Unexpected inhibitory regulation of glutamate release from rat cerebrocortical nerve terminals by presynaptic 5-hydroxytryptamine-2A receptors
Abstract
Presynaptic 5-HT2A receptor modulation of glutamate release from rat cerebrocortical nerve terminals (synaptosomes) was investigated by using the 5-HT2A/2C receptor agonist (±)-1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI). DOI potently inhibited 4-aminopyridine (4AP)-evoked glutamate release. Involvement of presynaptic 5-HT2A receptors in this modulation of 4AP-evoked release was confirmed by blockade of the DOI-mediated inhibition by the 5-HT2A receptor antagonist ketanserin but not by the 5-HT2C receptor antagonist RS102221. Inhibition of glutamate release by DOI was associated with a reduction of 4AP-evoked depolarization and downstream elevation of cytoplasmic free calcium concentration ([Ca2+]C) mediated via P/Q- and N-type voltage-dependent Ca2+ channels (VDCCs). In contrast to the DOI effect on 4AP-evoked release, the agonist had no effect on high external [K+] (30 mM)-induced (KCl) stimulation of VDCCs or glutamate release. Likewise, release mediated by direct Ca2+ entry with Ca2+ ionophore (ionomycin) or by hypertonic sucrose was unaffected by DOI. Mechanistically, DOI modulation of 4AP-evoked glutamate release appeared to involve a phospholipase C/protein kinase C signaling cascade, insofar as pretreatment of synaptosomes with the phospholipase C inhibitor U73122 or protein kinase C inhibitors Ro320432 or GF109203X all effectively occluded the inhibitory effect of the agonist. Together, these results suggest that presynaptic 5-HT2A receptors present on glutamatergic terminals effect an unexpected depression of glutamate release by negatively modulating nerve terminal excitability and downstream VDCC activation through a signaling cascade involving phospholipase C/protein kinase C. These observations invoke presynaptic inhibitory 5-HT2A receptor function as a potential target for drugs to mitigate the effects of excessive glutamatergic transmission. © 2006 Wiley-Liss, Inc.
Serotonin (5-hydroxytryptamine; 5-HT) functions as a neurotransmitter or neuromodulator to influence neuronal activity in the mammalian central nervous system (CNS). In the CNS, serotonergic neurons are located mainly in the dorsal and medial raphe nuclei and send neural projections to a number of brain regions (Oleskevich and Descarries, 1990; Bennett-Clarke et al., 1991). The 14 subtypes of 5-HT receptors currently identified are classified into seven families based on their pharmacological properties and primary amino acid sequences (Hoyer and Martin, 1997). A wide variety of 5-HT receptors are involved in cognitive behaviors in normal and pathological conditions (Buhot, 1997) and memory (Buhot et al., 2000). The 5-HT2A receptor subtype is widely expressed in cerebral cortex (Mengod et al., 1990), suggesting its involvement in crucial roles underlying higher brain function. Moreover, 5-HT2A receptors are the focus of distinct attention from the clinical and pharmaceutical research community, because this receptor subtype is involved in the actions of hallucinogenic drugs and has been implicated in the pathogenesis and treatment of psychiatric diseases (Aghajanian and Marek, 2000).
It is well known that 5-HT2A receptors are G-protein-coupled receptors (GPCRs) acting via Gq/11-mediated stimulation of phospholipase C-mediated phosphoinositide hydrolysis to facilitate synaptic transmission by reducing outward potassium currents (Barnes and Sharp, 1999). Indeed, data from in vitro electrophysiological experiments, as well as in vivo microdialysis studies, have shown that 5-HT2A receptor activation can enhance the release of glutamate in cortical regions (Aghajanian and Marek, 1997, 1999b; Marek and Aghajanian, 1999; Marek et al., 2000; Scruggs et al., 2003). Notwithstanding the body of evidence supporting a facilitatory role for 5-HT2A heteroreceptors, there has been some suggestion that 5-HT2A receptors can induce inhibition of synaptic transmission and glutamate release in several neuronal preparations (Edagawa et al., 2000; Marcoli et al., 2001), with the precise mechanism subserving this inhibitory modulation remaining undefined. The mechanisms by which 5-HT2A receptors act to modulate glutamate release are particularly pertinent given that aberrant glutamatergic transmission (Wiley et al., 1995; Skolnick et al., 1996; Danysz and Parsons, 1998) has been proposed to be involved in the putative participation of 5-HT2A receptors in numerous emotional disorders, including depression, anxiety, psychosis, and schizophrenia (Naughton et al., 2000). To address the issue of the contradictory effects of 5-HT2A receptors on synaptic transmission and glutamate release observed previously, in the present study we used isolated nerve terminals (synaptosomes) prepared from the rat cerebral cortex to investigate the function of 5-HT2A receptors in the presynaptic regulation of glutamate release. The synaptosome preparation provides a useful system to study directly the specific presynaptic regulation of neurotransmitter release given that the preparation is devoid of functional glial and nerve cell body elements (Dunkley et al., 1986) that might obfuscate interpretation because of modulatory loci at nonneuronal, postsynaptic, or network levels. We examined the effect of the phenethylamine (±)-1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane) (DOI; a 5-HT2A/2C agonist; Smith et al., 1998) on glutamate release and the nature of the modulatory coupling involved. Our results show that DOI acts at 5-HT2A receptors present on cerebrocortical nerve terminals to effect a reduction of nerve terminal excitability and calcium influx into nerve terminals. This latter effect in turn results in depression of 4-aminopyridine-evoked glutamate release. Surprisingly, in common with the established facilitatory modulation by 5-HT2A receptors, presynaptic inhibition by DOI through 5-HT2A receptors also appears to involve a phospholipase C (PLC)/protein kinase C (PKC) signal transduction pathway.
MATERIALS AND METHODS
Materials
Fura-2-acetoxymethyl ester and 3,3′-dipropylthiadicarbocyanine iodide [DiSC3(5)] were obtained from Molecular Probes (Eugene, OR). Percoll was obtained from Pharmacia. Ketanserin, RS102221, U73122, U73343, Ro320432, GF109203X, ω-conotoxin GVIA, and ω-conotoxin MVIIC were obtained from Tocris Cookson (Bristol, United Kingdom). Glutamate dehydrogenase, ω-agatoxin IVA, L-trans-pyrollidine-2,4-dicarboxylic acid, Ro320432, and all other reagents were obtained from Sigma (Poole, United Kingdom) or Merck (Poole, United Kingdom).
Synaptosomal Preparation
Synaptosomes were prepared as described previously (Dunkley et al., 1986; Sihra, 1997). Briefly, the cerebral cortex from 2-month-old male Sprague-Dawley rats was isolated and homogenized in a medium containing 320 mM sucrose, pH 7.4. The homogenate was spun for 2 min at 3,000g (5,000 rpm in a Beckman JA 25.5 rotor) at 4°C, and the supernatant was spun again at 14,500g (11,000 rpm in a JA 25.5 rotor) for 12 min. The pellet was gently resuspended in 8 ml of 320 mM sucrose, pH 7.4. Two millilitres of this synaptosomal suspension was placed into 3-ml Percoll discontinuous gradients containing 320 mM sucrose; 1 mM EDTA; 0.25 mM DL-dithiothreitol; and 3%, 10%, and 23% Percoll, pH 7.4. The gradients were centrifuged at 32,500g (16,500 rpm in a Beckman JA 20.1 rotor) for 7 min at 4°C. Synaptosomes placed between the 10% and the 23% Percoll bands were collected and diluted in a final volume of 30 ml HEPES buffer medium (HBM) consisting of 140 mM NaCl, 5 mM KCl, 5 mM NaHCO3, 1 mM MgCl2 · 6H2O, 1.2 mM Na2HPO4, 10 mM glucose, 10 mM HEPES (pH 7.4) before centrifugation at 27,000g (15,000 rpm in a Beckman JA 25.5 rotor) for 10 min. The pellets thus formed were resuspended in 3 ml HBM, and the protein content was determined by the Bradford assay (Bradford, 1976); 0.5 mg of synaptosomal suspension was diluted in 10 ml of HBM and spun at 3,000g (5,000 rpm in a Beckman JA 25.5 rotor) for 10 min. The supernatants were discarded, and the synaptosomal pellets were stored on ice and used within 4–6 hr.
Glutamate Release Assay
Glutamate release from purified cerebrocortical synaptosomes was monitored online, with an assay employing exogenous glutamate dehydrogenase and NADP+ to couple the oxidative deamination of the released glutamate to the generation of NADPH detected fluorometrically (Nicholls and Sihra, 1986). Synaptosomal pellets (0.5 mg protein) were resuspended in 1.5 ml HBM containing 16 μM bovine serum albumin (BSA) and incubated in a stirred and thermostated cuvette maintained at 37°C in a Perkin-Elmer LS-50B spectrofluorimeter. NADP+ (2 mM), glutamate dehydrogenase (GDH; 50 U/ml), and CaCl2 (1 mM) were added after 3 min. Glutamate release was monitored by measuring the increase of fluorescence (excitation and emission wavelengths of 340 nm and 460 nm, respectively) resulting from NADPH being produced by the oxidative deamination of released glutamate by GDH. In the absence of axonal connectivity, synaptosomes are not amenable to electrical stimulation, so several biochemical secretagogues have been developed, including the use of depolarization protocols involving potassium channel blockers such as 4-aminopyridine (4AP) or high external [K+] or direct mediation of Ca entry using Ca ionophores such as ionomycin (Sihra, 1997). 4AP destabilizes the membrane potential and is thought to cause repetitive spontaneous Na+ channel-dependent depolarization (Tibbs et al., 1989) that closely approximates in vivo depolarization of the synaptic terminal that leads to the activation of voltage-dependent Ca2+ channels (VDCCs) and neurotransmitter release. Elevated extracellular KCl concentrations depolarize the plasma membrane by shifting the K+ equilibrium potential above the threshold potential for activation of voltage-dependent ion channels. While Na+ channels are inactivated under these conditions (Barrie et al., 1991), VDCCs are activated nonetheless to mediate Ca2+ entry supporting neurotransmitter release. Comparison of the effects of modulators under 4AP and KCl stimulation protocols therefore makes it possible to distinguish between modulatory pathway that target: 1) ionic channels involved in maintaining the plasma membrane potential and shaping the action potential waveform vs. 2) the VDCCs coupled to glutamate release directly (Nicholls, 1998).
Secretagogues 4AP (3 mM), high external KCl (KCl; 15 mM or 30 mM), or ionomycin (5 μM) were added after 10 min of incubation to stimulate glutamate release. Data were accumulated at 2-sec intervals. A standard of exogenous glutamate (5 nmol) was added at the end of each experiment, and the fluorescence response used to calculate released glutamate was expressed as nanomoles glutamate per milligram synaptosomal protein (nmol/mg). Values quoted in the text and expressed in bar graphs represent levels of glutamate cumulatively release after 5 min of depolarization and are indicated as nmol/mg/5 min. Cumulative data were analysed in Lotus 1-2-3 and MicroCal Origin. Estimation of the IC50 was based on a one-site model [Inhibition = (InhibitionMAX × [DOI])/(IC50 + [DOI])] using the nonlinear curve-fitting function in MicroCal Origin. Statistical analysis was performed by two-tailed Student's t-tests or one-way ANOVA, followed by post hoc two-tailed t-tests.
Membrane Potential Measurement With DiSC3(5)
The synaptosomal membrane potential can be monitored by positively charged membrane potential-sensitive carbocyanine dyes such as DiSC3(5) (Enkvist et al., 1988). The dye becomes incorporated into the synaptosomal plasma membrane lipid bilayer. Upon depolarization with 4AP, the release of the dye from the membrane bilayer is indicated as an increase in fluorescence. Synaptosomes were preincubated and resuspended as described for the glutamate release experiments. After 3 min of incubation, 4 μM DiSC3(5) was added and allowed to equilibrate before the addition of CaCl2 (1 mM) after 4 min of incubation. 4AP was added to depolarize the synaptosomes at 10 min, and DiSC3(5) fluorescence was monitored at excitation and emission wavelengths of 646 nm and 674 nm, respectively, and data accumulated at 2-sec intervals. Cumulative data were analyzed in Lotus 1-2-3, and results are expressed in fluorescence units.
Cytosolic Ca2+ Measurements
[Ca2+]C was measured with the Ca2+ indicator Fura-2. Synaptosomes (0.5 mg/ml) were preincubated in HBM with 16 μM (1 mg/ml) BSA in the presence of 5 μM Fura-2-acetoxymethyl ester and 0.1 mM CaCl2 for 30 min at 37°C in a stirred test tube. After Fura-2 loading, synaptosomes were centrifuged in a microcentrifuge for 30 sec at 3,000g (5,000 rpm in a Beckman JA 25.5 rotor). The synaptosomal pellets were resuspended in HBM with BSA and the synaptosomal suspension stirred in a thermostatted cuvette in a Perkin-Elmer LS-50B spectrofluorimeter. CaCl2 (1 mM) was added after 3 min, and further additions were made after an additional 5 min, as described in the legends to the figures. Fluorescence data were accumulated at excitation wavelengths of 340 nm and 380 nm (emission wavelength 505 nm) at data accumulated at 7-sec intervals. Calibration procedures were performed as described previously (Sihra et al., 1992), using 0.1% sodium dodecyl sulfate to obtain the maximal fluorescence with Fura-2 saturation with Ca2+, followed by 10 mM EGTA (Tris buffered) to obtain minimum fluorescence in the absence of any Fura-2/Ca2+ complex. Cytosolic free Ca2+ concentration ([Ca2+]C, nM) was calculated with equations described previously (Grynkiewicz et al., 1985). Cumulative data were analyzed in Lotus 1-2-3 and MicroCal Origin. Statistical analysis was performed by two-tailed Student's t-tests.
RESULTS
Effect of DOI on 4AP-Evoked Glutamate Release From Rat Cerebrocortical Synaptosomes
To investigate the influence of 5-HT2 receptors on glutamate release, we examined whether 5-HT2 receptor activation by the 5-HT2A/2C receptor partial agonist DOI could affect the release of glutamate evoked by 4AP. Under control conditions, 4AP (3 mM) evoked a glutamate release of 18.6 ± 0.8 nmol/mg/5 min from synaptosomes incubated in the presence of 1 mM CaCl2. Application of DOI (50 μM) produced an inhibition of 4AP-evoked glutamate release to 8.2 ± 0.3 nmol/mg/5 min (n = 8; P < 0.01), without altering the basal release of glutamate (Fig. 1A). The DOI-induced inhibition of 4AP-evoked glutamate release was concentration dependent, with an IC50 value derived from a dose-response curve (Fig. 1C) of about 29 μM. Given the latter value and the robust 53% depression of 4AP-evoked glutamate release seen with 50 μM DOI, this concentration of DOI was used in subsequent experiments to evaluate the mechanisms underlying the ability of the agonist to reduce glutamate release.
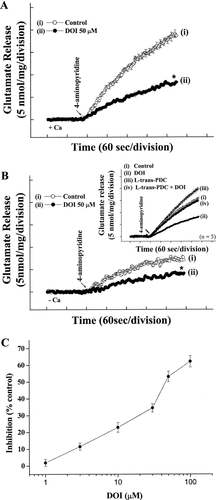
DOI inhibits 4AP-evoked glutamate release from cortical synaptosomes. Synaptosomes were resuspended in incubation medium at a final protein concentration of 0.5 mg/ml and incubated for 3 min before the addition of 1 mM CaCl2. 4AP (3 mM) was added after a further 10 min to effect depolarization (arrow). Ca2+-independent release was assayed by omitting CaCl2 and adding 0.2 mM EGTA 30 sec prior to depolarization. Glutamate release was assayed by online fluorometry, as described in Materials and Methods. Total glutamate release (+Ca; A) and Ca2+-independent glutamate release (−Ca; B) were measured under control conditions (open circles) or in the presence (solid circles) of 50 μM DOI added 10 min prior to the addition of 4AP. Inset shows the effect of 50 μM DOI (solid symbols) in the absence (circles) and presence (triangles) of 10 μM L-trans PDC. C: Dose-response curve for DOI inhibition of 4AP-evoked Ca2+-dependent glutamate release showing percentage inhibition compared with controls. Results are mean ± SEM values of independent experiments, using synaptosomal preparations from six to eight animals. Means and SEMs were calculated at each time point (2 sec), but error bars are shown only every 10 sec for clarity. Total glutamate release (A) and Ca2+-independent glutamate release evoked by 4AP (B), in the presence of DOI, were significantly different from their control conditions (*P < 0.01, two-tailed Student's t-test).
Glutamate release produced by the depolarization of isolated nerve terminals is known to have two components: a physiologically relevant Ca2+-dependent component, which is produced by the exocytosis of synaptic vesicles containing glutamate, and a Ca2+-independent component that results from prolonged depolarization causing a membrane potential-mediated shift of the glutamate transporter steady-state toward the outward direction to effect cytosolic glutamate efflux (Nicholls, 1989). To examine the degree to which the observed modulation of total release by 5-HT2 receptor activation reflected the Ca+-independent efflux of glutamate, we analyzed glutamate efflux by the addition of 0.1 mM EGTA to synaptosomes (incubated in the absence of external Ca2+) prior to depolarization with 4AP. This cytosolic release of glutamate amounted to less than 8 nmol/mg/5 min. Notably, this component of release was also affected by the prior addition of 50 μM DOI (4.1 ± 0.2 nmol/mg/5 min; n = 8; P < 0.01; Fig. 1B), suggesting that the overall inhibition of glutamate release by DOI reflects effects on both the Ca2+-dependent exocytosis of glutamate and the Ca2+-independent nonvesicular efflux. To examine the effect of DOI with the latter component abrogated, we looked at its effect in the presence of L-transpyrrolidine-2,4-dicarboxylic acid (L-trans-PDC), a potent inhibitor of the plasma membrane glutamate transporter, which should block or severely reduce the Ca2+-independent nonvesicular efflux by transporter reversal. In the presence of L-trans-PDC, although 4AP-evoked glutamate release was increased by the inhibitor (because of inhibition of reuptake of released glutamate), DOI continued to reduce significantly the secretagogue-induced release of glutamate. This indicates that a major proportion of the inhibition produced by DOI reflects an effect on the physiologically relevant, exocytotic pool of glutamate release. Similar results and conclusions could be drawn from analysis with the nontransportable glutamate uptake inhibitor DL-threo-beta-benzyloxyaspartate (DL-TBOA; Supplementary Fig. 1S).
Inhibition by DOI Is Mediated by 5-HT2A Receptors: Pharmacology
The somewhat surprising finding that DOI causes an inhibition of glutamate release in synaptosomes, in contrast to facilitatory effects observed in slice and in vivo studies, led us to next ask whether the effects were indeed receptor mediated. We pursued this question by examining the effect of the potent 5-HT2A receptor antagonist ketanserin (which has moderate affinity for the 5-HT2C receptor type; Baxter et al., 1995) and the 5-HT2C receptor antagonist RS102221 on the action of DOI. As shown in Figure 2, the inhibitory effect of 50 μM DOI on 4AP-evoked glutamate release was completely prevented when synaptosomes were pretreated with 10 μM ketanserin, previously shown to be sufficient for blocking synaptosomal 5-HT2 receptor responses (Maura et al., 1991; Garro et al., 2001). Thus, compared with the approximately 51% decrease of 4AP-evoked release produced by DOI (control 18.1 ± 0.5 nmol/ mg/5 min; DOI 8.8 ± 0.6 nmol/mg/5 min), in the presence of 10 μM ketanserin, the effect of DOI was completely blocked (ketanserin + DOI 17.5 ± 0.5 nmol/ mg/5 min). The application of 10 μM ketanserin alone elicited no significant decrease in the release of glutamate evoked by 4AP (ketanserin, 17.7 ± 0.5 nmol/mg/5 min). In contrast to the effect of 5-HT2A receptor antagonism by ketanserin, the 5-HT2C receptor antagonist 5 μM RS102221 influenced neither 4AP-evoked glutamate release, per se, nor the ability of DOI to depress this release (control 4AP 18.6 ± 1.1 nmol/mg/5 min; RS102221 18.3 ± 0.9 nmol/mg/5 min; RS102221 + DOI, 7.9 ± 1.2 nmol/mg/5 min) by 58% (Fig. 2), a value comparable that observed in the absence of RS102221 (Figs. 1A, 2). These results suggest first that DOI-mediated inhibition of 4AP-mediated release is indeed receptor-mediated and not a nonspecific effect of the compound. Second, these experiments invoke regulatory 5-HT2A receptors, rather than 5-HT2C receptors, in the DOI-mediated inhibition of glutamate release. The other Gq-coupled member in the 5HT2 class, the 5-HT2B receptor, is absent from the cerebrocortex (Duxon et al., 1997).
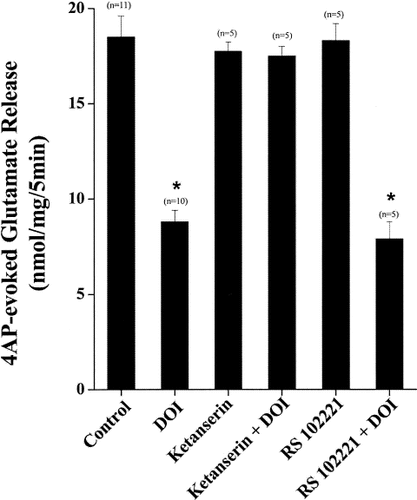
Blockade of DOI inhibition of 4AP-evoked Ca2+-dependent glutamate release by 5-HT2A receptor antagonists. Experiments were carried out as described in Materials and Methods and the legend to Figure 1. Glutamate release was evoked by the addition of 3 mM 4AP under the control conditions (control) or in the presence of 50 μM DOI, 10 μM ketanserin, 10 μM ketanserin + 50 μM DOI, 5 μM RS102221, or 5 μM RS102221 + 50 μM DOI added 10 min before the addition of 4AP. Columns are the mean ± SEM values of independent experiments, using synaptosomal preparations from five to 11 animals. Asterisks denote conditions under which release was significantly different from control 4AP-evoked release (P < 0.05, ANOVA followed by post hoc two-tailed Student's t-tests).
Effect 5-HT2A Receptor Activation on Nerve Terminal Excitability and Ca2+ Influx
We next addressed the potential mechanisms underlying the 5-HT2A receptor-mediated inhibition of glutamate release by assessing the effects of DOI on synaptosomal plasma membrane potential and Ca2+ influx. Because inhibition of Na+ channels or activation of K+ channels is known to stabilize membrane excitability and consequently cause a reduction in the evoked entry of Ca2+ and neurotransmitter release (Rehm and Tempel, 1991; Li et al., 1993; Pongs et al., 1999), we reasoned that the observed inhibitory effect of DOI on 4AP-evoked glutamate release could be due to an alteration of nerve terminal excitability. To test this possibility, we examined the effects of DOI on synaptosomal plasma membrane potential under resting conditions and on depolarization, with the membrane potential-sensitive dye DiSC3(5). As shown in Figure 3A, 4AP (3 mM) caused an increase in DiSC3(5) fluorescence of 3.1 ± 0.05 fluorescence units/5 min (n = 7). Preincubation of synaptosomes with 50 μM DOI did not alter the resting plasma membrane potential; however, in the presence of DOI, the 4AP-induced elevation of DiSC3(5) fluorescence was significantly decreased (2.1 ± 0.08 fluorescence units/5 min; n = 7; P < 0.01; Fig. 3A). This suggests that an attenuation of synaptosomal plasma membrane potential depolarization produced by 4AP, at least in part, underlies the observed inhibition of 4AP-evoked glutamate release by 5-HT2A receptor activation.
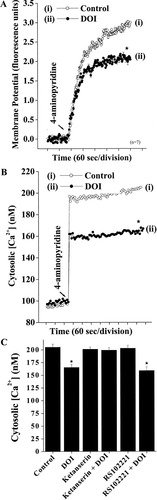
DOI inhibits 4AP-induced plasma membrane depolarization and voltage-dependent Ca2+ influx. Synaptosomes (0.5 mg/ml) were incubated as described in Materials and Methods. A: Synaptosomal membrane potential was monitored with 5 μM DiSC3(5) on depolarization with 4AP (3 mM) in the absence (control) or in the presence of 50 μM DOI added 10 min before depolarization. Each trace is the mean ± SEM values of independent experiments, using synaptosomal preparations from seven or eight animals. Means and SEMs were calculated at each time point (2 sec), but error bars are shown only every 10 sec for clarity. Asterisk indicates the difference in synaptosomal membrane potential after 4AP depolarization were significantly different in the absence and presence of DOI (P < 0.05, two-tailed Student's t-test). Differences in resting membrane potential in the absence and presence of DOI were not significantly different. B,C: [Ca2+]C was monitored by using Fura-2 loaded as described in Materials and Methods. Synaptosomes were stimulated with 3 mM 4AP in the absence (control) or presence of 50 μM DOI, 10 μM ketanserin, 10 μM ketanserin + 50 μM DOI, 5 μM RS102221, or 5 μM RS102221 + 50 μM DOI added 10 min before stimulation. Results are the mean ± SEM values of independent experiments, using synaptosomal preparations from eight animals. [Ca2+]C in the presence of DOI was significantly different from control (☆P < 0.01, ANOVA followed by post hoc two-tailed Student's t-tests).
To examine whether the attenuation of depolarization-induced synaptosomal membrane potential reflected a decreased entry of Ca2+ through the VDCCs, we used the Ca2+ indicator Fura-2 to assess what effect DOI has on the 4AP-induced increase in cytosolic Ca2+ levels ([Ca2+]C). After addition of 4AP (3 mM), [Ca2+]C in synaptosomes was elevated to a plateau level of 205.1 ± 6.2 nM (Fig. 3B). Preincubation of synaptosomes with 50 μM DOI reduced the 4AP-evoked [Ca2+]C increase by 19% (165.3 ± 5.7 nM; n = 8; P < 0.01; Fig. 3C). Importantly, the inhibition of 4AP-evoked increase of [Ca2+]C by DOI was suppressed by 10 μM ketanserin preincubation (ketanserin 201.1 ± 4.2 nM; ketanserin + DOI 199.1 ± 5.1 nM). In contrast to this effect of 5-HT2A receptor antagonism on DOI-mediated Ca2+ responses, 5-HT2C receptor blockade with 5 μM RS102221 was without effect on the DOI-mediated suppression of 4AP-evoked Ca2+ influx (RS102221 158.9 ± 7.2 nM; RS102221 + DOI 202.8 ± 6.1 nM; Fig. 3C). These data indicate that DOI reduces 4AP-evoked changes in [Ca2+]C with a pharmacological profile similar to that observed for the reduction of release (Fig. 2).
Involvement of Presynaptic P/Q- and N-Type Ca2+ Channels in DOI-Induced Inhibition of Glutamate Release
In the rat cerebrocortical nerve terminal preparation, the release of glutamate is supported by N- and P/Q-type VDCCs (Vazquez and Sanchez-Prieto, 1997). To determine whether a specific VDCCs was involved in the inhibition of 4AP-evoked glutamate release by DOI, we examined the effects of DOI in the presence of Ca2+ channel blockers/toxins. As shown in Figure 4A, DOI was ineffective following the prior block of VDCCs with ω-CgTX GVIA (N-type) and ω-Aga IVA (P/Q-type). Specifically, in the absence of Ca2+ channel blockers, DOI inhibited the 4AP-evoked release from 18.9 ± 0.2 nmol/mg/5 min to 8.8 ± 0.3 nmol/mg/5 min (n = 10; P < 0.05; Fig. 4B). Application of 2 μM ω-CgTX GVIA or 500 nM ω-Aga IVA reduced 4AP-evoked glutamate release to 16.1 ± 0.2 (n = 5; P < 0.05) or 11.7 ± 0.4 nmol/mg/5 min (n = 6; P < 0.01), respectively, and to 8.3 ± 0.4 nmol/mg/5 min when both toxins were coapplied. In the presence of ω-CgTX GVIA alone, DOI could still effectively reduced release (from 16.1 ± 0.2 to 5.8 ± 0.4 nmol/mg/5 min); in the presence ω-Aga IVA, the inhibition by DOI (from 11.7 ± 0.4 to 9.2 ± 0.5 nmol/mg/5 min respectively) was somewhat diminished (Fig. 4B). After the combined application of ω-Aga IVA and ω-CgTX GVIA, when glutamate release was reduced to 8.3 ± 0.4 nmol/mg/5 min (n = 6; P < 0.001, Fig. 4B), DOI caused a marginal further decrease to 7.9 ± 0.5 nmol/mg/5 min, which represented a statistically insignificant change in 4AP-evoked glutamate release resulting from DOI in the presence of P/Q- and N-type VDCC block (4.8% ± 2.3%, cf. DOI alone, 53.4% ± 3.2%; n = 8; P < 0.01; Fig. 4B). Similarly, we found that 1 μM ω-CgTX MVIIC, a wide-spectrum blocker of N-, P-, and Q-type Ca2+ channels, reduced the 4AP-evoked glutamate release to 6.4 ± 0.8 nmol/mg/5 min and completely prevented any further inhibition of glutamate release by 50 μM DOI (MVIIC + DOI 6.7 ± 0.8 nmol/mg/5 min; n = 5; Fig. 4B). Similarly to the lack of effect of DOI in the presence of ω-CgTX MVIIC, the agonist had no effect on the residual release in the presence of all three channel toxins (see Supplementary Fig. 2S). Overall, these data point to the possibility that the effects of DOI are manifest through the direct or indirect attenuation of P/Q-type VDCC activity.
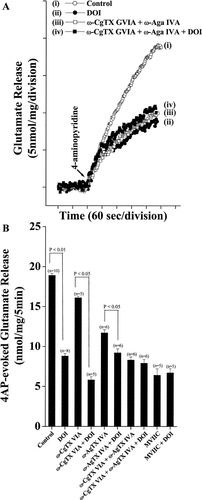
Blockade of P/Q- and N-type Ca2+ channels abolishes DOI inhibition of glutamate exocytosis. A: Glutamate release was evoked by 3 mM 4AP (arrow) in the absence (control) or presence of 50 μM DOI, 2 μM ω-CgTX GVIA + 500 nM ω-Aga IVA or 2 μM ω-CgTX GVIA + 500 nM ω-Aga IVA + 50 μM DOI. DOI was added 10 min before depolarization and ω-CgTX GVIA + ω-Aga IVA 10 min prior to this. B: Bar graph showing the release of glutamate evoked by 4AP (3 mM) in the absence (control) or presence of ω-CgTX GVIA (2 μM), ω-CgTX GVIA (2 μM) + DOI (50 μM), ω-Aga VIA (500 nM), ω-Aga VIA (500 nM) + DOI (50 μM), ω-CgTX GVIA (2 μM) + ω-Aga VIA (500 nM), ω-CgTX GVIA (2 μM) + ω-Aga VIA (500 nM) + DOI (50 μM), ω-CgTX MVIIC (1 μM) and ω-CgTX MVIIC (1 μM) + DOI (50 μM). Data represent mean ± SEM of five to 10 independent synaptosomal preparations (n in parenthesis). Significant differences in the absence and presence of DOI are as indicated (using ANOVA followed by post hoc two-tailed Student's t-tests).
Locus of 5-HT2A Receptor-Induced Inhibition of Glutamate Release
The data given above are consistent with the activation of 5-HT2A receptors reducing glutamate release by the attenuation of nerve terminal excitability, leading to a reduction in the influx of Ca2+ through largely P/Q-type VDCCs. The possibility remains that 5-HT2A receptor activation also directly impinges on these VDCCs to modulate their activity. Comparison of modulatory effects obtained with 4AP- and KCl-mediated depolarization has been used extensively to gain insight into the precise locus of modulation because of the different ways in which these paradigms achieve stimulation (Nicholls, 1993; see also Materials and Methods). In short, given that 4AP- and KCl-mediated depolarizations activate the same VDCCs (Vazquez and Sanchez-Prieto, 1997), DOI should arguably inhibit the Ca2+ influx mediated by either depolarizing agent and thereby inhibit glutamate release equally well. To examine this issue, we looked at the effect of DOI on the release evoked by high external KCl concentrations. In notable contrast to the modulation of 4AP-induced release by DOI, KCl (30 mM)-evoked glutamate release was not significantly affected by 50 μM DOI (control 25.8 ± 0.7 nmol/mg/5 min; DOI 25.4 ± 0.4 nmol/mg/5 min; n = 6; P > 0.05; Fig. 5A). This lack of effect of 5-HT2A receptor activation on KCl-evoked glutamate release was borne out in experiments looking at KCl-evoked Ca2+ entry. Again, in contrast to 4AP-evoked VDCC activation, 30 mM KCl-induced Ca2+ influx was not affected by DOI (Fig. 5B). The lack of effect of DOI on KCl-evoked Ca2+ influx is therefore in congruence with the ineffectiveness of the agonist with KCl evoked glutamate release (Fig. 5A). From the higher release obtained with 30 mM KCl compared with 3 mM 4AP, a plausible explanation for the lack of effect of DOI may be that the stimulation with 30 mM KCl is perhaps too strong to be down-modulated, as has been observed with de facto inhibitory receptors (Nicholls, 1998; Perkinton and Sihra, 1998). However, our observation that DOI failed to have any effect, even when 15 mM KCl was used to evoke synaptosomal depolarization (Fig. 5A, inset), argues against this possibility.
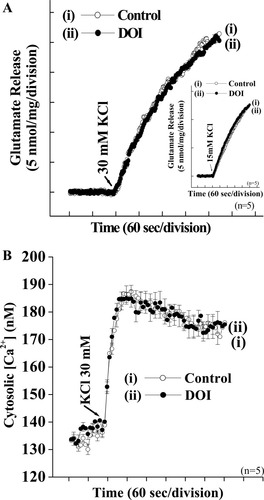
DOI fails to inhibit KCl-evoked glutamate release from cortical synaptosomes. Glutamate release experiments were carried as described in the legend to Figure 1 except that either 30 mM KCl (A) or 15 mM KCl (inset) was used to depolarize synaptosomes under the control conditions (open circles) or in the presence of 50 μM DOI (solid circles). B: KCl evoked increase in [Ca2+]C, in the absence (open circles) and presence (solid circles) of DOI was monitored by using Fura-2 as described in Materials and Methods and the legend to Figure 3. Results are mean ± SEM values of independent experiments, using synaptosomal preparations from five animals. Means and SEMs were calculated at each time point of release (2 sec), but error bars are shown only every 10 sec for clarity.
Although the above-mentioned experiments argue for 5-HT2A receptor-mediated effects on 4AP-induced Ca2+ influx and glutamate release being mediated through an effect on synaptosomal excitability, GPCRs have been reported to modulate the exocytotic process directly (Blackmer et al., 2001, 2005). To determine whether the regulation of glutamate release by DOI also reflects putative actions downstream of Ca2+ influx, we examined the effects of DOI on glutamate release induced by ionomycin (5 μM) or hypertonic solution (500 mM sucrose). The Ca2+-selective ionophore ionomycin causes a direct increase in intrasynaptosomal Ca2+ levels and triggers release of neurotransmitter without depolarization and Ca2+ channel activation (Sihra et al., 1992), whereas hypertonic solution increases the probability of release by emptying the readily releasable pool through a Ca2+-independent mechanism (Rosenmund and Stevens, 1996). These treatments therefore allow the assessment of only those modulatory influences directly affecting synaptic vesicle trafficking and exocytosis, without the involvement of upstream ion-channel function. As shown in Figure 6A, ionomycin (5 μM) caused a control glutamate release of 19.9 ± 0.6 nmol/mg/5 min. In the presence of 50 μM DOI, ionomycin-induced release of glutamate was not significantly affected (20.4 ± 0.8 nmol/mg/5 min; Fig. 6A). Similarly, the release evoked by 500 mM sucrose (23.9 ± 0.9 nmol/mg/5 min) was also unaltered by pretreatment with DOI (24.1 ± 1.1 nmol/mg/5 min; n = 5 Fig. 6B). Therefore, these results suggest that the DOI-mediated inhibition of glutamate release is not a consequence of a direct action of 5-HT2A receptor signaling on release events downstream of Ca2+ influx.
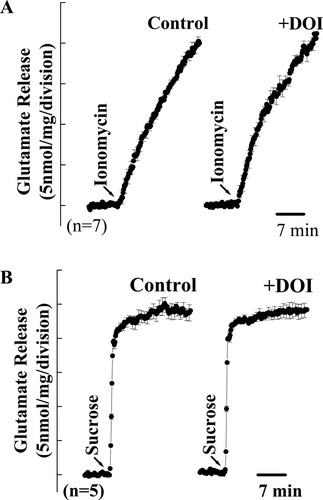
Lack of DOI effect on ionomycin- or hypertonic solution-induced glutamate release. Glutamate release was evoked by 5 μM ionomycin (A) or 500 mM sucrose (B) in the absence (control) or in the presence of 50 μM DOI (+DOI) added 10 min before stimulation. Each trace is the mean ± SEM values of independent experiments, using synaptosomal preparations from five to seven animals. Means and SEMs were calculated at each time point (2 sec), but error bars are shown only every 10 sec for clarity.
Involvement of a PLC/PKC Pathway in DOI-Induced Inhibition of 4AP-Evoked Glutamate Release
Molecular cloning has established that all 5-HT receptor subtypes, except 5-HT3 receptors, belong to the superfamily of the GPCRs (Hoyer and Martin, 1997). We therefore examined the signal transduction mechanism involved in the DOI-mediated inhibition of glutamate release through 5-HT2A receptor activation. For a number of systems, 5-HT2A receptors have been suggested to be coupled to PLC activation through the Gq proteins. As shown in Figure 7A, application of the PLC inhibitor U73122 (10 μM) itself reduced the 4AP-evoked glutamate release from 20.6 ± 0.7 nmol/mg/5 min to 12.7 ± 0.3 nmol/mg/5 min, a substantial inhibition (63.2 ± 3.2% control; Fig. 7C) indicating a basal PLC activity. Notably, however, when U73122 (10 μM) was applied together with DOI (50 μM), the inhibitory effect of DOI on 4AP-evoked glutamate release was significantly occluded (DOI 57.7% ± 2.1% control; U73122 + DOI 60.7% ± 2.8% control; Fig. 7A,C). The specificity of the effect of U73122 was evaluated by using the inactive analog U73343. U73343 (10 μM) had no effect on either control 4AP-evoked glutamate release (U73343 97% ± 1.9% control; Fig. 7C) or inhibition thereof by DOI (U73343 + DOI 59.2% ± 2.5% control; Fig. 7C).
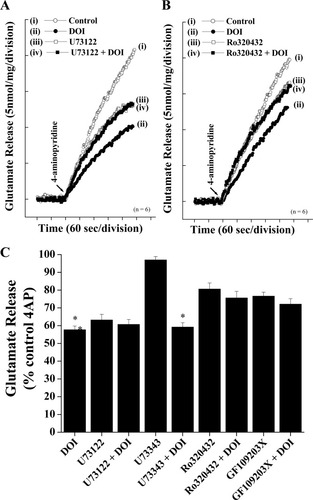
PLC and PKC inhibitors occlude DOI-mediated inhibition of glutamate release. Glutamate release was evoked by 3 mM 4AP in the absence (control) or presence of 50 μM DOI, 10 μM U73122, 10 μM U73122 + 50 μM DOI (A), or 50 μM DOI, 10 μM Ro320432, or 10 μM Ro320432 + 50 μM DOI (B). DOI was added 10 min before depolarization and U73122 and Ro320432 30 min prior to this. Results are averages of seven measurements obtained from an equal number of nerve terminal preparations and were calculated at each time point (2 sec). C: Quantitative comparison of the extent of glutamate release by 3 mM 4AP in the absence and presence of 50 μM DOI (added 10 min before depolarization) and absence and presence of 10 μM U73122, 10 μM U73343, 10 μM Ro320432, or 10 μM GF109203X (added 30 min before DOI). Results are presented as percentage of the control 4AP-evoked release and are mean ± SEM of experiments carried out with five to eight independent synaptosomal preparations. Asterisks denote significant effect by DOI compared with controls in absence of agonist (P < 0.05, ANOVA followed by post hoc two-tailed Student's t-tests).
Downstream of PLC activation, the two possibilities of diacylglycerol-mediated activation of PKC- and/or IP3-mediated liberation of Ca2+ stores exist as mediators of the modulation by 5-HT2A receptor/PLC activation. Although there is a paucity of data supporting the involvement intrasynaptosomal Ca2+ stores in glutamate release, there is a plethora of data implicating PKC as a key modulator of presynaptic function (Barrie et al., 1991; Sihra and Nichols, 1993). To examine the involvement of PKC in the modulation seen with 5-HT2A receptor activation, we performed experiments to test the effect of DOI following inhibition of PKC. Treatment of synaptosomes with the PKC inhibitor Ro320432 (10 μM for 30 min) itself attenuated control 4AP-evoked glutamate release from 20.1 ± 0.8 nmol/mg/5 min to 16.2 ± 0.4 nmol/mg/5 min (n = 6; P < 0.05; Fig. 7A,C). Under this condition, DOI (50 μM) further reduced release to only 15.3 ± 1.0 nmol/mg/5 min (Fig. 7C). In the presence of Ro320432, therefore, DOI induced a statistically insignificant inhibition of glutamate release of 4.9% ± 4.5% compared with the 42.3% ± 3.6% inhibition produced by DOI alone. This occlusion of the effect of DOI by PKC inhibition with Ro320432 was also obtained with another PKC inhibitor, GF109203X. As with Ro320432, although GF109203X (10 μM) attenuated control 4AP-evoked glutamate release by 23.4% ± 3.3%, it also occluded the inhibitory effect of DOI to 4.5% ± 3.1%, a statistically insignificant change (Fig. 7C).
These results with PLC and PKC inhibitors together indicate that 5-HT2A receptor-inhibited glutamate release involves a PLC/PKC-dependent pathway. However, these results and those described above are at odds with previous reports invoking a facilitatory function of 5-HT2A receptors operating through PLC/PKC and, indeed, our own studies over the years showing that activation of this signaling cascade largely facilitates the release of glutamate from rat cerebrocortical nerve terminals (Coffey et al., 1993, 1994; Wang and Sihra, 2004). Inhibitory modulation has largely been attributed to inhibitory GPCRs coupled to Gi/o (Wang et al., 2002; Wang and Sihra, 2003) that are pertussis (PTX) sensitive. We could, however, find no evidence for the inhibitory coupling of 5-HT2A receptor observed herein being through a PTX-sensitive G-protein (data not shown).
DISCUSSION
Inhibition of Glutamate Release by Presynaptic 5-HT2A Receptors
Although many previous studies have found that glutamatergic synaptic transmission and glutamate release in the cerebral cortex are increased by 5-HT2A receptors (Aghajanian and Marek, 1997, 1999b; Marek et al., 2000; Scruggs et al., 2003), activation of 5-HT2A receptors has also been reported to inhibit synaptic plasticity in the visual cortex (Edagawa et al., 2000) as well as glutamate release from cerebellar mossy fibers (Marcoli et al., 2001). These disparities and the relatively few reports of effects of 5-HT2A receptors on glutamate release studied directly in nerve terminals (Marcoli et al., 2001) prompted this investigation. In the present study, the principal finding was that isolated cerebrocortical glutamatergic nerve terminals have functional 5-HT2A receptors. Intriguingly, the activation of these receptors by DOI inhibited 4AP-evoked glutamate release through a mechanism that involves the suppression of synaptosomal excitability and voltage-dependent Ca2+-influx. These observations stand against the predominance of studies showing facilitatory effects 5-HT2A receptors on synaptic transmission, but perhaps even more surprising was our observation that the inhibitory effects of 5-HT2A receptor activation are also contingent on PLC/PKC activity.
Given the contentious nature of our primary observation of an inhibition of glutamate release by DOI, it was important to obviate the possibility that the agonist has nonspecific effects. Two lines of evidence argue against this parsimonious explanation. First, the lack of effect of DOI with alternative secretagogues such as KCl, ionomycin, or hypertonic sucrose argues against any spurious effect of the compound on nerve terminal function. Second, and most important, the DOI inhibition of glutamate release and Ca2+ influx was completely blockable by ketanserin, a selective 5-HT2A receptor antagonist with moderate affinity for 5-HT2C receptor (Baxter et al., 1995), strongly implying a receptor-mediated regulation.
DOI displays partial agonist activity at 5-HT2 receptors, which mainly reflects actions at 5-HT2A (Krebs-Thomson et al., 1998; Smith et al., 1998), although some activity of the agonist has also been attributed to 5-HT2C receptor stimulation (Chen et al., 2003). In the present study, the abrogation of the inhibitory effect DOI on 4AP-evoked glutamate release by ketanserin (a selective 5-HT2A receptor antagonist), together with the lack of effect of RS100221 (a 5-HT2C receptor antagonist), favors the presence of 5-HT2A- rather than 5-HT2C-receptor subtypes on glutamatergic nerve terminals in the rat cerebral cortex. Anatomical studies examining the presence of cortical 5-HT2A receptors have indicated a somatodendritic localization of 5-HT2A receptors on pyramidal and GABAergic neuron (Willins et al., 1997; Jakab and Goldman-Rakic, 1998; Santana et al., 2004), with a small proportion of receptors on axonal varicosities, which were apparently nonglutamatergic (Miner et al., 2003). Notwithstanding these observations, the present functional analysis argues for the presence of 5-HT2A receptors on glutamategic nerve terminals, in agreement with previous reports in which nerve terminals were studied in isolation (Marcoli et al., 2001). The apparent discrepancy between the receptor localization studies and the present report may reflect the relative high sensitivity of functional assays of neurotransmitter release modulation compared with immunocytochemical detection of expression levels of receptors. This is somewhat borne out by the functional demonstration of 5-HT2A receptors on glutamatergic terminals revealed by sensitive electrophysiological analyses of excitatory postsynaptic potentials in cortical slices (Aghajanian and Marek, 1997, 1999b; Marek and Aghajanian, 1999; Marek et al., 2000) and cortical microdialysis (Scruggs et al., 2003).
Mechanism of DOI-Induced Inhibition of Glutamate Release
Addressing the cellular mechanism of action of 5-HT2A receptors in the DOI-mediated inhibition of glutamate release, we considered three possibilities that might underlie the modulation: 1) alteration of synaptosomal excitability and downstream modulation of Ca2+ influx into the terminal, 2) direct regulation of VDCCs to affecting Ca2+ entry, and/or 3) direct modulation of the vesicular release machinery. The last of these possibilities was obviated by our observation that DOI did not affect ionomycin- or hypertonic sucrose-induced release. On the other hand, our demonstration, using the Ca2+ indicator Fura-2, that the 4AP-evoked increase in [Ca2+]C was reduced by DOI in a ketanserin-sensitive manner suggests that 5-HT2A receptor activation may be attenuating glutamate release by indirectly or directly reducing Ca2+ influx into cerebrocortical nerve terminals. We examined whether Ca2+ entry specifically through P/Q- and N- type VDCCs subserves the effect of DOI by assessing the effect of the agonist in the presence of Ca2+ channel toxins, singly or in combination. Our observation that the inhibitory effect of DOI on 4AP-evoked glutamate release was completely prevented only under conditions in which all of the release-coupled VDCCs had been blocked indicates that 5-HT2A receptors effectively suppress glutamate release components supported by P/Q- and N-type VDCCs. However, the fact that DOI remains largely effective in the presence of ω-CgTx GVIA (to block N-type VDCCs) seems to implicate P/Q-type VDCCs as being predominantly targeted by 5-HT2A receptor activation.
Given these results, the question remained of whether the suppression of release-coupled VDCCs by 5-HT2A receptor action reflected a direct effect on VDCC function. To address this issue, we compared the relative effects of DOI on the 4AP- vs. KCl-evoked activation of synaptosomal Ca2+ entry. Both of these depolarizing treatments are thought to activate P/Q- and N-type VDCCs coupled to glutamate similarly and thus should reflect this by qualitatively similar modulation, if this occurs at the level of VDCCs. Where the two depolarizing paradigms differ is in that, whereas 4AP-mediated depolarization/VDCC activation involves upstream ion (K+ and Na+)-channel activity, 30 mM external KCl effects experimental clamping of the K+ electrochemical potential and hence membrane potential (Nicholls, 1998). As such, the latter type of depolarization bypasses regulation at the level of K+ channels and also effectively removes voltage-dependent Na+ channel involvement, in that these channels desensitize in response to strong and continued depolarization (Barrie et al., 1991). Comparison of the two depolarizing agents revealed that 4AP- but not 30 mM KCl-evoked VDCC activation was inhibited by DOI stimulation of 5-HT2A receptors. The lack of effect of DOI on 30 mM KCl-induced Ca2+ influx was paralleled by the similar inability of DOI to affect 30 mM KCl-induced glutamate release.
Based on the mechanistic differences between the 4AP- and the KCl-mediated depolarization discussed above, the data presented are consistent with the regulation of glutamate release mediated by 5-HT2A receptor activation impinging upstream of VDCCs, possibly at the level of synaptosomal excitability. In line with this, 4AP-mediated depolarization, probed using the membrane potential-sensitive dye DiSC3(5), was found to be inhibited by DOI. Such an effect of DOI on synaptosomal excitability could be brought about as result of a 5-HT2A receptor-mediated regulation of hyperpolarizing K+ channels, as has been proposed previously (Bartrup and Newberry, 1994), or, alternatively, through a mechanism involving 5-HT2A receptor stabilization of Na+ channels to limit 4AP-mediated depolarization of the synaptosomal membrane potential.
Notwithstanding the rationale for the use of differential modulation of 4AP- and KCl-mediated as an indicator of the locus of regulatory action being upstream of Ca2+ entry, it is prudent to consider an important alternative scenario. It is possible that the modulatory effects of presynaptic metabotropic receptors impinge on the activation kinetics of presynaptic ion channels. In such a situation, modulation by 5-HT2A receptor activation would be apparent only with 4AP-depolarization, during which channels would be repetitively opening and closing, and not with KCl depolarization, when constantly clamped/activated. In the absence of any means to address this directly in synaptosomes, we would therefore be circumspect about stating that 5-HT2A receptor activation affects only loci upstream of VDCC activation rather than VDCCs directly. Having said this, direct metabotropic receptor inhibition of the VDCC is generally thought to involve Gi/o coupling. Not only are 5-HT2A receptors proposed to tranduce via Gq but we could find no evidence for Gi/o involvement on the basis of pertussis toxin sensitivity of the 5-HT2A receptor-mediated inhibition reported herein (data not shown). Our tentative conclusion is therefore that the inhibition of glutamate release by 5-HT2A receptor activation occurs primarily by suppression of synaptosomal excitability.
In synaptic terminals, the activation of K+ channels is recognized to result in presynaptic inhibition resulting from the enhancement of K+ conductance bringing about nerve terminal hyperpolarization. This causes a subsequent decrease in voltage-dependent presynaptic Ca2+ influx and a consequent reduction of neurotransmitter release (Nicoll, 1988). Such a mechanism could potentially subserve the 5-HT2A receptor-mediated inhibition 4AP-mediated glutamate release from cerebrocortical synaptosomes. Apart from the directly observed attenuation of 4AP-evoked synaptosomal plasma membrane depolarization by DOI, our observation that DOI inhibits Ca2+-independent glutamate release also points to a suppression of synaptosomal excitability. Together with the Ca2+-dependent exocytosis of glutamate, Ca2+-independent, nonvesicular/cytosolic efflux can also be elicited directly as a result of the depolarization of the nerve terminals by using biochemical methods (Nicholls et al., 1987). It is known that, under such conditions (possibly resembling those occurring during brain ischaemia), the uptake carrier can work in reverse to cause the efflux of glutamate out of the neuron and thus essentially serve to invoke a Ca2+-independent, nonvesicular mechanism for glutamate release (Nicholls, 1993; Szatkowski and Attwell, 1994). The magnitude of this efflux of glutamate is a direct reflection of the extent of depolarization-dependent reversal of the electrogenic glutamate transporter produced by biochemical secretagogues (Nicholls et al., 1987). Thus our observation that DOI inhibits this efflux supports the hypothesis that 5-HT2A receptor activation leads to a suppressive effect on nerve terminal excitability, although a direct receptor-mediated inhibition of the glutamate carrier itself cannot be ruled out at this point. Experiments using the glutamate transport blockers L-trans-PDC and DL-TBOA, though inconclusive on the latter point, do serve to show that, even in the absence of transporter activity, an effect of DOI on the physiological, Ca2+-dependent release of glutamate persists, albeit at a reduced level.
The inhibitory effect of 5-HT2A receptor activation on glutamate release through a reduction in synaptosomal excitability and/or alteration in VDCC activity is surprising in that it contrasts with several previous reports utilizing slice and in vivo preparations. Thus, although 5-HT2A receptors have been proposed to exist on glutamatergic terminals from whole-cell patch clamp studies of rat prefrontal cortical slices (Aghajanian and Marek, 1997, 1999b; Marek and Aghajanian, 1999; Marek et al., 2000) and cortical microdialysis analysis (Scruggs et al., 2003), the modulation observed has been found to be facilitatory. Moreover, the regulation was suggested to operate by the inhibition of voltage-gated K+ channels, which would increase neuronal excitability (Lambe and Aghajanian, 2001). Where inhibitory responses to 5-HT2A receptors have been observed in the past, they have been attributed to 5-HT2A receptors on GABAergic interneurons facilitating GABA release to hyperpolarize distal pyramidal neurons (Tanaka and North, 1993; Marek and Aghajanian, 1994; Zhou and Hablitz, 1999). Although the latter mechanism clearly cannot operate within synaptosomal preparation and under the conditions of our experiments, a previous study has shown direct inhibitory 5-HT2A receptor coupling to hyperpolarizing K+ channels (Bartrup and Newberry, 1994). The latter type of influence of 5-HT2A receptors would be in line with our observation with synaptosomes, but, notably, the aforementioned study was carried out with C6 glioma cells and without looking at neurotransmitter release. In another notable case in which isolated nerve terminals were utilized, in congruence with the current observations, DOI was found to inhibit synaptosomal glutamate release, albeit through an undefined mechanism (Marcoli et al., 2001). What then might be the reason(s) for the aforementioned diametrically conflicting observations? Nothwithstanding the contrasting techniques used to demonstrate the facilatatory effects of 5-HT2A receptor in previous studies and the inhibitory activity reported herein, we sought to understand the reasons for the discrepancy by examining whether this could be due to differential signaling mechanisms evoked by 5-HT2A receptor activation.
Second Messenger Involvement in 5-HT2A Receptor-Mediated Inhibition of Glutamate Release
5-HT2A receptors are representative of a family of neurotransmitter receptors known to couple functionally to Gq/G11 proteins to stimulate PLC and phosphatidylinositide turnover, resulting in the activation of PKC (Hoyer et al., 1994). Consistently with this, in the present study, a G-protein coupled to the PLC/PKC pathway seems to be involved in the inhibitory action of DOI on 4AP-evoked glutamate release from cerebrocortical synaptosomes. Two lines of evidence support this notion. First, the inhibitory effect of DOI on 4AP-evoked glutamate release was occluded by inhibiting PLC with the specific inhibitor U73122, but not by the inactive analogue U73343. Second, in synaptosomes treated with the specific PKC inhibitors Ro320432 or GF109203X, the inhibition of release by DOI was substantially reduced (∼4% of inhibition remained). The lack of complete suppression of the DOI-induced inhibitory action may be explained by the incomplete block of PKC by 10 μM Ro320432 or GF 109203X. These results with PLC and PKC inhibitors, taken together with the possibility that DOI reduced 4AP-induced synaptosomal excitability, suggest that a 5-HT2A receptor-PLC/PKC activity either increases K+ conductance or decreases Na+ conductance to reduce Ca2+ influx through P/Q- and N-type Ca2+ channels and, in turn, reduces glutamate release. Despite the inhibitory coupling, we found no evidence for the 5-HT2A receptor modulation of 4AP-evoked glutamate release being PTX-sensitive (data not shown), as has been the case for number of other inhibitory presynaptic receptors that we and others have shown to modulate glutamate release (Sanchez-Prieto et al., 1996; Wang et al., 2002; Wang and Sihra, 2003).
The conclusion from this analysis is that the presynaptic inhibitory action of 5-HT2A receptors on glutamate release from synaptosomes is mediated by a G-protein-coupled PLC/PKC-dependent pathway. The question is how the inhibitory effect of 5-HT2A receptor activation reported herein can be consolidated with the previous nonsynaptosomal studies. In these earlier studies, using electrophysiological techniques in rat cortical neurons and in vivo microdialysis, 5-HT2A receptor activation was reported to facilitate excitatory synaptic transmission and glutamate release, respectively, again, through Gq/PLC/PKC coupling (Aghajanian and Marek, 1999a; Lambe and Aghajanian, 2001; Scruggs et al., 2003). Given that the slice and in vivo preparations would not delineate pre- and postsynaptic receptor responses, one possibility is that these reports reflect the overall effect of 5-HT2A receptors at multiple localizations, whereas the synaptosomal studies exclusively reflect the behavior of a presynaptic form of the receptor. However, given that there is little evidence for alternative forms of the 5-HT2A receptor in different neuronal localizations, this situation seems unlikely. Another tenable hypothesis to explain the divergent data would be that the coupling of presynaptically localized 5-HT2A receptor through a Gq/PLC/PKC mechanism is switched to an inhibitory state. There is some precedence for switching of Gq/PLC/PKC signaling from facilitatory to inhibitory modes from work with metabotropic glutamate receptors (mGluRs). Thus, although type 1 mGluRs are well-established facilitators of glutamate release through Gq/PLC/PKC coupling (Sanchez-Prieto et al., 1996), following prolonged receptor activation they are able to undergo a temporal switch to effect release inhibition by the same Gq/PLC/PKC pathway (Rodriguez-Moreno et al., 1998; Herrero et al., 1998). The switching appears to be contingent on the balance between kinase and phosphatase activity (Sistiaga and Sanchez-Prieto, 2000). It remains to be seen whether the reported discrepancies in the direction of 5-HT2A receptor-mediated regulation of glutamate release reflect such a mode switching of a Gq/PLC/PKC pathway, depending on the particular experimental scenario.
One final possibility to explain the dichotomy of modulation seen pertains to the potential specific pharmacological actions of DOI. It is well established that basal/constitutive PLC/PKC in isolated nerve terminals is high in the absence of any agonist (Barrie et al., 1991; Coffey et al., 1993). If, as has be indicated for several G-protein-coupled receptors, this constitutive activity is in part produced by the unliganded stimulatory activity of 5-HT2A receptor/Gq/PLC/PKC cascade, then there is the potential for an agonist to produce a paradoxical suppression of this signaling pathway. Given this, one possible mechanism by which DOI might cause an inhibition of glutamate release is by acting on the 5-HT2A receptor/Gq/PLC/PKC cascade as an inverse agonist. The paucity of specific agonists at 5-HT2A receptors makes the examination of this possibility problematic at the present time, but it is interesting to note, in this regard, that a 5-HT2A receptor ligand, LY367265, purported to be a receptor antagonist has an inhibitory effect similar to that of DOI with respect to glutamate release (Wang, 2005).
Therapeutic Considerations
Better knowledge of the 5-HT2A receptor-induced inhibition of glutamate release in cerebrocortical nerve terminals is of potential interest for a number of pathological conditions including neurodegenerative and psychiatric disorders. In fact, excessive glutamate release has been proposed to be involved in the pathogenesis of these diseases (Beal, 1992), and suppression of glutamate release from nerve terminals is considered to be a novel treatment strategy for CNS disorders. On the basis of the results presented, we would suggest that presynaptic 5-HT2A receptors might have a physiological role in preventing the excessive accumulation of extracellular glutamate occurring as a result of repetitive activity and thereby potentially act in a neuroprotective manner in cerebral cortex.




