin immunoprecipitation kit (Upstate, Lake Placid, NY) according to the manufacturer's protocol. Chromatin from 6-day-differentiated MN9D cells was used. One microliter of preimmune serum or anti-NPAS1 antibody was used as a negative control or the experiment, respectively. The primer sequences used were for mouse TH promoter (5′-ctggtttgattagagagctctaga-3′ and 5′-ttaaaggccaggctgacgtcaaag-3′) and mouse Nkx2.2 (5′-atgtcgctgaccaacacaaa-3′ and 5′-gggag tattggaggccctcg-3′).
RESULTS
n-Butyric Acid Induces Differentiationof MN9D Cells
MN9D cells exhibit an immature dopaminergic neuronal phenotype (Choi et al., 1991; Wallen et al., 1999). To study the development of these immature neurons, it is crucial that we have an efficient way to differentiate them into mature neurons. We found that, upon treatment with 1 mM n-butyric acid, they exhibited a mature neuronal morphology, characterized by a flattened cell body and the extension of neurites (Fig. 1A). Furthermore, these treated cells expressed a mature neuronal marker, growth-associated protein (GAP43), which was undetectable in the nontreated MN9D cells (Fig. 1B). The cells were found to be arrested in G1 stage (Fig. 1C). Significantly, the level of TH was found to decrease over the course of MN9D differentiation (Fig. 1D).
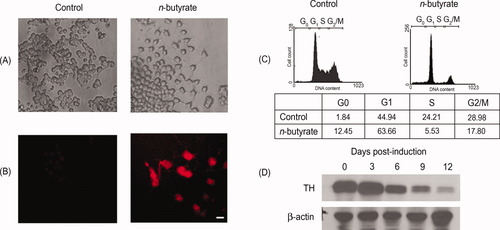
n-Butyrate induces differentiation in MN9D cells. A: MN9D cells were treated with 1 mM n-butyrate. Morphological changes can be observed 6 days after treatment. Untreated MN9D cells were round and only occasionally extended short neurites, whereas n-butyrate-treated cells had flattened cell bodies and extension of longer neurites. B:n-Butyrate-treated cells expressed the neuronal marker, GAP43. Immunostaining showed that n-butyrate treated MN9D cells expressed GAP43, which was not expressed in the control cells. C: MN9D cells were arrested in G1/G2 phase upon treatment with n-butyrate. Cells were stained with propidium iodide and analyzed by FACS. The percentage of cells in G1/G2 phase of the cell cycle is shown. D: Tyrosine hydroxylase level decreased upon n-butyrate treatment. Western blot was carried out with total cell lysates isolated from undifferentiated, first, second, third, and fourth split MN9D cells for TH. β-Actin was used as a loading control. Scale bar = 10 μm.
Neuronal PAS Domain Protein 1 Is Up-Regulated During MN9D Differentiation
Previously, we have constructed a library of genes up-regulated upon differentiation of the MN9D treated with 1 mM n-butyric acid (results not shown). One of the genes identified was NPAS1 (with an up-regulation of approximately twofold on a cDNA chip), a transcription factor reported to be expressed exclusively in the mouse adult central nervous system (Zhou et al., 1997). In this study, quantitative real-time PCR was carried out to trace the expression profile of NPAS1 during the course of MN9D differentiation. The transcript level of NPAS1 increased to at least twofold as early as 1 hr postinduction (see Fig. 3A). The transcript level continued to increase steadily until 1 day posttreatment, when it reached 15-fold. This large increase in NPAS1 transcript level indicates that it may be playing a crucial role during dopaminergic neuronal differentiation.
Because there is no commercial NPAS1 antibody available, we decided to generate our own mNPAS1 antibody. A hydrophilic region within the C-terminus of NPAS1 (QAVPADQDKDKDPQ), which is not found in the closely related mNPAS3 protein, was chosen for peptide synthesis, and subsequently this peptide was used to generate NPAS1 antibody. The specificity of the antibody was tested in a Western blot analysis of HEK293 cells expressing FLAG-tagged mouse NPAS1. HEK293 cells were used instead of MN9D cells because the recombinant NPAS1 protein was found to be expressed better in HEK293 compared with MN9D cells (results not shown). A single band of about 60 kDa, the expected size of the recombinant NPAS1 protein, was observed (Fig. 2Bi, lane 2). On the other hand, no band was observed in cell lysate of HEK293 cells expressing FLAG-tagged ARNT (Fig. 2Bi, lane 1), indicating the specificity of the antibody.
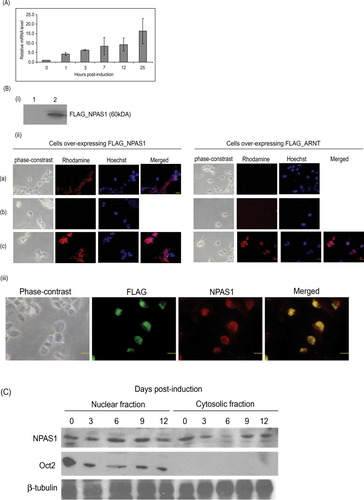
Changes in NPAS1 level during MN9D differentiation. A: Real-time PCR was carried out for NPAS1 with cDNAs from undifferentiated MN9D cells and 1, 3, 7, 12, and 25 hr after treated with 1 mM n-butyrate. NPAS1 mRNA level increased to fivefold as early as 3 hr after treatment and reached 15-fold after 1 day. HPRT gene was used for normalization. The average and SE values are calculated from the results of three independent experiments. B: NPAS1 antibody can recognize both denatured and native NPAS1 protein. i: Western blot was carried out with protein lysates from HEK293 cells overexpressing either FLAG_ARNT (lane 1) or FLAG_NPAS1 (lane 2) fusion proteins. A specific band of about 60 kDa was observed in lane 2, which was absent in lane 1. ii: Immuocytochemistry was performed on HEK293 cells overexpressing either FLAG_NPAS1 or FLAG_ARNT using either NPAS1 antibody (a) or FLAG antibody (c). As a negative control, no primary antibody was used (b). Specific binding was detected with rhodamine-conjugated secondary antibody. In cells overexpressing FLAG_NPAS1, the cytoplasm is stained, but not the nucleus, and the same staining pattern was observed when FLAG antibody was used. In cells overexpressing FLAG_ARNT, no staining was observed when NPAS1 antibody was used, but the nuclei were stained when FLAG antibody was used. In the absence of primary antibody, no staining was observed. iii: Overlap of FLAG and NPAS1 antibodies in HEK293 cells overexpressing FLAG-tagged NPAS1 protein. Cells were stained with FLAG antibody that is directly conjugated to FITC, and NPAS1 antibody as described for ii. There is an exact overlap of the two staining. C: There was increased NPAS1 level in the nucleus during MN9D differentiation. Western blot for NPAS1 was carried out on nuclear and cytosolic fractions from 0, 3, 6, 9, and 12 days posttreatment MN9D cells. There is a gradual increase in NPAS1 level in the nuclear fraction from 0 to 9 days, followed by an increase on 12 days. In the cytosol, NPAS1 level decreased from 0 to 9 days with an increase on day 12. Western blots for Oct2 and β-tubulin were carried out to show no contamination of the fractions and equal loading, respectively. Scale bars = 10 μm.
We went on to determine whether the antibody is able to recognize native NPAS1. For this purpose, immunocytochemistry was carried out in HEK293 cells overexpressing either FLAG-tagged NPAS1 or FLAG-tagged ARNT (Fig. 2Bii). As a positive control, the cells were also stained with FLAG antibody to ensure that the FLAG_NPAS1 and FLAG_ARNT fusion proteins were properly expressed. When stained with NPAS1 antibody, a positive signal was observed in the cytoplasm but not in the nucleus (consistent with the staining pattern with FLAG antibody) in cells overexpressing FLAG_NPAS1. The nuclear exclusion of NPAS1 is consistent with our published data showing that NPAS1 actually requires ARNT for nuclear localization (Teh et al., 2006). We also performed double staining with both FLAG and NPAS1 antibody in HEK293 cells overexpressing FLAG-tagged NPAS1. The results showed an almost exact overlap for the two antibodies (Fig. 2Biii). In the case of cells overexpressing FLAG_ARNT, no signal was observed with NPAS1 antibody, confirming the specificity of the antibody to NPAS1. A positive signal was observed in the nucleus with FLAG antibody, indicating that FLAG_ARNT is localized to the nucleus (Fig. 2Bii). From these results, it is confirmed that the NPAS1 antiserum can recognize native NPAS1.
Being a transcription factor, NPAS1 has to be localized in the nucleus in order to carry out its function. We found that there is a gradual increase in NPAS1 level in the nuclear fraction from 0 to 9 days postinduction, followed by a decrease on 12 days after differentiation, whereas the level in the cytosolic fraction showed a reverse trend (Fig. 2C). This indicates that there is a translocation of NPAS1 into the nucleus during MN9D differentiation.
NPAS1 Repressed TH Level
NPAS1 (Ohsawa et al., 2005) and HIF1 (Semenza and Wang, 1992) have been separately reported to regulate the same gene, erythropoietin (EPO). Because HIF1 is also known to up-regulate TH expression (Schnell et al., 2003), we postulate that NPAS1 might also play a role in regulating TH expression during MN9D differentiation. We therefore tested the effect of overexpression of NPAS1 on TH transcript and protein levels in MN9D cells. In this experiment, we also cotransfected the cells with an ARNT-expressing plasmid, because it was previously shown that NPAS1 is able to interact with ARNT (Teh et al., 2006), suggesting that the two proteins have to heterodimerize for NPAS1 to bind to DNA and thereby carry out its role as a transcription factor (Ohsawa et al., 2005). As shown in Figure 3A, overexpression of NPAS1_GFP with either FLAG (lane 1) or FLAG_ARNT (lane 2) resulted in a decrease in TH transcript and protein level compared with control cells overexpressing GFP and FLAG (lane 3) or cells overexpressing GFP and FLAG_ARNT (lane 4). These indicate that NPAS1 is able to regulate the expression of TH negatively.
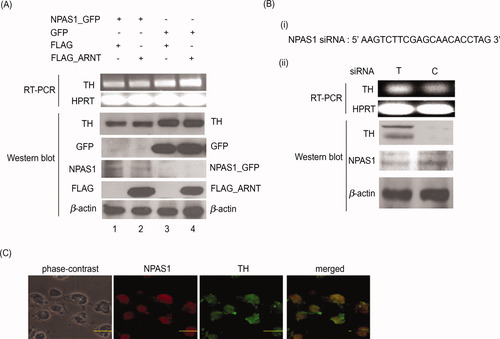
NPAS1 represses TH expression level. A: Overexpression of NPAS1 decreased TH level in MN9D cells. MN9D cells were coexpressed with either NPAS1_GFP or GFP with FLAG or FLAG_ARNT. When the cells were overexpressed with NPAS1 and FLAG (lane 1), the TH transcript and protein levels decreased compared with control cells overexpressing GFP and FLAG (lane 3). There was also a decrease in TH transcript and protein levels in cells overexpressing NPAS1 and ARNT (lane 2) compared with cells expressing GFP and ARNT (lane 4). Western blot was carried out with GFP and FLAG antibodies to show the proper expression of the fusion proteins. B: Silencing of NPAS1 increased TH level in MN9D cells. i: Region of NPAS1 mRNA targeted for siRNAs synthesis and silencing. ii: Differentiated MN9D cells were transfected with NPAS1 siRNA (lane T) or control siRNA (lane C). Upon transfection with NPAS1 siRNA, TH transcript and protein levels increased compared with cells transfected with the control siRNA. Western blot with NPAS1 confirmed a decrease in NPAS1 in cells transfected with NPAS1 siRNAs compared with control. β-Actin was used as a loading control. The results are representative of three separate experiments. C: Colocalization of NPAS1 and TH in the same cell. MN9D cells were stained with NPAS1 and TH antibodies and detected with secondary antibodies conjugated to rhodamine and FITC, respectively. Both NPAS1 and TH are expressed in all the MN9D cells. Scale bar = 20 μm.
We further confirmed the repressive effect of NPAS1 on TH expression by silencing NPAS1 expression in differentiating MN9D cells. A small interfering RNA targeting a specific region of NPAS1 mRNA was designed according to standard protocol and subsequently synthesized (Fig. 3Bi). A control siRNA, which encodes a random sequence not targeting any mammalian sequence, was used as a negative control. Upon transfection of differentiated MN9D cells with NPAS1 siRNA, the level of TH transcript and protein increased significantly compared with cells transfected with the control siRNA (Fig. 3Bii). Immunostaining of MN9D cells showed that NPAS1 and TH are expressed in the same cells (Fig. 3C), suggesting that the repressive effect of NPAS1 on TH expression might be a direct one.
NPAS1 Binds to the Promoter Region of TH and Represses Its Expression
There are three hypoxia response elements (HREs) found on the mouse TH promoter within the first 250 bp upstream of the transcription start site (Fig. 4A). It is possible that NPAS1 may bind to these HREs to inhibit transcription of TH gene. We first tested whether the repressive effect of NPAS1 on TH expression is due to negative transcriptional regulation of NPAS1 on TH promoter. It is well established that the N-terminus domain of bHLH-PAS protein, which consists of the bHLH and PAS region, is responsible for binding to DNA for transcriptional regulation. To determine whether this is also the case for NPAS1, a plasmid containing the TH promoter fused upstream of a luciferase reporter gene (Thiel et al., 2005) was transfected into HEK293 cells, together with plasmids overexpressing NPAS1 or deletion mutants of NPAS1 or ARNT in different combinations. Overexpression of HA_ARNT increased the luciferase level, indicating that ARNT has a positive regulatory role on TH promoter (Fig. 4B). However, upon coexpression with FLAG_NPAS1 (lane 4) or FLAG_NPAS1(N; lane 6), the luciferase level decreased drastically, suggesting that NPAS1 can override the transactivation function of ARNT and has a repressive effect on the TH promoter. On the other hand, overexpression of FLAG_NPAS1(C) with HA_ARNT (lane 8) did not decrease the luciferase level, indicating that the N-terminus of NPAS1 is crucial for mediating the repressive effect.
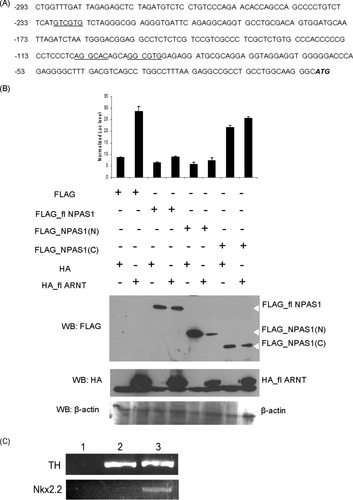
NPAS1 has a repressive effect on TH promoter by directly binding to it. A: The mouse TH promoter contains three hypoxia response elements (underscored) within the first 250 bp upstream of the translation start site. B: HEK293 cells were cotransfected with plasmids expressing FLAG, FLAG_NPAS1, FLAG_NPAS1(N), or FLAG_NPAS1(C) with plasmids overexpressing either HA or HA_ARNT in different combinations as indicated on the X-axis, together with the pTH_Luc reporter plasmid and pSV40_Luc internal control plasmid. Upon overexpression of ARNT, the reporter luciferase level increased drastically. When NPAS1 or NPAS1(N) was coexpressed with ARNT, the level of reporter luciferase decreased to control level. Overexpression of fl NPAS1 or NPAS1(N) with HA has no effect on the reporter level. NPAS1(C) has a positive effect on the TH promoter, but it did not repress the transactivation effect of ARNT. The results are from one representative experiment (n = 3) and are represented as mean and SEM (n = 3). Western blot was carried out to show overexpression of the different fusion proteins. β-Actin was included as a loading control. C: Chromatin from 6-day-differentiated MN9D cells was immunoprecipitated with the anti-NPAS1 antibody (lane 2) and NPAS1-binding DNA was examined by 39 cycles of PCR. Preimmune serum was used was negative control (lane 1). DNA extracted from 6-day-differentiated cells was used as positive control (lane 3). TH promoter was detected in anti-NPAS1 antibody immunoprecipitated DNA and positive control but not in preimmune serum precipitated sample. The region of an unrelated Nkx2.2 gene exon was also used as a negative control.
Because ARNT is able to activate the TH promoter and overexpression of NPAS1 in the presence of ARNT represses this effect (Fig. 4B), it is possible that the effect of NPAS1 on TH promoter reporter is just an antagonistic effect to ARNT, or the effect of NPAS1 is dependent on only nuclear localization of NPAS1 (which is dependent on ARNT) and not NPAS1 binding directly to TH promoter. Alternatively, the effect of NPAS1 could be a result of its translocation into the nucleus (aided by ARNT) and subsequent binding to the TH promoter as a NPAS1_ARNT heterodimer. Previously, our group and others have shown that NPAS1 forms a heterodimer with ARNT both in vitro and in vivo (Ohsawa et al., 2005; Teh et al., 2006). To determine the mechanism by which NPAS1 regulates TH promoter, we also carried out a chromatin immunoprecipitation (ChIP) assay on 6-day-differentiated MN9D cells. Chromatin was immunoprecipitated by anti-NPAS1 antibody, and the precipitated chromatin was tested for the presence of TH promoter region. The TH promoter region was detected by anti-NPAS1 antibody but not by the preimmune serum (Fig. 4C). The region of an unrelated Nkx2.2 gene exon, which was shown not to be bound by NPAS1 (Ohsawa et al., 2005), was not detected by this ChIP analysis. These findings indicate that NPAS1 binds to the TH promoter region in 6-day-differentiated MN9D. NPAS1 is thus likely to repress TH expression through direct binding to the TH promoter region.
Developmental Expression of NPAS1 in the Mouse
It has been reported that NPAS1 is expressed exclusively in the central nervous system on Northern blot analysis (Zhou et al., 1997). Subsequently, NPAS1 was found to be expressed in neurons of E16.5 mouse embryo (Ohsawa et al., 2005). We are interested in the role of NPAS1 during development, so the expression profile of NPAS1 in the mouse was determined. Western blot was carried out with protein lysates from different regions of E12.5, E14.5, E16.5, and E18.5 embryos. As shown in Figure 5, during embryonic development, NPAS1 was found to be expressed in all the tissues tested, which include the forebrain, midbrain, hindbrain, spinal cord, tail, liver, and limb. However, in the adult stage, NPAS1 expression is restricted to the central nervous system and liver. This expression pattern is contrary to a previous report using Northern blotting in which NPAS1 was not found to be expressed in the liver (Zhou et al., 1997). This discrepancy suggests that NPAS1 protein is relatively stable and remains abundant in the liver, despite reduced mRNA levels. Overall, this expression profile of NPAS1 suggests that it may be playing a wide role during embryonic development.
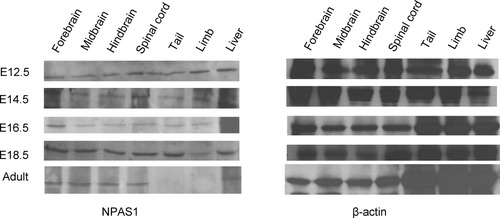
NPAS1 is expressed in many regions of the mouse embryo but has restricted expression in the adult stage. Proteins were isolated from different regions—forebrain, midbrain, hindbrain, spinal cord, tail, limb, and liver—at different embryonic (E12.5, 14.5, 16.5, and 18.5) stages as well as the adult stage. Western blotting was then carried out with the anti-NPAS1 antibody. NPAS1 was found to be expressed in the all the regions tested at all developmental stages. In the adult stage, NPAS1 expression is restricted to the central nervous system and liver. β-Actin was used as a loading control.
Developmental Changes of TH and NPAS1 Levels in the Midbrain
To determine whether NPAS1 plays a physiological role during the development of dopaminergic neuron in the midbrain, quantitative real-time PCR was carried out using cDNAs from both the dorsal midbrain (DM) and the ventral midbrain (VM), respectively, of mouse embryos at different stages (Fig. 6Ai). NPAS1 transcript level showed a single peak at E15.5 in the DM. In contrast, in the VM, NPAS1 expression began as early as E11.5 and increased steadily thereafter, up until E18.5. Western blot for NPAS1 was also carried out with protein lysates from the DM and VM at different prenatal stages (E12.5, 14.5, 16.5, and 18.5). As shown in Figure 6Aii, NPAS1 was detected in the DM only at E18.5. On the other hand, NPAS1 was detected in the VM as early as E14.5. Such expression pattern suggests that NPAS1 may play an important role during development of the VM.
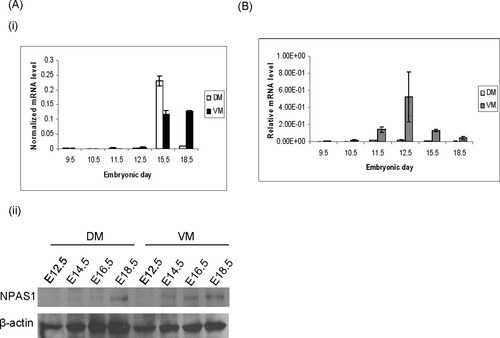
Changes in NPAS1 and TH expression in the midbrain during prenatal stages. A: Expression of NPAS1 transcript and protein in the DM and VM at different embryonic stages. i: Real-time PCR was carried out for NPAS1 with cDNAs isolated from the DM and VM at different embryonic stages (E9.5, 10.5, 11.5, 12.5, 15.5, and 18.5). In the VM, NPAS1 was first detected at E11.5 and increased steadily throughout. In the DM the NPAS1 transcript level showed a single peak at E15.5. HPRT was used for normalization. ii: Western blot was carried out for NPAS1 with protein lysates isolated from the DM and VM at different embryonic stages (E12.5, 14.5, 16.5, and 18.5). NPAS1 was observed only at E18.5 in the DM. There was a steady increase in NPAS1 protein level from E14.5 onward in the VM. β-Actin was used as loading control. B: TH level decreases during late prenatal stages in the ventral midbrain. Real-time PCR was carried out for TH with cDNAs from the DM and VM of different embryonic stages. TH transcript level peaked at E12.5 and began to decrease thereafter. The level of TH transcript was normalized to HPRT level. Results shown are the average and SEM of three independent experiments.
We have shown that NPAS1 is expressed in the VM and it down-regulates TH expression, so we further traced the changes of TH level during VM development in the mouse. Real-time PCR was carried out with cDNAs from the DM and VM from different embryonic stages: E9.5, 10.5, 11.5, 12.5, 15.5, and 18.5. TH was found to be expressed at about E10.5 in the VM (Fig. 6B). The transcript level then increased steadily until E12.5, after which it started to decrease from E15.5 onward until E18.5. This indicates that there is a dynamic change in TH transcript level in the mouse VM during prenatal development. On the contrary, TH was expressed at very low level in the DM, which is not known to have any dopaminergic neurons. In summary, there is an increase of NPAS1 in the VM during development, accompanied by a corresponding decrease in TH level.
DISCUSSION
Our study revealed that there is a decrease in TH level during the MN9D differentiation process. A previous study also reported a decrease in dopamine content in MN9D upon treatment with another differentiating agent, retinoic acid (Hermanson et al., 2003). This suggests that the decrease in TH and subsequently dopamine level during DA neuron differentiation may be a de novo phenomenon regardless of the inducer used. In our study, we showed that TH level does not increase continuously throughout development in the VM; although there is an increase in TH level in the midprenatal stages, its level decreases in the later stages. It may be argued that the decrease could be due to the migration of TH-positive neurons away from the VM at later stages (Kawano et al., 1995). However, there are different lines of evidence to suggest that the dynamic change in TH level is bone fide during development in the VM.
Previous investigators have shown that there is a decrease in TH level in the rat VM from E15.5 onward (Solberg et al., 1993). Moreover, results from transgenic mice that carried green fluorescent protein (GFP) gene under the control of a 9-kb rat TH promoter have shown that both the typical expression frequency and the relative fluorescence intensity of GFP in the VM decrease during late prenatal stages (at E12.5 and E14.5, respectively) and increase again after postnatal day 7 (Matsushita et al., 2002). These results are consistent with our real-time PCR result for TH, which shows a decrease in the later prenatal stages. Taken together, our results combined with published data showed that the MN9D cell model is suitable for the study of TH regulation during VM dopaminergic neurons differentiation, because the decrease in TH during its differentiation mirrors what is happening in vivo.
In this study, we have shown that NPAS1 down-regulates the expression of TH in the MN9D and that this down-regulation is due to a direct effect on the TH promoter, where the N-terminus (which contains the DNA binding domain) of NPAS1 is required for a repressive effect. The result suggests that the N-terminus of NPAS1 is sufficient to repress transcription, which is in agreement with our published data showing the presence of an autonomous repression domain within the N-terminus of NPAS1 (Teh et al., 2006).
Midbrain DA neurons first appear in the intermediate zone of the developing neural tube and then migrate first ventrally and then laterally to form the SNc and VTA (Shults et al., 1990; Kawano et al., 1995). In mice, DA neurons appear in the ventral midbrain between E11 and E13 and then migrate ventrolaterally and rostrally during subsequent embryonic development (Kawano et al., 1995). The onset of expression of NPAS1 in the ventral midbrain (from E12.5 onward) coincides very well with the time when TH starts to decrease (after E12.5). The fact that NPAS1 transcript level increases in the VM during a time of active dopaminergic neuron development and the inverse relationship between its expression with TH level (Fig. 6), plus our in vitro results showing that NPAS1 is able to repress TH expression (Figs. 3, 4), provide us with very strong evidence that NPAS1 actually regulates TH expression in dopaminergic neurons during development in the VM. This time window also correlates with the stages during which midbrain DA neurons undergo migration. The role of NPAS1 in cell migration is further supported in a study by Levesque et al. (2007) showing that down-regulation on NPAS1 resulted in the down-regulation of genes involved in cell migration. The only missing evidence here is the colocalization of NPAS1 and TH in the same cell of the VM. We have actually performed this immunohistochemical staining but could not show such colocalization. Our failure to show colocalization might be due to technical difficulties with immunostaining resulting from the fact that the level of NPAS1 protein is really very low in the VM, making it very hard to detect; and also because the level of NPAS1 and TH is inversely proportional, making it very tricky to catch them at a stage when both can be detected in the same cell. However, we did show that NPAS1 and TH are expressed in the same MN9D cells (Fig. 3C), providing strong evidence that this could also be the case in the VM.
More recently, NPAS3, a gene highly similar to NPAS1 was postulated to be involved in schizophrenia-like behavior (Erbel-Sieler et al., 2004). It was found that NPAS3 regulates hippocampal neurogenesis (Pieper et al., 2005). The involvement of NPAS3 in schizophrenia is supported by later findings of decreased hippocampal neurogenesis in post-mortem human brain tissue from patients with schizophrenia (Reif et al., 2006). The symptoms of schizophrenia have also been linked to an increased forebrain DA transmission (Sesack and Carr, 2002). One reason for this hyperactivation of the forebrain may be an increase in DA content. NPAS3 and NPAS1 are highly similar transcription factors that might have similar targets, and we have shown that NPAS1 negatively regulates TH, the rate-limiting enzyme for the synthesis of dopamine, so our findings here suggest that NPAS3 could also be involved in schizophrenia by means of regulating forebrain DA transmission.
Acknowledgements
The authors thank Dr. Jacques Michaud (Research Center, Hôpital Sainte-Justine, Canada) and Dr. Chen-Ming Fan (Department of Embryology, Carnegie Institution of Washington) for providing us with the relevant plasmids. We also thank Dr. Alfred Heller (University of Chicago) for the use of MN9D cells.




