Neuronal precursors within the adult rat subventricular zone differentiate into dopaminergic neurons after substantia nigra lesion and chromaffin cell transplant
Abstract
Neurogenesis in the adult mammalian brain continues in the subventricular zone (SVZ). Neuronal precursors from the SVZ migrate along the rostral migratory stream to replace olfactory bulb interneurons. After the destruction of the nigro-striatal pathway (SN-lesion), some SVZ precursors begin to express tyrosine hydroxylase (TH) and neuronal markers (NeuN). Grafting of chromaffin cells (CCs) into the denervated striatum increases the number of TH+ cells (SVZ TH+ cells; Arias-Carrión et al., 2004). This study examines the functional properties of these newly differentiating TH+ cells. Under whole-cell patch-clamp, most SVZ cells recorded from lesioned and grafted animals (either TH+ or TH−) were non-excitable. Nevertheless, a small percentage of SVZ TH+ cells had the electrophysiologic phenotype of mature dopaminergic neurons and showed spontaneous postsynaptic potentials. Dopamine (DA) release was measured in SVZ and striatum from both control and SN-lesioned rats. As expected, 12 weeks after SN lesion, DA release decreased drastically. Nevertheless, 8 weeks after CCs graft, release from the SVZ of SN-lesioned rats recovered, and even surpassed that from control SVZ, suggesting that newly formed SVZ TH+ cells release DA. This study shows for the first time that in response to SN-lesions and CC grafts neural precursors within the SVZ change their developmental program, by not only expressing TH, but more importantly by acquiring excitable properties of mature dopaminergic neurons. Additionally, the release of DA in a Ca2+-dependent manner and the attraction of synaptic afferents from neighboring neuronal networks gives further significance to the overall findings, whose potential importance is discussed. © 2006 Wiley-Liss, Inc.
The subventricular zone (SVZ) is one of the regions where production of new neurons continues in the adult mammalian brain (Hinds, 1968; Altman, 1969; Kaplan and Hinds, 1977; Lois and Alvarez-Buylla, 1994; Lois et al., 1996; Thomas et al., 1996; Alvarez-Buylla and García-Verdugo, 2002). This region comprises stem cells and progenitors committed to a neuronal or glial lineage (Doetsch et al., 1997; Levison and Goldman 1997; Garcia-Verdugo et al., 1998; Luskin 1998). Neuronal progenitors migrate from the SVZ through the rostral migratory stream (RMS), and on reaching the olfactory bulb they originate olfactory granule and periglomerular interneurons (Doetsch et al., 1997; Luskin, 1998). Granule neurons are homogenously GABAergic, whereas 40% of the periglomerular neurons are GABAergic and, of those, 65% are also dopaminergic (Kosaka et al., 1997). Under normal conditions, neuronal progenitors in the SVZ remain as non-differentiated non-excitable cells. They only began to display properties of mature interneurons on their arrival to the olfactory bulb (Belluzzi et al., 2003).
SVZ neuronal precursors have been the subject of considerable interest as a potential source of cells capable of replacing adult neurons lost during neurodegenerative diseases (Richardson et al., 2005a, b). Neurogenesis in the SVZ is regulated physiologically (Cameron and McKay, 1999; Kirschenbaum et al., 1999), and can be modified pharmacologically (Chen et al., 2000). Neural progenitors can proliferate and differentiate in the adult brain in response to pathologic events (Jin et al., 2004) or brain injury (Szele and Chesselet, 1996; Mehler and Gokhan, 1999; Arvidsson et al., 2002).
We reported recently that unilateral 6-hydroxydopamine (6-OHDA) destruction of the substantia nigra (SN), in conjunction with subsequent striatal grafting of chromaffin cells (CCs), induces the appearance of numerous TH-immunoreactive cells (SVZ TH+ cells) in the ependymal and subependymal regions of the lateral ventricles (Arias-Carrión et al., 2004). Because TH+ cells are completely absent in the lateral ventricles of intact animals, this finding suggests that lesion and grafting induce cell differentiation. Accordingly, the aim of this study was to determine the functional properties of these newly appearing SVZ TH+ cells.
This study confirms that SN lesion and subsequent CCs transplant induces neural precursors from the SVZ to change their developmental program, and differentiate into TH+ cells. Furthermore, our data suggests that these newly appearing TH+ cells are capable of releasing dopamine (DA) in a Ca2+-dependent manner, and that some of them develop electrophysiologic properties largely indistinguishable from those of substantia nigra pars compacta dopaminergic neurons.
MATERIALS AND METHODS
6-OHDA Nigro-Striatal Lesion and Behavioral Tests
Studies were conducted in accordance with procedures approved by the Committee of Bioethics and Care of Experimental Animals of The National University of México, and the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH, 1985). Under ketamine-xylazine anesthesia (87 and 13 mg/kg, respectively) male Wistar rats, weighing 180–200 g were injected stereotaxically with 8 μg of 6-OHDA (Sigma, St. Louis, MO) stabilized with 0.2 μg/μl of L-ascorbate into the left substantia nigra (SN; 4.80 mm caudal, 1.6 mm lateral to bregma, 8.2 mm ventral to the skull surface (Dunnett et al., 1981). Coordinates were set according to the atlas of Paxinos and Watson. Lesioned animals were selected based on their rotational behavior in response to amphetamine (4 mg/kg i.p). One month after lesion, all animals were placed (randomized) into automated rotometer bowls, and left and right full-body turns were monitored by a home-made computerized activity monitor system. Animals showing >500 turns ipsilateral toward the lesioned side after a single dose of amphetamine were considered having >97% striatal dopaminergic lesion (Ungerstedt and Arbuthnott, 1970; Dunnett et al., 1981).
Transplant of Chromaffin Cells
Chromaffin cells (CCs) were obtained as described previously (Arias-Carrión et al., 2004). Briefly, newborn 3–5 day-old rats (∼200 pups), were sacrificed by ice exposure, and the adrenal medulla was dissected under a stereomicroscope. Tissue fragments were placed in Ca2+-free Spinner's saline solution (Sigma) supplemented with (1 mg/ml) bovine serum albumin (BSA; Sigma). Fragments were then digested in Spinner's saline solution supplemented with 2 mg/ml of collagenase (Worthington, Type I) and 1.5 mg/ml of deoxyribonuclease Type II (Sigma) for 45 min at 37°C. Freshly dissociated CCs were suspended in culture medium (Dulbecco's modified Eagle medium: DMEM; GIBCO), supplemented with 4.5 μg insulin (Sigma), 100 U/ml penicillin (Sigma), 100 mg/ml streptomycin (Sigma) and 2.5 mg/ml fungizone (Gibco), and kept in a tissue culture incubator until the transplant, usually within <4 hr. Transplantation of CCs took place 1 week after the pre-test behavioral evaluation (i.e., 5 weeks after SN-lesion). Sixty-four lesioned rats were divided into two groups: Group 1: SN-lesioned with no transplant, n = 30, Group 2: SN-lesioned with CCs transplant, n = 34. Group 1 included animals receiving the following treatments: 1) lesion only (n = 14); 2) introduction of the 27-gauge needle into the striatum but with no injection (n = 4); 3) injection of 4 μl of sterile saline (n = 4); 4) injection of 4 μl of sterile culture medium (n = 4); and 5) injection of 4 μl of culture medium containing dead chromaffin cells (sonication for 1 min; n = 4). None of these procedures increased the formation of SVZ-TH+ cells significantly above that of lesion alone (data not shown). Additionally, a control group was formed with 24 untreated rats.
Host 6-OHDA-lesioned animals were anesthetized with ketamine-xylazine (87 and 13 mg/kg, respectively), and placed in a Kopf stereotaxic frame (Kopf Instruments, Tujunga, CA). A burr hole was drilled in the skull and CCs were aspirated using 27-gauge needle attached to a 10 μl Hamilton syringe mounted in a manually driven micro injector (Kd Scientific, Holliston, MA). Each animal received 4 μl of DMEM, containing a suspension of 1 × 106 CCs, injected at a flow rate of 1 μl/min into the head of the caudate nucleus on the SN-lesioned side of the brain (from bregma: anterior 0.0 mm, lateral 3.0 mm, ventral 5.0 mm, incisor bar 0). This position for grafting was chosen to completely rule out the possibility of recording electrophysiologically or measuring dopamine release from grafted CCs. Brain slices used for functional experiments were obtained form a region at least 1 mm anterior to the grafting site.
TH Immunofluorescence in Brain Frozen Sections
At the end of behavioral tests (evaluation at 4 weeks after SN lesion and again 9 weeks thereafter), 24 animals (eight from each experimental group: control untreated, SN-lesioned, and SN-lesioned with CC transplant), were deeply anesthetized with Nembutal and perfused transcardially with saline followed by ice-cold 4% paraformaldehyde (PFA). Brains were then removed, post-fixed in buffered-4% PFA with 15% and 30% sucrose (each for 24 hr), and finally cut in 30 μm slices with a cryostat (Leica CM 1900). After blockade with 1.5% horse serum, sections were incubated for 24 hr in the presence of a rabbit anti-tyrosine hydroxylase antibody at 1:1,000 dilution (Chemicon), and then for 2 hr at room temperature in the presence of a fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG (Zymed) at 1:500 dilution.
Confocal Microscopy
Immunostained brain slices were examined with a MCR 1024 Bio-Rad laser scanning system equipped with a Kr/Ar laser attached to an inverted Nikon TMD 300 microscope. Images were collected with fluorescence oil-immersion objectives (45× and 60×, 1.4 NA, Plan Apo, Nikon, Japan). For imaging of FITC-immunofluorescence, samples were excited at 488 nm and emitted light was band-passed with a 522/DF32 nm filter. For imaging of TRITC-immunofluorescence, samples were excited at 568 nm and emitted light was band-passed at 605/DF32 nm. Images at 488 nm and 568 nm excitation were obtained by two separate photomultiplier channels, either concurrently or in separate runs. Analysis and processing of confocal images was carried out with software written by Todd Clark Breile (Confocal Assistant 4.02). Final image composition was made with Adobe Photoshop 7.0, Adobe Illustrator 10, and Microsoft Power Point 2003.
Electrophysiology
Preparation of brain slices
Coronal slices containing the SVZ and SN were acutely prepared as described elsewhere (Galarraga et al., 1999; Wang et al., 2003) (Fig. 6A). Briefly, 20 animals SN-lesioned with CCs transplant were anesthetized with an overdose of chloral hydrate solution and decapitated 4 weeks after transplant. The extent of nigrostriatal denervation in these rats was evaluated 4 weeks after lesion of the SN and 1 week after the last behavioral evaluation these group was transplanted with CCs. Additionally, a group of 10 control untreated rats was used in parallel.
Their brains were removed quickly and placed into ice-cold saline containing (in mM): 125 NaCl, 3 KCl, 25 NaHCO3, 1.25 Na2HPO4, 2 MgCl2, 2 CaCl2, 25 glucose, pH = 7.3 with NaOH, 300 mOsm/l; and saturated with 95% O2 and 5% CO2. Coronal slices (350 μm thick) containing either the lateral ventricles and subventricular zone (SVZ) or the substantia nigra (SN) were cut using a Vibratome 3000 (Vibratome Company, St. Louis, MO), transferred to saline and let to recover for 1 hr at room temperature (∼24°C). Individual slices were transferred to a plexiglas recording chamber and superfused with saline at a rate of 3–4 ml/min at 32°C by using a gravity-fed sewer pipe system.
Whole Cell Recordings
Recordings were carried out with the whole-cell modality of the patch-clamp technique either in current clamp or voltage clamp mode using a Multiclamp 700A amplifier and a PC running Clampex 8 software (Axon Instruments, Union City, CA). Recordings were obtained, under visual guidance, either from SVZ cells located at ≤100 μM from the ciliated epithelia of the lateral wall of the left lateral ventricle, or from cells in the SNc. Slices were viewed with a 40× water-immersion Nikon objective, in an upright microscope (Nikon Eclipse E600 FN, Japan) equipped with a CCD camera (CCD100S, DAGE.MTI, Michigan City, IN), under infrared illumination and enhanced differential interference contrast (DIC). Micropipettes were pulled from borosilicate glass (4–7 MΩ). The internal solution consisted of (in mM): 119 KMeSO4, 1 MgCl2, 10 HEPES, 0.5 EGTA, 2 Na2-ATP, 0.7 Na2GTP, biocytin 0.5%, pH = 7.3 with KOH, and 280–300 mOsm/l.
Because some SVZ cells fired action potentials on membrane depolarization, we examined their electrophysiologic characteristics using protocols suitable to obtain intensity-frequency relationships (I–F plots) of depolarization-induced action potential firing, as well as with current-voltage relationships (I–V plots) in both current- and voltage-clamp modes. For comparison, the same protocols were used on SNc neurons. The presence of spontaneous synaptic currents was also examined by recording at a holding potential of −70 mV and under voltage clamp conditions.
Dopamine Release Experiments
Coronal slices containing the SVZ or the striatum were prepared as in Wang et al. (2003). Briefly, animals were anesthetized with an overdose of chloral hydrate solution and decapitated 4 weeks after transplant (SN-lesioned with chromaffin cells transplant, n = 6) or the last behavioral evaluation (SN-lesioned, n = 6). Additionally, a group of control untreated rats was used in parallel (n = 6). The brain was quickly removed and chilled (0–4°C) in 95% O2/5% CO2 saturated saline containing (in mM): 125 NaCl, 3 KCl, 25 NaHCO3, 1.25 Na2HPO4, 2 MgCl2, 2 CaCl2, 25 glucose, pH = 7.3 with NaOH, 300 mOsm/l. Coronal slices 300 μm thick were cut with a Vibratome 3000 (Vibratome, Saint Louis, MO) in cold, oxygenated ACSF (approximately six slices were obtained per animal). After 1 hr recovery in ACSF at room temperature, slices were dissected to obtain longitudinal strips from the SVZ or the dorsal striatum and equal wet weight and 5 mm in length (Fig. 2). The six SVZ and striatal strips were transferred separately to small glass holders that allowed their rapid manipulation and exposure to different solutions in succession, for periods of 10 min to measure dopamine release as described in Galarraga et al. (1999). Tissue strips corresponding to SVZ and striatum (Fig. 2B,C) were cut and placed in two separate holders, one for each region. Plastic Eppendorf tubes were prepared as follows: Tube 1: control condition, 200 μl of normal ACSF; Tube 2, depolarizing condition, 200 μl of ACSF containing 20 mM KCl (20 mM instead of 20 mM NaCl to maintain osmolarity); Tube 3, calcium deficient condition, 200 μl of ACSF containing 20 mM KCl and no added CaCl2; Tube 4, control condition, 200 μl of normal ACSF. All solutions were continuously gassed with 95%O2 and 5% CO2, the pH was adjusted to 7.3, and the osmolarity to 290 mOsm/l (Galarraga et al., 1999).
The media remaining in tubes 1–4 after the sequential exposure of plastic holders containing strips of SVZ or striatum, were analyzed for dopamine content using HPLC-ED detection. The mobile phase was 1 L of filtered aqueous solution containing 12.9 g citric acid (0.05 M), 4.1 g sodium acetate (0.05 M) and 186 mg disodium ethylenediaminetetraacetate dehydrate (0.05 mM). The pH was adjusted to 5.2 with 1 M HClO4. Twenty microliters of each sample were injected for analysis using a Rheodyne 712S auto injector. Electrochemical detection was carried out using a BAS 4 C detector (EDC) prototype 650 mV vs. Ag/AgCl, glassy carbon electrode. A Gilson pump provided a flow rate 1 ml/min trough the column and guard columns (Spherisorb 150 mm × 4.6 mm, ODS-1 5 μm). Dopamine was identified and measured against an external reference (625 fmol of 3,4-hydroxytyramine; dopamine, Sigma). This standard was run before sample analysis. The HPLC software (Unipoint, System Software, Gilson, Inc.) automatically calculated the area under each peak. Dopamine determinations were carried out by a lab technician who was unaware of the differences between experimental groups.
Statistics
ANOVA and Kruskal-Wallis or Friedman statistics (for unpaired and paired samples, respectively) were used to compare mean concentration data of release samples (fmol/20μl) between groups and post hoc Tukey or Student-Newman-Keuls tests for pairwise comparisons. Significance was established at P < 0.05.
RESULTS
Behavioral Tests
Animals were selected on the basis of amphetamine-induced rotational behavior 4 weeks after SN-lesion. As reported previously (Arias-Carrión et al., 2004), the number of turns elicited by amphetamine increased further within the following 8 weeks. In contrast, SN-lesioned animals that received transplants of CCs, exhibited a substantial improvement of their motor asymmetry compared to their own pre-transplant counterparts, or with SN-lesioned animals that received no transplant (data not shown, but see Arias-Carrión et al., 2004).
Tyrosine Hydroxylase Immunostaining
Control experiments confirmed that the unilateral 6-hydroxy-dopamine (6-OHDA) destruction of the substantia nigra (SN), evaluated by amphetamine-induced rotational behavior, induced the appearance of TH-immunoreactive (TH+) cells in the SVZ and neighboring striatum of adult rats. TH-immunoreactive cells (SVZ TH+ cells) were more abundant in the lateral ventricles of animals with SN lesion and subsequent graft of CCs. These SVZ TH+ cells were completely absent in the ventricles of control, non-treated animals (data not shown). It has been shown previously that SVZ TH+ cells do not correspond to CCs that migrated from the graft (Arias-Carrión et al., 2004).
Electrophysiologic Findings
To increase the likelihood of recording from TH-immunoreactive cells in the SVZ, experiments were carried out in brain slices containing the lateral ventricles from SN-lesioned rats that subsequently received striatal CCs graft. This condition produces the highest number of SVZ TH+ cells (Arias-Carrión et al., 2004). Eighty-seven cells recorded from the SVZ were accepted for analysis. After fixation and immunostaining, ∼54% of the recorded cells were found immunoreactive to TH (n = 47/87).
The majority of SVZ TH− cells and SVZ TH+ cells lacked membrane excitability. Figure 1A shows confocal fluorescence micrographs from an area of the SVZ where a cell was recorded electrophysiologically. Figure 1A1 identifies the recorded cell filled with biocytin (asterisk). Figure 1A2 shows the TH-immunoreactivity of the same field. Several TH+ cells are visible in this picture. Superimposition of both confocal micrographs in Figure 1A3, demonstrates that the biocytin-filled cell was not TH-immunoreactive. As shown in Figure 1B this SVZ TH− cell was unable to fire action potentials on depolarization. Injection of strong depolarizing current only elicited a small rudimentary spike at the beginning of the pulse (arrow). The majority of SVZ TH− cells (32/40) and SVZ TH+ cells (42/47) recorded electrophysiologically and identified with the same procedure, lacked excitable behavior. These electrophysiologic records resemble responses obtained from SVZ neuronal precursors and migrating neuroblasts of the rostral migratory stream in control animals (Wang et al., 2003; Belluzzi et al., 2003). Several cells recorded in the SVZ lacked TH immunoreactivity, but displayed excitable behavior (8/40). Given their position relative to the ventricle lining (up to 100 μm from the ciliated endothelium), and their electrophysiologic properties (data not shown), it was assumed that they possibly correspond to pre-existing striatal neurons (however, see discussion).
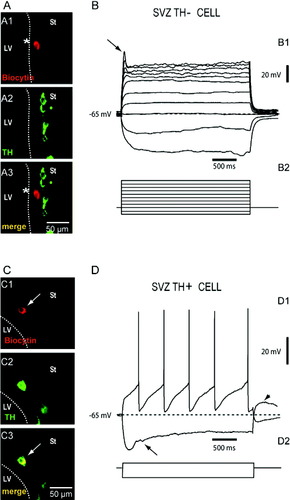
Electrophysiologic characteristics of SVZ cells. A: Confocal micrographs obtained from the SVZ of a rat with SN-lesion and CCs transplant. A1: Biocytin staining allows identification of the recorded cell. A2: TH immunostaining micrograph from the same field. A3: Merge of micrographs shown in A1 and A2, demonstrating that the recorded cell lacked TH immunoreactivity. B1: Voltage responses from the cell shown in A1 to depolarizing and hyperpolarizing current pulses shown in B2. The majority of SVZ cells recorded were non-excitable. A small, rudimentary spike could be recorded occasionally. C: Confocal micrographs from the SVZ of a rat with SN-lesion and CCs transplant. C1: Identification of the recorded cell by its biocytin staining. C2: TH immunostaining micrograph from the same field. C3: Merge of C1 and C2. In this case, the recorded cell expressed TH immunoreactivity. The dotted line represents the border of the lateral ventricle (LV), Stratium (St). D1: Voltage responses from the cell show in C1 to depolarizing and hyperpolarizing current pulses shown in D2. A minority of SVZ TH+ cells recorded were excitable and exhibited electrophysiologic responses similar to dopaminergic neurons, such as low frequency tonic firing on membrane depolarization, a sag after hyperpolarizing current injection and a rebound depolarizing hump after the end of the hyperpolarizing current pulse.
Some SVZ TH+ cells showed excitable properties. As mentioned earlier, only a small fraction of SVZ TH+ cells (n = 5/47 or 11%) displayed excitability. An example is shown in Figures 1C,D. Confocal micrographs analogous to those illustrated in Figure 1A, demonstrate that, in this case, the biocytin-filled cell was immunoreactive to TH (arrows in Fig. 1C1,C3). As shown in Figure 1D, the injection of a depolarizing current step produced regular firing of action potentials at low frequency (<10 Hz), and a time-dependent relaxation (sag) on membrane hyperpolarization (Fig. 1D, arrow; see below). As shown in Figure 2A, SVZ TH+ cells continuously fire action potentials at regular intervals after a small depolarization by DC current injection, with a spike threshold at around −40 ± 4 mV (n = 5). The gap between action potentials show membrane after-hyperpolarizations (AHPs) with a mean peak amplitude of 21 ± 2.3 mV in (n = 4) and 100–500 msec in duration. These AHPs were biphasic, with a brief initial, fast rising component, followed by a long-lasting, slowly rising component (arrow in Fig. 2A). These electrophysiologic properties, including interspike AHPs are similar to those reported for dopaminergic midbrain neurons (Grace and Bunney, 1983a, b, 1984a, b; Tepper et al., 1987; Grace and Onn, 1989). Excitable SVZ TH+ cells also displayed a characteristic inflection in the rising phase of the action potential (Fig. 2B). Figure 2C illustrates a similar recording from a SNc neuron, showing a similar inflection (arrow), which in this case, results from a delay between the activation of the initial segment and the firing of the somato-dendritic spike (Grace and Bunney, 1983b).
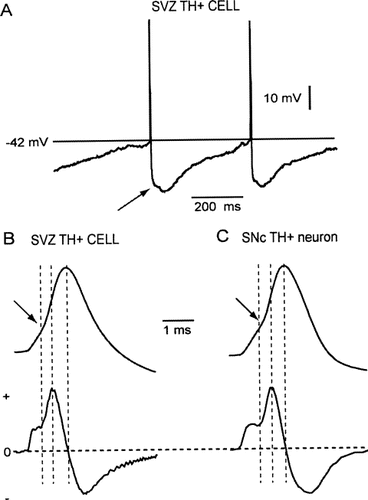
Comparison of action potentials recorded from SVZ TH+ cells and SNc neurons. A: Current clamp recordings of a SVZ TH+ cell shows continuously firing of action potentials in response to the injection of a small depolarizing current. Interspike intervals show large, long-lasting after hyperpolarizations (arrow). B: Fast time-scale recording of an action potential from a SVZ TH+ cell. The first derivative of this record is shown below. The arrow indicates the inflection in the rising phase of the action potential, which can be seen more clearly in the first derivative record (first dotted line). C: Fast time-scale recording of an action potential from a SNc dopaminergic neuron. The first derivative of this record is also shown. Notice the similarities between both records, including the inflection in the rising phase of the action potential.
A more detailed comparison of the firing properties of a SVZ TH+ cell and a substantia nigra compacta (SNc) dopaminergic neuron is exemplified in Figure 3, where the left column (Fig. 3A) illustrates membrane potential responses from a SVZ TH+ cell and the right column (Fig. 3C) illustrates responses from a SNc neuron. Cells were subject to 2 sec-long depolarizing current pulses of increasing intensity (Fig. 3A,C). A weak depolarization gives rise to low-frequency trains of action potentials with occasional spike failures (Fig. 3A,C top traces; asterisks). With moderate depolarizations, trains of action potentials of increasing frequency were generated (Fig. 3A,C, middle traces). Firing frequency increased further with stronger stimulus, but repetitive firing did not last until the end of the depolarizing pulse (firing blockade; Fig. 3A,C bottom traces; double asterisks). The intensity-frequency relationship (Fig. 3I–F plots) obtained from both cell types are compared in Figures 3B,D. The highest frequency achieved by the group of SVZ TH+ cells was 8.9 ± 0.9 Hz (n = 4).The I-F plot obtained from a SNc neuron (Fig. 3D) closely resembled that of excitable SVZ TH+ cells (Fig. 3B). In both cases, firing frequency did not exceed ∼10 Hz.

Comparison of intensity-frequency relationship between SVZ TH+ cells and SNc neurons. A: Representative traces of action potential firing recorded from a SVZ TH+ cell in response to 2 sec depolarizing current steps of increasing intensity (current steps are shown at the bottom). Notice that this cell shows action potential failures with weak depolarizations (*) and stops firing action potentials on high-intensity stimulation (**). B: Intensity-frequency relationship (I–F plot) constructed from data obtained from SVZ TH+ cells using protocols similar to those shown in (A). C: Representative traces of action potential firing recorded from a SNc neuron in response to depolarizing current steps of increasing intensity (current steps shown at the bottom). This SNc neuron also shows failures with weak depolarization (*) and action potentials blockade after high-intensity stimulation (**). D: Intensity-frequency relationship constructed from voltage traces shown in (C). Action potentials were clipped in some voltage traces for clarity.
As shown earlier, excitable SVZ TH+ cells display a time dependent voltage relaxation (sag) in response to hyperpolarizing current injection (Fig. 3D). This feature is also a characteristic of dopaminergic neurons (Lacey et al., 1989; Grace and Onn, 1989). Figures 4A,D exemplify voltage traces obtained from a SVZ TH+ cell and a SNc neuron in response to depolarizing and hyperpolarizing current steps. The relaxation produced after hyperpolarizing current injection, is indicated with asterisks. The rebound depolarization after the end of the hyperpolarizing step is also evident in both sets of recordings. Then, the same cells were recorded under voltage-clamp, and current traces in response to depolarizing and hyperpolarizing voltage commands were recorded. Figure 4B exemplifies current traces obtained from the SVZ TH+ cell in response to depolarizing and hyperpolarizing voltage steps. Figure 4E shows corresponding current traces obtained from a SNc neuron. Depolarizing voltage steps elicited outward currents and the firing of several unclamped action currents, whereas a slowly-activating inward current develops in response to hyperpolarizing voltage steps (asterisks in Fig. 4B,E). The time course and voltage dependence of the inward current recorded in SVZ TH+ cells resembles the hyperpolarization-activated cationic current (HCN current) seen in dopaminergic neurons and several other nerve cells (Mercuri et al., 1995; Watts et al., 1996). A Boltzmann fit to the differentiated I–V plots of the inward current recorded from SVZ TH+ cells (data not shown) yielded the following parameters: Voltage threshold = −74 ± 4.2 mV; half-activation voltage; Vhalf = −93 ± 2.8 mV and peak conductance; Gmax ∼18 nS (n = 5). The same parameters, obtained from a group of SNc neurons, were: −73 ± 5 mV; −98 ± 6 mV and ∼16 nS, respectively (n = 6). Similar values have been reported in other studies of SNc dopaminergic neurons (Mercuri et al., 1995; Watts et al., 1996).
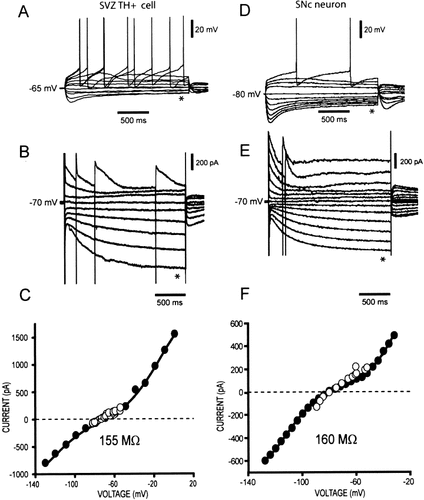
Comparison of current-voltage relationships of SVZ TH+ cells and SNc neurons. A,D: Set of voltage traces recorded from a SVZ TH+ cell (A) and a SNc neuron (B), in response to the injection of depolarizing and hyperpolarizing current steps (not shown). These recordings were done under current-clamp conditions. B,E: Set of current traces obtained from the same cells shown in (A) and (D), in response to depolarizing and hyperpolarizing voltage commands. The holding potential was kept at −70 mV. Experiments were done under voltage-clamp conditions (voltage steps not shown). A time and voltage dependent inward current activated on hyperpolarization is apparent from these records. C,F: Current-voltage (I–V) plots obtained from traces shown in (A) and (B) and (D) and (E), respectively. Empty circles correspond to voltage traces (A,D) and filled circles to current traces (B,E). A polynomial function was fitted to these data points. The indicated input resistances were obtained from the slopes of the I–V plots around zero current.
Remarkably, recordings obtained from both cell types are almost indistinguishable; the main differences being a bigger sag and a more prominent persistent outward current elicited by depolarizing commands in the SNc cell. Finally, Figures 4C,F illustrate current–voltage relationships (I–V plots), obtained from measurements on the family of voltage traces shown in Figures 4A,B (empty circles), and current traces in Figures 4B,E (closed circles), respectively. Note the superimposition of both data sets around the cell's resting potential, and the similarities between the I–V plots of the two cell types. From these I–V plots, the reciprocal of the minimal slope conductance was obtained: The mean input resistance (Rin) measured in SVZ TH+ cells was 156 ± 1 MΩ (n = 5), whereas in SNc neurons it was 160 ± 5 MΩ (n = 6).
Spontaneous Synaptic Currents Recorded in SVZ TH+ Cells
To address the question of whether SVZ TH+ cells have the potential to integrate to the neighboring neuronal circuits in the host brain, spontaneous postsynaptic events were recorded under voltage-clamp conditions. As exemplified in Figure 5A, spontaneous synaptic currents were absent (either with or without 4-AP), in voltage clamp recordings from SVZ cells lacking TH immunoreactivity, or of non-excitable TH+ SVZ cells (n = 7). Nonetheless, when similar recordings were obtained from excitable SVZ TH+ cells (Fig. 5B), spontaneous synaptic currents were readily observed. The frequency of these events increased after the application of 100 μM 4-aminopyridine (4-AP; see Flores-Hernández et al., 1994). Similar recordings were obtained from two additional SVZ TH+ cells. Examples of spontaneous synaptic events before and after 4-AP application are shown at higher magnification and faster time speed in Figure 5C. These results suggest that besides differentiating into neurons similar to midbrain dopaminergic neurons SVZ TH+ cells are also capable of attracting synaptic afferents from the neighboring neuronal networks.
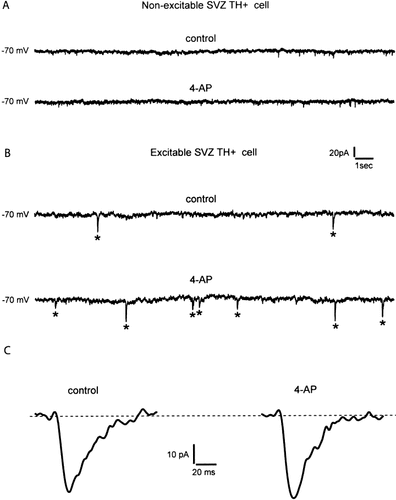
Recording of spontaneous postsynaptic currents from an excitable SVZ TH+ cell. A: Current traces obtained at a holding potential of −70 mV from a SVZ TH+ cell that lacked membrane excitability. No synaptic potentials were visible in these records, either before (upper record) or after (lower record) the application of 100 μM of the potassium channel blocker 4-AP. B: Similar current traces obtained from a SVZ TH+ cell that fired action potentials regularly. Spontaneous inward postsynaptic currents were observed at a low frequency in normal bathing solution (upper record), but the frequency of these spontaneous events increased significantly after the application of 4-AP to the bath (lower record). C: Fast time-scale recording of spontaneous synaptic currents before (left) and after (right) application of 4-AP, showing that 4-AP does not affect the kinetics of these synaptic currents.
Dopamine Release
To further explore the functionality of TH+ cells, dopamine (DA) release was measured in tissue strips from SVZ and striatum. A freshly cut coronal section of the rat brain containing the lateral ventricles (LV) and the striatum is illustrated in Figure 6A. The white outlines in this picture identify the regions (SVZ; striatum), that were cut-out for DA release determinations (see low magnification micrographs of these tissue fragments in B and C: SVZ and striatum, respectively). The amount of basal and stimulated DA release obtained from control (non-lesioned) striatal fragments is shown in Figure 6D. The first white bar represents basal, spontaneous DA release. The black bar next to it shows the amount of DA released after bathing striatal fragments with ACSF containing 20 mM KCl. The depolarizing medium increased DA release by 55% (P < 0.001). Incubation with the same depolarizing solution, but with no added Ca2+, elicited only 19% of the DA release obtained from the control as shown with the gray bar in Figure 6D (P < 0.001). The amount of basal DA release recovered partially after bathing striatal fragments with normal ACSF (Fig. 6D, rightmost white bar). Similar, Ca2+-dependent release of ACh from striatal tissue has been documented using the same technique (Galarraga et al., 1999).
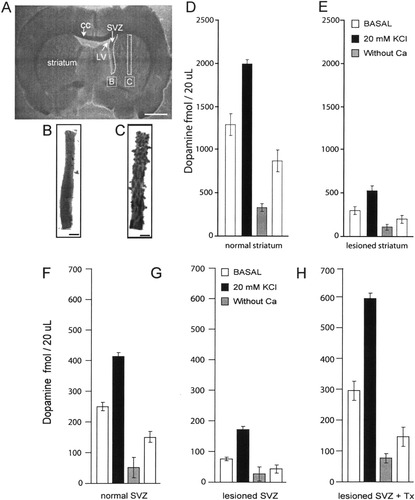
Dopamine release from striatum and subventricular zone. A: Low magnification micrograph of a freshly cut coronal brain slice. The white outlines show the tissue strips that were dissected for dopamine release determinations (SVZ: subventricular zone; LV: lateral ventricle; CC: corpus callosum (see Materials and Methods). B,C: Examples of tissue strips that were dissected from the slice B: SVZ; C: striatum. Scale bars = 100 μm D–H: Dopamine release determinations under the different experimental conditions. First white bar: Basal, non-stimulated release after incubation with control medium. Black bar: Dopamine release after stimulation with depolarizing medium. Gray bar: Dopamine release after stimulation with depolarizing medium in calcium-deficient medium. Second white bar: Basal dopamine release after returning to control medium. D: Release determinations of striatum obtained from a control, untreated rat. E: Release determinations from the denervated striatum of a rat with nigro-striatal lesion. F: Release determinations from SVZ obtained from of a control, untreated rat. G: Release determinations from SVZ obtained from a rat with nigro-striatal lesion alone. H: Release determinations from the SVZ of a rat with nigro-striatal lesion and subsequent transplant of chromaffin cells.
The denervated striatum of SN-lesioned animals released ∼70% and ∼73% less DA than the control striatum (basal and stimulated release, respectively, see white and black bars in Figure 6E; P < 0.004 in both cases). Nevertheless, incubation with the depolarizing medium still induced 64% additional release of DA above the basal level (black bar in Fig. 6E). A significant fraction (∼77%) of the stimulated release depends on the presence of extracellular Ca2+ (see grey bar in Fig. 6E).
When SVZ fragments from control animals were similarly assayed, it was found that they released ∼80% less DA (both basal and stimulated), than corresponding fragments of the control striatum (compare Fig. 6D,F; P < 0.0001 for both comparisons). These results are in agreement with anatomic reports indicating that dopaminergic innervation of the SVZ is considerably less dense than in the striatal interior (Hoglinger et al., 2004). Although of smaller magnitude, DA release from the control SVZ resembled that of the striatum in that depolarization with K+ increased DA release by ∼60%, (P < 0.003) and stimulated DA release diminished by ∼87% after the removal of external Ca2+ (P < 0.0001; see gray bars in Fig. 6D,F). After 6-OHDA lesion of the SN, DA release (both basal and stimulated) from the SVZ adjacent to the denervated striatum also declined significantly (Fig. 6F,G). Remarkably, when DA release was assayed in SVZ fragments from SN-lesioned animals that subsequently received CCs graft, we found that they released ∼18% and ∼27% more DA (basal and stimulated, respectively) than control SVZ slices (P < 0.04 and P < 0.0001, respectively).
The main conclusion that emerges from these results is that the graft of CCs in the denervated striatum of animals with SN-lesion, not only reverses the loss of DA release from the SVZ, but increases DA release above that of the SVZ of control, non-treated animals. The appearance of an additional component of DA release, regulated by membrane depolarization and requiring the presence of external Ca2+, suggests that newly-appearing TH+ cells in the SVZ are the source of supplementary DA.
DISCUSSION
Induction of TH Expression in Cells Derived From the SVZ
SN-lesion induces the differentiation of TH+ cells in the SVZ of adult rats in particular, when the animals receive a striatal graft of CCs. Because DA-containing cell bodies are normally absent in the rodent lateral ventricles or striatum, and TH is essential for DA synthesis, the presence of TH-immunoreactive cells in animals with striatal dopaminergic denervation is potentially of considerable significance. TH+ cells become visible initially in the walls of the lateral ventricles and therefore, it was hypothesized that they derive from neuronal precursors from the SVZ, which continuously proliferate in this region (Alvarez-Buylla and García-Verdugo, 2002). This suggestion received support from experiments showing that at least some of these cells divided during the period of BrdU availability (Arias-Carrión et al., 2004).
In principle, these findings are not entirely surprising because SVZ neuronal precursors give rise to olfactory bulb periglomerular interneurons, some of which are dopaminergic (Lois and Alvarez-Buylla, 1994; Doetsch et al., 1997; Luskin, 1998). Nevertheless, dopaminergic differentiation in the SVZ has never been reported under normal conditions: in studies with adult mice expressing a TH promoter to drive either a LacZ or an enhanced green fluorescent protein (EGFP) reporter gene, β-Gal- or EGFP-stained cells were not seen in either the SVZ or the RMS (Baker et al., 2001; Saino-Saito et al., 2004). Moreover, these precursors never functionally differentiate in the SVZ, or in the rostral migratory stream (Wang et al., 2003; Belluzzi et al., 2003).
Thus, we hypothesized that under the influence of factors appearing in SN-lesioned animals some neuronal precursors from the SVZ differentiate prematurely in situ into dopaminergic interneurons. To test this hypothesis, we carried out single-cell electrophysiology experiments to determine whether TH+ SVZ cells were excitable, and if their electrophysiologic properties resembled those of olfactory periglomerular interneurons (Puopolo and Belluzzi, 1998). In addition, DA release assays were conducted to ascertain if SVZ TH+ cells were capable of synthesizing and releasing DA.
Our experiments showed that SVZ fragments from SN-lesioned and CCs grafted animals released significantly more DA than SVZ fragments from animals with SN lesion alone and even those from control SVZ fragments. Nonetheless, the amount of DA released remains much less than that released from control striatal fragments of the same wet weight, and similar to that released from the denervated striatum. The increased DA release from SVZ fragments of lesioned and grafted animals, suggest that newly-appearing TH+ cells are the source of supplementary DA. Nevertheless, the amount of DA released from the TH+ SVZ cells remains quite small, when compared to the amount of DA released from the normal striatum (compare Fig. 6D,H). Electrophysiologic recordings partially explained why DA release from SVZ fragments of these animals was small: the majority of recorded TH+ SVZ cells were unable to fire action potentials. Patch-clamp recordings in mouse brain slices demonstrated that under normal conditions, neuronal progenitors in situ have electrophysiologic properties of immature neurons such as depolarized resting potentials, high input resistances, and lack of action potential firing on membrane depolarization (Belluzzi et al., 2003; Wang et al., 2003). Neuronal progenitors do not develop membrane excitability during migration either, and they display electrophysiologic properties typical of mature interneurons only when they enter the olfactory bulb. In contrast, our experiments demonstrate that, after SN lesion and CCs graft, some cells from the SVZ develop membrane properties quite different from those of neuronal progenitors in situ.
The induction of membrane excitability correlates with the expression of TH, at least in some of these cells. Here, one may wonder whether neuronal progenitors originally predestined to become dopaminergic neurons of the olfactory bulb undergo a premature differentiation. We believe that this is probably not the case: the majority newly arrived periglomerular neurons respond to depolarizing current steps with a single action potential, followed by a plateau, and their I-V relationships show little outward rectification (Belluzzi et al., 2003). These hallmarks of non-rectifying periglomerular cells (Puopolo and Belluzzi, 1998) differ from newly generated neurons in the SVZ, which produce repetitive action potentials and display outward rectification. The electrophysiologic properties of TH+ cells resemble more those of mesencephalic dopaminergic neurons while differing considerably from pre-existent striatal neurons of any type. Nonetheless, the possibility of premature, ectopic differentiation of TH+ periglomerular neurons in the SVZ, with atypical excitable properties must be ruled out in future experiments.
Some of the recorded SVZ cells were electrically excitable but lacked TH immunoreactivity. Although we assumed that they were pre-existing striatal neurons, we cannot rule out that they correspond to newly differentiated neurons that acquired a non-dopaminergic phenotype. Alternatively, it is also possible that the expression of the TH protein is a relatively late phenomenon. Studies with transgenic mice expressing a TH promoter to drive LacZ or EGFs reporter genes offered this explanation for cells expressing LacZ or EGFP reporter genes but no TH immunoreactivity (Baker et al., 2001; Saino-Saito et al., 2004). In addition, Belluzzi et al. (2003) reported that newly-generated periglomerular neurons in the olfactory bulb were excitable, but still lacked TH immunoreactivity after 5 weeks of retroviral-mediated labeling. Previous studies have established that receptor afferent stimulation is required for full expression of the DA phenotype. Thus, in the absence of innervation, TH transcription can be induced without translation (Baker et al., 2001). Further studies are needed to distinguish among these various possibilities.
As for the mechanism of induction of neuronal differentiation in the SVZ, it has been proposed that the chemical destruction of the nigrostriatal pathway may induce the appearance of trophic signals in the denervated striatum (Zhou et al., 1996; Hida et al., 1997), and also that denervation produces factors similar to those released after ischemia, both leading to neurogenesis in the SVZ. The further increase of differentiation of neuronal precursors after grafts of CCs described here could result from the injury produced by grafting itself, and the concomitant expression of additional local signals (Belmadani et al., 2006). Grafted CCs contain neurotrophic molecules (Krieglstein and Unsicker, 1997), which if released locally, could further induce differentiation of neuronal precursors in the SVZ.
SVZ neuronal progenitors isolated from the adult brain can differentiate into hippocampal granule cells after their transplantation in the hippocampus, indicating that SVZ cells are capable of heterotypic neuronal differentiation in an ectopic environment (Richardson et al., 2005b). The same cells failed to differentiate, however, when transplanted either into normal or denervated striatum from adult rats (Herrera et al., 1999; Richardson et al., 2005a). These results suggest that the striatum does not provide an adequate environment to promote neuronal differentiation of grafted adult SVZ precursors. Nonetheless, our data indicates that the denervation of the striatum does induce in situ differentiation of SVZ neuronal precursors into TH+ cells that not only express neuronal markers (Arias-Carrión et al., 2004), but also behave electrically as dopaminergic neurons and may also release dopamine. Because these cells were not grafted but differentiated in situ, it is possible that the combination of local SVZ signals with those from the denervated striatum, and perhaps from the CC graft, are required to induce local differentiation of SVZ precursors. Future studies should determine if trophic factors that regulate adult neurogenesis in the SVZ, like the fibroblast growth factor (bFGF or FGF-2), epidermal growth factor (EGF); transforming growth factor (TGF)α, brain-derived neurotrophic factor (BDNF) and morphogenetic factors like sonic hedgehog (Shh), and bone morphogenic proteins (BMPs) (Abrous et al., 2005), either released from CCs or induced after striatal denervation could lead to differentiation of dopaminergic neurons in the SVZ, and if blocking of their actions can prevent its generation.
Intrinsic dopaminergic interneurons have been identified in the adult striatum from human and nonhuman primates (Dubach et al., 1987; Betarbet et al., 1997; Porritt et al., 2000). Interestingly, the number of these cells increases several fold in monkeys, after dopaminergic denervation by MPTP (Betarbet et al., 1997), and in patients with Parkinson's disease (Porritt et al., 2000). The increased population of TH+ striatal neurons in Parkinson's disease patients and MPTP-treated monkeys may denote a homeostatic mechanism by which the adult striatum compensates dopaminergic striatal denervation. It has been proposed that these new TH+ cells originate from striatal neuronal precursors or from pre-existing non-dopaminergic interneurons. Our data suggest that neuronal precursors from the primate SVZ are just as good candidates for giving rise to these new striatal dopaminergic neurons.
In summary, we show for the first time that following nigro-striatal lesion and graft of CCs newly formed TH+ cells derived from SVZ neural progenitors can develop excitable properties and release dopamine in a voltage and Ca2+-dependent manner. It remains to be determined if these cells release locally sufficient dopamine to compensate to some extent for the loss of dopamine supply, thus explaining the slight behavioral improvement observed in SN-lesioned animals (Arias-Carrión et al., 2004). Understanding the molecular mechanisms underlying the in situ differentiation of neural progenitors and the production of dopaminergic cells in the adult germinal centers may be extremely relevant, particularly in regard to therapies aimed at replacing specific neurons lost in the course neurodegenerative diseases by stimulating neuronal differentiation of endogenous neural precursors.
Acknowledgements
The authors are indebted to A. Patrón, Head of the Microscopy Unit, and C.V. Rivera, Head of the Animal Facility. We also want to thank M. Palomero-Rivero, D. Tapia, F. Jandete-Garcia, F. Pérez-Eugenio, N. Jiménez, and D. Millán-Aldaco for expert technical assistance.




